Abstract
Purpose
To evaluate the chemical and enzymatic stabilities of methoxy, ethoxy and propylene glycol linker containing prodrugs in order to find a suitable linker for prodrugs of carboxylic acids with amino acids.
Methods
L-valine and L-phenylalanine prodrugs of model compounds (benzoic acid and phenyl acetic acid) containing methoxy, ethoxy and propylene glycol linkers were synthesized. The hydrolysis rate profile of each compound was studied at physiologically relevant pHs (1.2, 4, 6 and 7.4). Enzymatic hydrolysis of propylene glycol containing compounds was studied using Caco-2 homogenate as well as purified enzymes, valacyclovirase.
Results
It was observed that the stability of the prodrugs increases with the linker length (propyl>ethyl>methyl). The model prodrugs were stable at acidic pH as compared to basic pH. It was observed that the prodrug with the aliphatic amino acid promoiety was more stable as compared to its aromatic counterpart. The comparison between benzyl and the phenyl model compounds revealed that the amino acid side chain is significant in determining the stability of the prodrug whereas the benzyl or phenyl carboxylic acid had little or no effect on the stability. The enzymatic activation studies of propylene glycol linker prodrug in presence of valacyclovirase and cell homogenate showed faster generation of the parent drug at pH 7.4. The half life of prodrugs at pH 7.4 was more than 12 hours, whereas in presence of cell homogenate the half lives were less than 1 hour. Hydrolysis by Caco-2 homogenate generated the parent compound in two steps, where the prodrug was first converted to the intermediate, propylene glycol benzoate, which was then converted to the parent compound (benzoic acid). Enzymatic hydrolysis of propylene glycol containing prodrugs by valacyclovirase showed hydrolysis of the amino acid ester part to generate the propylene glycol ester of model compound (propylene glycol benzoate) as the major product.
Conclusion
The methoxy linker containing amino acid prodrugs were the least stable while propylene glycol linker containing prodrugs were most stable. This work suggests that the propylene glycol linker is an optimal linker for amino acids prodrugs since it has good chemical stability and is enzymatically hydrolyzed to yield the parent drug. This approach can be further extended to other non-amino acid prodrugs and to provide a chemical handle to modify lead molecules containing carboxylic group(s).
Keywords: Acyloxyester, prodrugs, aminoacids, propylene glycol, valacyclovirase
Introduction
Drugs containing ionizable polar groups like carboxylic acids are poorly absorbed from gastrointestinal (GI) tract due to their physicochemical properties.1 The absorption of these drugs can be improved by masking the carboxyl group by forming simple esters.2–6 Aliphatic or aryl esters prodrugs are sometimes successful in addressing the absorption issues, but most of the time does so by compromising the aqueous solubility of parent drug and/or decreasing the potency of parent drug by forming stable prodrugs leading to incomplete bioconversion. Another approach to enhance the absorption of these poorly absorbed carboxylic acids is to make them substrates for the carrier mediated transport. The oral bioavailability of poorly absorbed drugs can be enhanced by transport by oligopeptide transporters.7 Peptide transporters (PEPT1) are present in the epithelial cells of small intestine and are instrumental in transporting di/tri peptides and peptidomimetics across the membrane against concentration gradient.8 Conjugating specific amino acid with a carboxylic acid containing drug can make them substrates for PEPT1, thus enhancing the absorption of the parent drug.9, 10 An amino acid prodrug of the carboxylic acid drug can be synthesized by forming an amide between the amine of aminoacid and carboxylic acid of the drug. The amide prodrugs are mostly stable in-vivo; thereby risk decreasing the potency of the drug by incomplete bioconversion.11 An alternate approach is to link the two carboxylic acid groups through an alkyloxy linker. Acyloxy ester prodrugs are used to enhance the absorption of penicillins; the classic example is of bacampicillin an ethoxycarbonyloxyethyl ester prodrug of ampicillin.12 As with any prodrugs there should be right balance between chemical and metabolic stability. There are not enough evidence and studies on the stability of the acyloxy (alkyloxy) ester prodrugs of carboxylic acids using amino acid as a promoiety.
In this work, we report the effect of linker length on the stability of the amino acid alkyloxy ester prodrugs of carboxylic acids. We have used two model compounds; benzoic acid and phenylacetic acid as parent drugs representing aromatic model compound. The aromaticity of the model compounds provided the ease of analytical identification of the prodrugs and the metabolites. Here, we compare the effect of L-valine and L-phenylalanine as the promoieties. L-valine was chosen as one of the promoieties as it has been successfully used as the promoiety to enhance the transport of drugs by PEPT1 transporter e.g. valacyclovir and gancyclovir.7 Previous studies in our lab have shown that prodrugs with valine promoiety are better substrates for PEPT1 transporter.9, 13 Electron releasing or withdrawing properties of amino acid side chain affect stability of ester bond. An L-valine and L-phenylalanine side chain offers electron donating and electron withdrawing effects, respectively; therefore it will provide a good comparison of effect of amino acid on the stability of the prodrugs. The pH profile of hydrolysis and enzymatic activation of the model prodrugs are reported.
