Abstract
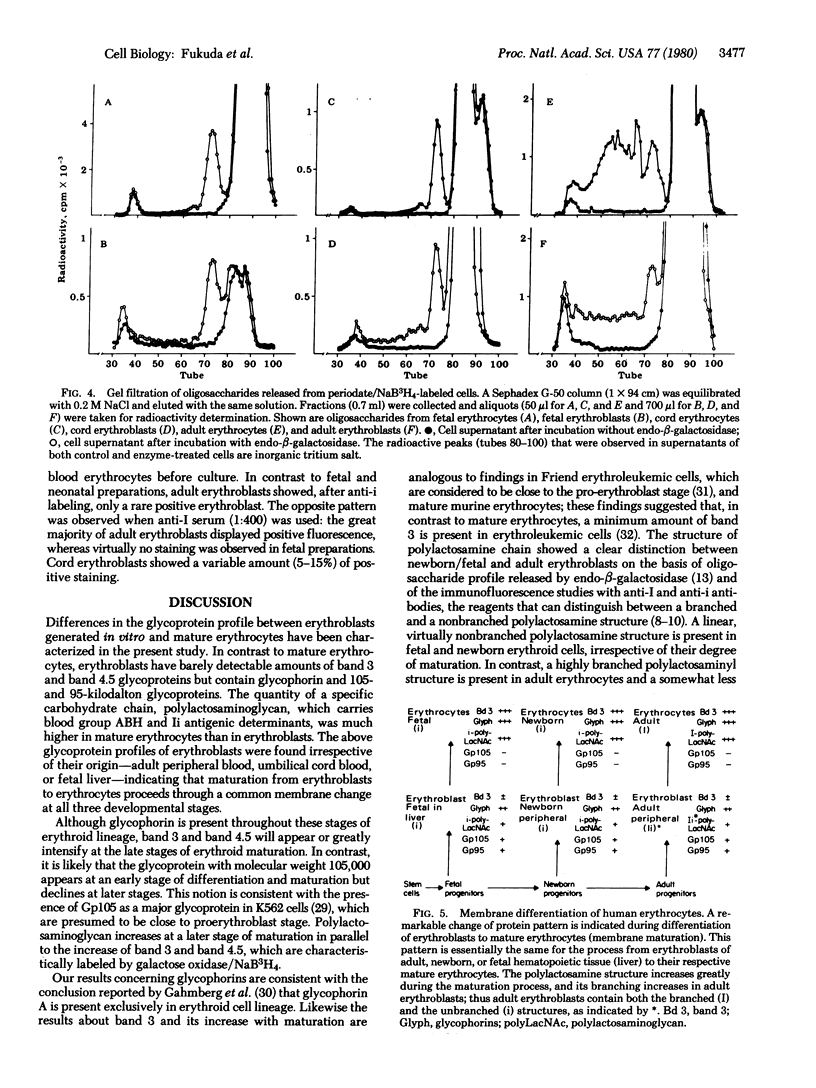
Human erythroblasts in culture, irrespective of the ontogenic stage of their progenitors, are characterized by: (i) the barely detectable amount of band 3 glycoprotein, (ii) the presence of two glycoproteins with molecular weights 105,000 and 95,000, (iii) the high concentration of glycophorin, and (iv) a minimum quantity of the carbohydrate chain susceptible to endo-β-galactosidase (“polylactosaminoglycan”). In contrast, mature erythrocytes, whether of fetal, neonatal, or adult origin, are characterized by a high concentration of band 3 glycoprotein, polylactosaminoglycan, and glycophorins, but do not contain 105- and 95-kilodalton-glycoproteins. Thus, the process of erythroid maturation from erythroblasts to erythrocytes is accompanied by the appearance of band 3, the disappearance of 105- and 95-kilodalton glycoproteins, and a great increase in the quantity of polylactosaminoglycan. The structure of polylactosaminoglycan may not be different between mature erythrocytes and erythroblasts from the same ontogenic stage, but it is distinctively different from one stage to the other. The profiles of oligosaccharides released by endo-β-galactosidase and immunofluorescence studies with anti-Ii antibodies indicated that a linear polylactosaminoglycan structure was present in erythroblasts as well as in erythrocytes of the fetal and newborn stage, whereas a branched polylactosaminoglycan structure was present in erythroblasts as well as erythrocytes of adult blood. Thus, two membrane characteristics are closely associated with the process of erythroid cell development; one—the membrane proteins band 3, band 4.5, and 95- and 105-kilodalton glycoproteins—determines the degree of maturation, and the other—polylactosaminoglycan—may determine the ontogenic stage of the erythroblast progenitors.