Materials and Methods
All chemicals were reagent, analytical or HPLC grade. The tert-butyloxycarbonyl (Boc) protected L-amino acids were obtained either from Calbiochem-Novabiochem (San Diego, CA, USA) or Alfa Aesar (Ward Hill, MA, USA). ACS grade solvents, trifluoroacetic acid, anhydrous N, N-dimethyl formamide (DMF), triethylamine and other chemicals were procured from Aldrich Chemical Company (Milwaukee, WI, USA). HPLC grade acetonitrile and trifluoroacetic acid were obtained from Fisher Scientific 9St. Louis, MO, USA). Valacyclovirase was previously cloned and purified by Amidon Lab and used as such for activation studies.
Experimental Section
Synthesis
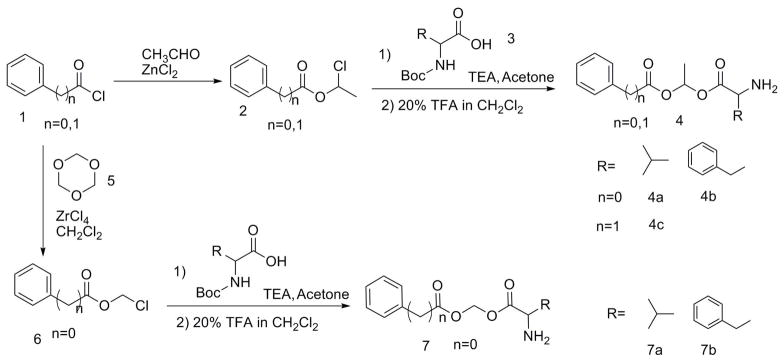
The acyloxy esters of L-valine and L-phenylalanine were synthesized according to scheme 1. Phenylacetic acid and benzoic acid were converted to their respective chloromethyl esters by reacting with either acetaldehyde or trioxane.14, 15 The chloromethyl ester was reacted with Boc-protected amino acids in DMF in presence of base to form acyloxyester of aminoacid with benzoic acid or phenylacetic acid.14 The Boc group of the esters is deprotected by trifluroacetic acid in dichloromethane to yield final esters as trifluroacetate salt.
Scheme 1.
Syntheses of Methoxy and Ethoxy Linker Prodrugs
(S)-(2-amino-3-phenylpropanoyloxy)methyl benzoate (BA-Et-Val, 4a)
1H NMR (CD3OD) δ (ppm) 0.98(m, 6H), 1.24(d, J = 8.2 Hz, 3H), 2.13(m, 1H), 4.03 (m, 1H), 7.13(m, 1H), 7.h1(m, 2H), 7.64(m, 1H), 8.03(m, 2H); ESI-MS: 266.2 (M+H)+
1-((S)-2-amino-3-phenylpropanoyloxy) ethyl benzoate (BA-Et-Phe,4b)
1H NMR (CD3OD) δ (ppm) 1.61 (d, J = 5.2 Hz, 3H), 3.06(m, 2H), 4.5(m, 1H), 7.09(q, J = 5.6 Hz, 1H), 7.1(m, 4H), 7.23(m, 1H), 7.4(m, 2H), 7.5(m, 1H), 8.2(m, 2H); ESI-MS: 314.1 (M+H)+
1-(2-phenylacetoxy) ethyl 2-amino-3-methylbutanoate (PA-Et-Val, 4c)
1H NMR (CD3OD) δ (ppm) 0.9(m, 6H), 1.59(d J = 4.6 Hz, 3H), 2.09(m, 1H), 3.6 (s, 2H), 4.1(m, 1H), 6.9(m, 1H), 7.2(m, 5H); ESI-MS: 280.2 (M+H)+
(2-amino-3-phenylpropanoyloxy) methyl benzoate (BA-Me-Phe, 7b)
1H NMR (CD3OD) δ (ppm) 2.9 (dd, J = 5.2, 8.2 Hz, 1H), 3.1 (dd J = 6, 7.6 Hz, 1H), 4.4(m, 1H), 6.0 (dd, J = 5.6, 10 Hz, 2H), 7.1(m, 5H), 7.5 (m, 2H), 7.6(m, 1H), 8.0(d, J = 7.2 Hz, 2H)
2-hydroxypropyl benzoate (10)
To a stirring solution of 2-methyloxirane, 9 (573 μL, 8.19mmol) in anhydrous acetonitrile were added benzoic acid, 8 (1.0 g, 8.19mmol) and catalytic amount of tert-butyl ammonium bromide (TBAB, 39mg, 0.254 mmol).16 Resulting solution was then refluxed overnight. When all benzoic acid was used up (TLC, EtOAc:Hexane 1:1), solvent was evaporated and reaction mixture was dissolved in water and then extracted with chloroform and dried over magnesium sulfate. Solution was then filtered and evaporated under reduced pressure to give 2-hydroxypropyl benzoate which was used as such for further reaction. Compound was >90% pure and 1.2 g was obtained with 88% yield.
BA-PG-L-Val (12a)
2-hydroxypropyl benzoate, 3 (200mg, 1.1 mmol), Boc-L-valine (289 mg, 1.33mmol), DMAP (162.7 mg, 1.33mmol) and EDCI (206.8mg, 1.33mmol) were mixed together and stirred in 10mL anhydrous DMF for 4–6 hours.17 After completion of the reaction, DMF was evaporated under reduced pressure and later purified using flash column chromatography using ethyl acetate/hexane (1/5) to give 301 mg of clear oil in 64 % yield. For the synthesis of final compound 5a, Boc-protected derivative was stirred with 40% trifluoroacetic acid in dichloromethane for about 2 hours.18 Following completion of reaction, compounds were vacuum dried and later lyophilized as clear oil. 1H NMR (CDCl3) δ (ppm) 0.96–1.08 (m, 6H), 1.40 (dd, J = 6.5, 3H), 2.33 (m, 1H), 3.95 (m, 1H), 4.23–4.47 (m, 2H), 5.35–5.41 (m, 1H), 7.46 (t, J = 7.0, 2H), 7.59 9t, J = 7.05, 1H), 8.00–8.04 (m, 2H); ESI-MS: 280.1 (M+H)+
BA-PG-Phe(12b)
Same procedure as used for synthesis of 12a. Compound was obtained as clear oil in an overall yield of 74%. 1H NMR (CDCl3) δ (ppm) 1.23–1.39 (m, 3H), 2.96–3.33 (m, 2H), 4.24–4.42 (m, 3H), 5.26–5.38 (m, 1H), 7.12–7.30 (m, 5H), 7.37–7.48 (m, 2H), 7.52–7.63 (m, 1H), 7.91–8.06 (m, 2H).); ESI-MS: 328.1 (M+H)+
Stability and Hydrolysis Studies
An ideal prodrug must be stable enough to be absorbed intact across intestinal mucosa but should be converted to parent drug following its active/passive transport. Chemical and metabolic stability helps in screening ideal candidate for further studies.
1. Chemical Stability
The chemical stability of prodrugs was studied in 100mM buffers at various pHs. For, pH 1.2 hydrochlorate buffer (85ml of 0.2M hydrochloric acid and 50ml of 0.2M potassium chloride), pH 4 acetate buffer (0.3g sodium acetate trihydrate and 3.9ml of 2N acetic acid), pH 6 phosphate buffer (50 ml of 0.2M monobasic potassium phosphate and 5.6ml of 0.2M sodium hydroxide) and pH 7.4 phosphate buffer (50 ml of 0.2M monobasic potassium phosphate and 39.1ml of 0.2M sodium hydroxide), final volume was made to 400ml. 50 μL samples was taken at time points and diluted with 0.1% ice cold trifluoroacetic acid and analyzed by HPLC.
2. Metabolic Stability
2a. Stability in Rat Intestinal Perfusate
Rat intestinal perfusate was collected as described earlier.19 Compounds were incubated with 90% of intestinal perfusate at final concentration of 100μM and analyzed by HPLC following sample preparation.
2a. Hydrolysis in cell homogenates
Caco-2 cells (passage 36) were seeded for 22 days and washed with phosphate buffer saline (pH 7.4). The cells were scrapped and collected in 100mM phosphate buffer (pH 7.4) and spun down by centrifugation. The cells were re-suspended in phosphate buffer and lysed by sonicating for a few seconds. The cell lysate was centrifuged at 10000rpm for 15 min and the supernatant was collected. The protein amount of supernatant was determined with the Bio-Rad DC Protein Assay using bovine serum albumin as the standard. Van Gelder et al have studied the linearity of esterase activity in intestinal tissue homogenates of various species including human intestine and had derived a protein concentration corresponding to the esterase activity in the respective tissue homogenates.20 The Caco-2 protein concentration (500μg/mL) corresponds to 100μg/mL protein concentration from human intestinal homogenate for similar esterase activity. All model compounds were incubated with Caco-2 cell extract (500 μg/ml of protein) at 37°C for over 2 hours. Sample was taken up at the specific time points (0, 5,10,15,20 and 30min) and mixed with acetonitrile containing 0.1% trifluoroacetic acid at 0 °C. The samples were spun at 10,000 rpm at 4°C for 15 min and the supernatant was filtered through polypropylene filter, 0.45μm, Whatman® for HPLC analysis.