Keywords: stem cell culture, band 3 protein, glycophorins, polylactosaminoglycan, Ii antigens
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzt K., Dubois P., Bennett D., Condamine H., Babinet C., Jacob F. Surface antigens common to mouse cleavage embryos and primitive teratocarcinoma cells in culture. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2988–2992. doi: 10.1073/pnas.70.10.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Feizi T., Childs R. A., Watanabe K., Hakomori S. I. Three types of blood group I specificity among monoclonal anti-I autoantibodies revealed by analogues of a branched erythrocyte glycolipid. J Exp Med. 1979 Apr 1;149(4):975–980. doi: 10.1084/jem.149.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. N., Fukuda M., Hakomori S. Cell surface modification by endo-beta-galactosidase. Change of blood group activities and release of oligosaccharides from glycoproteins and glycosphingolipids of human erythrocytes. J Biol Chem. 1979 Jun 25;254(12):5458–5465. [PubMed] [Google Scholar]
- Fukuda M. N., Matsumura G. Endo-beta-galactosidase of Escherichia freundii. Purification and endoglycosidic action on keratan sulfates, oligosaccharides, and blood group active glycoprotein. J Biol Chem. 1976 Oct 25;251(20):6218–6225. [PubMed] [Google Scholar]
- Fukuda M., Eshdat Y., Tarone G., Marchesi V. T. Isolation and characterization of peptides derived from the cytoplasmic segment of band 3, the predominant intrinsic membrane protein of the human erythrocyte. J Biol Chem. 1978 Apr 10;253(7):2419–2428. [PubMed] [Google Scholar]
- Fukuda M., Fukuda M. N., Hakomori S. Developmental change and genetic defect in the carbohydrate structure of band 3 glycoprotein of human erythrocyte membrane. J Biol Chem. 1979 May 25;254(10):3700–3703. [PubMed] [Google Scholar]
- Furthmayr H., Marchesi V. T. Subunit structure of human erythrocyte glycophorin A. Biochemistry. 1976 Mar 9;15(5):1137–1144. doi: 10.1021/bi00650a028. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gahmberg C. G., Jokinen M., Andersson L. C. Expression of the major red cell sialoglycoprotein, glycophorin A, in the human leukemic cell line K562. J Biol Chem. 1979 Aug 10;254(15):7442–7448. [PubMed] [Google Scholar]
- Gahmberg C. G., Jokinen M., Andersson L. C. Expression of the major sialoglycoprotein (glycophorin) on erythroid cells in human bone marrow. Blood. 1978 Aug;52(2):379–387. [PubMed] [Google Scholar]
- Glickman R. M., Bouhours J. F. Characterization, distribution and biosynthesis of the major ganglioside of rat intestinal mucosa. Biochim Biophys Acta. 1976 Jan 22;424(1):17–25. doi: 10.1016/0005-2760(76)90045-x. [DOI] [PubMed] [Google Scholar]
- Harper P. A., Knauf P. A. Comparison of chloride transport in mouse erythrocytes and Friend virus-transformed erythroleukemic cells. J Cell Physiol. 1979 Feb;98(2):347–357. doi: 10.1002/jcp.1040980211. [DOI] [PubMed] [Google Scholar]
- Harrison P. R. Analysis of erythropoeisis at the molecular level. Nature. 1976 Jul 29;262(5567):353–356. doi: 10.1038/262353a0. [DOI] [PubMed] [Google Scholar]
- Järnefelt J., Rush J., Li Y. T., Laine R. A. Erythroglycan, a high molecular weight glycopeptide with the repeating structure [galactosyl-(1 leads to 4)-2-deoxy-2-acetamido-glucosyl(1 leads to 3)] comprising more than one-third of the protein-bound carbohydrate of human erythrocyte stroma. J Biol Chem. 1978 Nov 25;253(22):8006–8009. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liao T. H., Gallop P. M., Blumenfeld O. O. Modification of sialyl residues of sialoglycoprotein(s) of the human erythrocyte surface. J Biol Chem. 1973 Dec 10;248(23):8247–8253. [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- MARSH W. L. Anti-i: a cold antibody defining the Ii relationship in human red cells. Br J Haematol. 1961 Apr;7:200–209. doi: 10.1111/j.1365-2141.1961.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Gachelin G., Damonneville M., Delarbre C., Jacob F. Cell surface carbohydrates of embryonal carcinoma cells: polysaccharidic side chains of F9 antigens and of receptors to two lectins, FBP and PNA. Cell. 1979 Sep;18(1):183–191. doi: 10.1016/0092-8674(79)90367-2. [DOI] [PubMed] [Google Scholar]
- Niemann H., Watanabe K., Hakomori S. Blood group i and I activities of "lacto-N-norhexaosylceramide" and its analogues: the structural requirements for i-specificities. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1286–1293. doi: 10.1016/0006-291x(78)91275-5. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Chen P., Maniatis A., Stamatoyannopoulos G. Simultaneous assessment of i-antigenic expression and fetal hemoglobin in single red cells by immunofluorescence. Blood. 1980 Feb;55(2):221–232. [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Buckley J., Kurachi S., Nute P. E., Stamatoyannopoulos G. Erythroid progenitors circulating in the blood of adult individuals produce fetal hemoglobin in culture. Science. 1978 Mar 24;199(4335):1349–1350. doi: 10.1126/science.628844. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Rosenblum B. B., Papayannopoulou T., Brice M., Nakamoto B., Shepard T. H. HbF and HbA production in erythroid cultures from human fetuses and neonates. Blood. 1979 Aug;54(2):440–450. [PubMed] [Google Scholar]
- Watanabe K., Hakomori S. I., Childs R. A., Feizi T. Characterization of a blood group I-active ganglioside. Structural requirements for I and i specificities. J Biol Chem. 1979 May 10;254(9):3221–3228. [PubMed] [Google Scholar]
- Watanabe K., Hakomori S. I. Status of blood group carbohydrate chains in ontogenesis and in oncogenesis. J Exp Med. 1976 Sep 1;144(3):644–653. doi: 10.1084/jem.144.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison K. R., Stern P. L. Expression of a Forssman antigenic specificity in the preimplantation mouse embryo. Cell. 1978 Aug;14(4):785–793. doi: 10.1016/0092-8674(78)90334-3. [DOI] [PubMed] [Google Scholar]