2b. Hydrolysis with Valacyclovirase
Possible hydrolysis of model compound prodrugs was evaluated in presence valacyclovirase as described earlier.21 Hydrolysis due to buffers was normalized and Kcat and Vmax were calculated using GraphPad Prism v4.0 software.
Data Analysis
HPLC Analysis
The samples from enzymatic and chemical stability studies of all model prodrugs were analyzed by Agilent 1100 Series HPLC system (Agilent Technologies, Inc. Santa Clara CA) equipped with two Agilent 1100 pumps, a Agilent autosampler maintained at 4 °C and a Agilent PDA detector. The system was operated by Agilent Chemstation software. Waters Xterra® C18 reversed-phase (3.5 μ, 4.6 × 50 mm) and Agilent Zorbax Sb-Aq (3.5 μ, 4.6 × 150 mm) columns were used for sample analysis. The analytes were eluted using a gradient/isocratic method at 1mL/min. The mobile phases (water and acetonitrile) contained 0.1% TFA as modifier.
Stability Studies
Apparent first order kinetics and rate constants were determined by using initial rates of hydrolysis. The apparent first-order degradation rate constants of prodrugs at 37°C were determined by plotting the logarithm of prodrug remaining as a function of time. The relation between the rates constant, k, and slopes of the plots are explained by the equation:
The degradation half-lives were then calculated by the equation:
Results
Synthesis
Model compounds involving linkers (methyl, ethyl, and propyl) were successfully synthesized in overall yield of approximately 65%. The compounds containing stereocenters were used as a racemic mixture. Final compounds were lyophilized to give clear oil. All the compounds were analyzed for their purity by HPLC. All compounds had a purity of greater than 95%. The identity of all the intermediates and final compounds was confirmed by ESI-MS and [1H] NMR.
Hydrolysis Studies
Chemical Stability and Kinetics
The chemical stability and hydrolysis kinetics were determined by evaluating the hydrolysis rates in 100mM isotonic buffers (pHs 1.2, 4.0, 6.0 and 7.4). Physiologically relevant pHs were chosen to study the hydrolysis of model compounds. All the compounds displayed first order kinetics while methoxy linker containing compounds could not be studied due to their instability at pH 7.4. Apparent first order kinetics and rate constants were determined by using initial rates of hydrolysis. The first-order degradation rate constants of model compounds at 37°C were determined by plotting the logarithm of prodrug concentration remaining as a function of time.
Comparison of half lives of methoxy, ethoxy and propylene glycol linkers containing model compounds at different pH is summarized in Table 1. All the compounds underwent acid/base catalyzed hydrolysis characteristic of esters. The propylene glycol containing compounds were found to be far more stable at any given pH when compared to the methoxy and ethoxy esters. The pronounced difference in half lives is evidenced by the comparison of the half lives of the prodrugs at pH 7.4. The Estimated t1/2 for BA-PG-Phe and BA-PG-Val at pH 7.4 was found to be around 21 hours and 24 hours respectively, which is >20 fold stable as compared to methoxy and ethoxy linker prodrugs. The pH dependent degradation of BA-PG-Phe and BA-PG-Val shows that these prodrugs are notably stable at lower pH (pH 6 or lower) producing half lives > 30 hours (Figure 1 and 2). Chemical stability increased with increase in linker length so the order of stability depending on linker length was as PG linker> acyloxy ethyl linker > acyloxy methyl linker. Valine esters were more stable than the phenylalanine counterpart as expected. There were no noticeable differences in the stability between acyloxy ethoxy valine ester of benzoic acid and phenylacetic acid.
Table 1.
Half Lives for the Methoxy, Ethoxy and Propylene Glycol Linker Containing Prodrugs in Isotonic Buffers at pH 1.2, 4, 6 and 7.4 as well as in Rat Lumenal Perfusate and Rat Intestinal Homogenate at 37°C (n=3)
| Compounds | Half lives, t1/2 (min) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1.2 | 4.0 | 6.0 | 7.4 | Rat Lumenal Perfusate | Rat Intestinal Homogenate | |
| BA-Et-Phe | 1732 | 3465* | 385±19 | 24±2 | ND** | ND** |
| BA-Me-Phe | 193 | 385 | 2.1 | 0.13 | ND** | ND** |
| BA-Et-Val | 8662* | 17325* | 2772* | 46.2 | ND** | ND** |
| BA-Me-Val | 630 | 1307 | 3.5 | 0.14 | ND** | ND** |
| PA-Et-Val | 11550* | 23100* | 1732.5 | 50± 14.8 | ND** | ND** |
| BA-PG-Phe | >1800 | >1800 | >1800 | 1278.4±265.4 | > 120 | <60 |
| BA-PG-Val | >1800 | >1800 | >1800 | 1454.4±165.6 | >120 | <60 |
Estimated half lives
Not determined
Figure 1.
pH dependent degradation of BA-PG-Phe at 37°C. The percentage of prodrug remaining at given time is plotted against time.
Figure 2.
pH dependent degradation of BA-PG-Val at 37°C. The percentage of prodrug remaining at given time is plotted against time.
The pH rate profiles for all compounds were evaluated. The degradation of compounds at a given pH is catalyzed by hydronium and/or hydroxide ions and water. The reactions dependent on hydronium or hydroxide usually follows pseudo-first-order kinetics and the rate is described by the first order rate constant, kobs. Plotting kobs against pH gave the pH rate profile for the model compounds. The pH profile showed V-shaped curve suggesting hydrolysis by both hydronium and hydroxide as expected for any ester hydrolysis.
Stability in Rat Intestinal Perfusate
To test the stability of prodrugs in the intestine before absorption, rat intestinal perfusate was used. It was found that both BA-PG-Val and BA-PG-Phe have estimated half life to be >2h in 90% perfusate while the control compound leu-enkephalin, which is a well known substrate for luminal enzymes22 was completely hydrolyzed within 1h of incubation under similar conditions. This study shows that reasonable stability can be achieved using PG linker which can further be fine tuned using various promoieties.
Cell-homogenate Stability
The tested compounds were incubated with Caco-2 cell homogenates (500μg/mL) and samples were analyzed by HPLC. Half-life was estimated from linear regression of prodrug concentration remaining vs. time. Half-life in buffer at pH 7.4 was estimated as control and values were corrected to yield half-lives values in cell homogenate. BA-PG-PHE was rapidly hydrolyzed with estimated t1/2 found to be <5min. For BA-PG-VAL estimated t1/2 was approximately 43 minutes. Figure 4a shows the disappearance of BA-PG-PHE and appearance of metabolites. As shown in Figure 4b, time course for the disappearance of BA-PG-Val, for first 60 min both metabolites BA-PG and BA are increasing followed by sharp increase in BA while decrease in BA-PG. This indicates that BA-PG is simultaneously converted to BA thus generating the compound of interest. This also demonstrates sequential activation of model compound first from BA-PG-VAL to BA-PG and then to BA.
Figure 4.
Time course for disappearance prodrug and appearance of metabolites in Caco-2 cell homogenate a) Degradation of BA-PG-Phe in presence of Caco-2 homogenate at pH 7.4 at 37°C b) Degradation of BA-PG-Val in presence of Caco-2 homogenate at pH 7.4 at 37°C
This type of Pseudo-First-Order consecutive reaction can be represented by following equation.
In this equation, prodrug (PD) first converts to intermediate PD′ which is then converted to desired therapeutic agent, D. Following mathematical model can be applied for this type of reactions as represented in Figure 4.
By fitting the Caco-2 data for BA-PG-Val and BA-PG-Phe, k1 for BA-PG-Val and BA-PG-Phe was found to be 0.021 and 0.192 min−1, respectively. k2 for BA-PG was found to be 0.0016 min−1. This shows that prodrug gets converted at a faster rate to BA-PG which eventually hydrolyzes to BA at a relatively slower rate. Thus, concentration of BA-PG can be determined at any time point using above equation. As compared to various pH buffers, fast degradation in cell homogenate suggests that the parent drug may be rapidly generated in vivo by action of cellular enzymes for example esterases and valacyclovirase.
Hydrolysis with Valacyclovirase
BA-PG-Val and BA-PG-Phe model compounds when incubated with valacyclovirase, rapidly hydrolyzes to BA-PG suggesting potential activation mechanism for the similar prodrugs. For BA-PG-Val and BA-PG-Phe Km was found to be 0.46 mM and 2.37 mM, respectively and Km/Kcat was found to be 39.13 and 5.46mM−1s−1, respectively (Table 2). Both compounds when incubated with porcine carboxyesterases I with and without valacyclovirase generates BA exclusively and rapidly (data not shown). This suggests their direct and/or sequential hydrolysis to generate desired compound BA (Scheme 2). This result demonstrates potential activation mechanism which can be utilized to design similar prodrugs.
Table 2.
Kinetic Parameters for Valacyclovirase Hydrolysis of Propylene Glycol Linker Prodrugs
| Propylene Glycol Linker Prodrugs | Km (mM) | Vmax (mM/min) | Kcat/Km (mM−1s−1) |
|---|---|---|---|

|
0.46 | 0.0033 | 39.13 |

|
2.55 | 0.0025 | 5.46 |
Scheme 2.
Synthesis of Propylene Glycol Linker Prodrugs
Hydrolysis studies shows that model compounds may be stable enough to be absorbed intact but have the potential to release active compound immediately after absorption. Kinetics with valacyclovirase and Caco-2 homogenate suggests that these can be potential substrates for the enzymes which can catalyze the hydrolytic activation of prodrugs to give parent drug. Thus prodrugs with PG linkers may be good candidates for further developments.
Discussion
Low toxicity of aminoacids makes them attractive carriers for development of prodrugs of poorly absorbed therapeutic agents containing carboxylic group. In addition to making the prodrug a substrate for carrier mediated transport, amino acid promoiety also balances the water solubility of the prodrug. Acyloxy ester linkers are mainly used to make ester prodrugs of compounds containing carboxyl groups. Major hurdle in developing the acyloxy ester prodrugs of amino acid is the low stability of the prodrug. The prodrug is destabilized by the presence of α-amino group of amino acids either due to the strong electron-withdrawing effect of the protonated amino group at physiological pH or due to the ability of unprotonated amino group to increase hydrolysis through nucleophilic and general-base catalysis.23 An alternate approach to circumvent the instability is to develop a carbonate of the amino acids.24 Although this approach is successful in improving the stability of the prodrug it might compromise the affinity of the prodrug for the amino acid transport in-vivo thereby affecting the absorption. Therefore, we decided to alter the type of linker and tune the stability accordingly.
To evaluate the linker effect on stability of prodrugs, we synthesized model prodrugs containing methoxy, ethoxy and propylene glycol linkers. The methoxy linker containing prodrugs are less stable than ethoxy and propylene glycol linker containing prodrugs. The phenylalanine prodrugs were less stable as compared to the valine counterpart as expected due to destabilization by electron withdrawing phenyl ring. The stability profile of valyl prodrugs of benzoic acid and phenylacetic acid model compound were more or less identical suggesting that the α-amino group of the aminoacid is the destabilizing factor and the proximity of the aromatic ring on the other side of the molecule has little or no effect. The stability of the prodrugs is slightly increased in case of ethoxy linker as compared to the methoxy linker containing model compound but it may not be stable enough for a compound to be suitable for oral delivery. Hence, we developed the propylene glycol linker containing prodrugs as the data with methoxy and ethoxy linker suggested that the increase in spacer length between the two carbonyls increased stability. Propylene glycol was chosen as it is a commonly used excipient in pharmaceutical formulations with no known toxic effects. The model compounds containing propylene glycol was very stable, as evident from their half lives at pH 7.4. Propylene glycol linker containing model compound prodrugs has shown more than 20 fold increase in stability at pH 7.4 as compared to methoxy and ethoxy counterparts.
All the model compounds were hydrolyzed by cell homogenate and purified valacyclovirase. As the methoxy and ethoxy linker containing model compounds were fairly unstable at pH 7.4 the detailed enzymatic hydrolysis study for those compounds were not pursued. Propylene glycol (PG) linker compounds were well stable at pH 7.4 so their bioactivation was critical to determine the ability of these compounds to generate the parent compound in-vivo. The enzymatic hydrolysis of PG containing compounds with valacyclovirase was studied. Valacyclovirase is an α-amino acid ester hydrolase;25 therefore it hydrolyzed only the aminoacid ester part of the model compound generating propylene glycol benzoate (BA-PG). This observation was in accordance with the mechanism of action of valacyclovirase.25 The PG containing model compounds were hydrolyzed exclusively to give the parent carboxylic acid when incubated with porcine carboxyesterases I. These compounds were also hydrolyzed by Caco-2 cell homogenate sequentially (half life < 1 hour) to generate the parent compound BA. The Caco-2 cell homogenate hydrolysis kinetics showed disappearance of PG prodrugs over 1 hour followed by increase in metabolites BA-PG and BA, which is then followed by increase in BA and decrease in BA-PG, indicating the complete conversion of prodrug to parent drug. The hydrolysis of PG prodrugs was also studied in rat intestinal homogenates, most of the prodrug was hydrolyzed within 1 hour of incubation suggesting that these prodrugs can be successfully activated to parent drug in-vivo. Thus, owing to the ester nature, these prodrugs can be substrate for various ubiquitously present esterases in rats as well as in humans.
The chemical and enzymatic hydrolysis studies of PG linker prodrugs show that these prodrugs are chemically robust but can be activated to parent drug in presence of enzymes. This satisfies the most important criterion for a compound to be a prodrug. On the other hand, a successful prodrug strategy not only depends on stability but also significant solubility and permeability. This work was mainly instrumental in devising a suitable linker which would impart chemical stability to the prodrugs of carboxylic acid with amino acids. The permeability of the model compounds was not the primary focus of this work, but it is certainly of immense value in our design of prodrugs of actual drugs. Our preliminary results of Caco-2 monolayer permeability of various prodrugs of antiviral agents with different linkers showed that PG linker did not have any negative effect on permeability. In fact permeability seems higher than acyloxy linker mainly because of increased stability of PG linker allowing more prodrug to be present in donor solution. Thus PG linker does not have significant influence on the permeability and transport of prodrugs (manuscript in preparation).
Here we have reported a stability profile for amino acid ester prodrugs with various acyloxy linkers and have shown that the PG linker can be used to increase the stability of the prodrugs without compromising the bioactivation. In conclusion, the PG linker can be used to develop the amino acid ester prodrugs of various poorly absorbed carboxylic acid containing drugs.
Figure 3.
pH rate profile for degradation of model compounds a) comparison of pH rate profile for methoxy linker containing phenylalanine and valine model prodrugs b) comparison of pH rate profile for ethoxy linker containing phenylalanine and valine model prodrugs d) pH rate profile of propylene glycol containing valine and phenyl alanine prodrugs
References
- 1.Valentino J, Stella RTB, Hageman Michael J, Oliyai Reza, Maag Hans, Tilley Jefferson W. Prodrugs Challenges and Rewards Part 1. V. Springer; New York: 2007. pp. 703–729. [Google Scholar]
- 2.Testa B, Mayer JM. Hydrolysis in Drug and Prodrug Metabolism: Chemistry, Biochemistry, and Enzymology. 1. John Wiley & Sons Canada Ltd; 2003. p. 800. [Google Scholar]
- 3.Fleisher D, Bong R, Stewart BH. Improved oral drug delivery: solubility limitations overcome by the use of prodrugs. Advanced Drug Delivery Reviews. 1996;19(2):115–130. [Google Scholar]
- 4.Kearney AS. Prodrugs and targeted drug delivery. Advanced Drug Delivery Reviews. 1996;19(2):225–239. [Google Scholar]
- 5.Sinhababu AK, Thakker DR. Prodrugs of anticancer agents. Advanced Drug Delivery Reviews. 1996;19(2):241–273. [Google Scholar]
- 6.Stella VJ, Borchradt RT, Hageman MJ, Oliyai R, Maag H, Tilley JW. Prodrugs: Challenges and Rewards, Parts 1 and 2. 1. Springer-Verlag New York, LLC; 2007. p. 1470. [Google Scholar]
- 7.Han HK, Oh DM, Amidon GL. Cellular uptake mechanism of amino acid ester prodrugs in Caco-2/hPEPT1 cells overexpressing a human peptide transporter. Pharm Res. 1998;15(9):1382–6. doi: 10.1023/a:1011945420235. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Aliaga I, Daniel H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol Sci. 2002;23(9):434–40. doi: 10.1016/s0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- 9.Song X, Lorenzi PL, Landowski CP, Vig BS, Hilfinger JM, Amidon GL. Amino acid ester prodrugs of the anticancer agent gemcitabine: synthesis, bioconversion, metabolic bioevasion, and hPEPT1-mediated transport. Mol Pharm. 2005;2(2):157–67. doi: 10.1021/mp049888e. [DOI] [PubMed] [Google Scholar]
- 10.Landowski CP, Vig BS, Song X, Amidon GL. Targeted delivery to PEPT1-overexpressing cells: acidic, basic, and secondary floxuridine amino acid ester prodrugs. Mol Cancer Ther. 2005;4(4):659–67. doi: 10.1158/1535-7163.MCT-04-0290. [DOI] [PubMed] [Google Scholar]
- 11.Chandrasekaran S, Al-Ghananeem AM, Riggs RM, Crooks PA. Synthesis and stability of two indomethacin prodrugs. Bioorg Med Chem Lett. 2006;16(7):1874–9. doi: 10.1016/j.bmcl.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Bodin NO, Ekstrom B, Forsgren U, Jalar LP, Magni L, Ramsay CH, Sjoberg B. Bacampicillin: a new orally well-absorbed derivative of ampicillin. Antimicrob Agents Chemother. 1975;8(5):518–25. doi: 10.1128/aac.8.5.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landowski CP, Song X, Lorenzi PL, Hilfinger JM, Amidon GL. Floxuridine amino acid ester prodrugs: enhancing Caco-2 permeability and resistance to glycosidic bond metabolism. Pharm Res. 2005;22(9):1510–8. doi: 10.1007/s11095-005-6156-9. [DOI] [PubMed] [Google Scholar]
- 14.Nudelman A, Gnizi E, Katz Y, Azulai R, Cohen-Ohana M, Zhuk R, Sampson SR, Langzam L, Fibach E, Prus E, Pugach V, Rephaeli A. Prodrugs of butyric acid. Novel derivatives possessing increased aqueous solubility and potential for treating cancer and blood diseases. Eur J Med Chem. 2001;36(1):63–74. doi: 10.1016/s0223-5234(00)01199-5. [DOI] [PubMed] [Google Scholar]
- 15.Mudryk B, Rajaraman S, Soundararajan N. A practical synthesis of chloromethyl esters from acid chlorides and trioxane or paraformaldehyde promoted by zirconium tetrachloride. Tetrahedron Letters. 2002;43(36):6317–6318. [Google Scholar]
- 16.Khalafi-Nezhad A, MNSRAK An Efficient Method for the Chemoselective Preparation of Benzoylated 1,2-Diols from Epoxides. 2004;35 [Google Scholar]
- 17.Schmidt R, Carrigan JG, DeWolf CE. Synthesis and characterization of a series of novel phenol- and polyphenol-based glycerolipids. Canadian Journal of Chemistry. 2006;84:1411–1415. [Google Scholar]
- 18.Masuda T, Yoshida S, Arai M, Kaneko S, Yamashita M, Honda T. Synthesis and anti-influenza evaluation of polyvalent sialidase inhibitors bearing 4-guanidino-Neu5Ac2en derivatives. Chem Pharm Bull (Tokyo) 2003;51(12):1386–98. doi: 10.1248/cpb.51.1386. [DOI] [PubMed] [Google Scholar]
- 19.Han HK, Stewart BH, Doherty AM, Cody WL, Amidon GL. In vitro stability and intestinal absorption characteristics of hexapeptide endothelin receptor antagonists. Life Sci. 1998;63(18):1599–609. doi: 10.1016/s0024-3205(98)00429-9. [DOI] [PubMed] [Google Scholar]
- 20.Van Gelder J, Annaert P, Naesens L, De Clercq E, Van den Mooter G, Kinget R, Augustijns P. Inhibition of intestinal metabolism of the antiviral ester prodrug bis(POC)-PMPA by nature-identical fruit extracts as a strategy to enhance its oral absorption: an in vitro study. Pharm Res. 1999;16(7):1035–40. doi: 10.1023/a:1018931631912. [DOI] [PubMed] [Google Scholar]
- 21.Kim I, Chu X-y, Kim S, Provoda CJ, Lee KD, Amidon GL. Identification of a Human Valacyclovirase. Biphenyl Hydrolase-like Protein as Valacyclovir Hydrolase. Journal of Biological Chemistry. 2003;278(28):25348–25356. doi: 10.1074/jbc.M302055200. [DOI] [PubMed] [Google Scholar]
- 22.Friedman DI, Amidon GL. Oral absorption of peptides: influence of pH and inhibitors on the intestinal hydrolysis of leu-enkephalin and analogues. Pharm Res. 1991;8(1):93–6. doi: 10.1023/a:1015842609565. [DOI] [PubMed] [Google Scholar]
- 23.Bundgaard H. Drugs of the Future. 1991;16:443. [Google Scholar]
- 24.Mendes E, Furtado T, Neres J, Iley J, Jarvinen T, Rautio J, Moreira R. Synthesis, stability and in vitro dermal evaluation of aminocarbonyloxymethyl esters as prodrugs of carboxylic acid agents. Bioorg Med Chem. 2002;10(3):809–16. doi: 10.1016/s0968-0896(01)00336-4. [DOI] [PubMed] [Google Scholar]
- 25.Lai L, Xu Z, Zhou J, Lee KD, Amidon GL. Molecular basis of prodrug activation by human valacyclovirase, an alpha-amino acid ester hydrolase. J Biol Chem. 2008;283(14):9318–27. doi: 10.1074/jbc.M709530200. [DOI] [PMC free article] [PubMed] [Google Scholar]