Abstract
Indian mustard (Brassica juncea) plants exposed to Pb and EDTA in hydroponic solution were able to accumulate up to 55 mmol kg−1 Pb in dry shoot tissue (1.1% [w/w]). This represents a 75-fold concentration of Pb in shoot tissue over that in solution. A threshold concentration of EDTA (0.25 mm) was found to be required to stimulate this dramatic accumulation of both Pb and EDTA in shoots. Below this threshold concentration, EDTA also accumulated in shoots but at a reduced rate. Direct measurement of a complex of Pb and EDTA (Pb-EDTA) in xylem exudate of Indian mustard confirmed that the majority of Pb in these plants is transported in coordination with EDTA. The accumulation of EDTA in shoot tissue was also observed to be directly correlated with the accumulation of Pb. Exposure of Indian mustard to high concentrations of Pb and EDTA caused reductions in both the transpiration rate and the shoot water content. The onset of these symptoms was correlated with the presence of free protonated EDTA (H-EDTA) in the hydroponic solution, suggesting that free H-EDTA is more phytotoxic than Pb-EDTA. These studies clearly demonstrate that coordination of Pb transport by EDTA enhances the mobility within the plants of this otherwise insoluble metal ion, allowing plants to accumulate high concentrations of Pb in shoots. The finding that both H-EDTA and Pb-EDTA are mobile within plants also has important implications for the use of metal chelates in plant nutritional research.
The synthetic chelate EDTA forms a soluble complex with many metals, including Pb (Kroschwitz, 1995), and can solubilize Pb from soil particles (Means and Crerar, 1978). Recently, application of EDTA to Pb-contaminated soils has been shown to induce the uptake of Pb by plants (Jøgensen, 1993; Huang and Cunningham, 1996; Blaylock et al., 1997; Huang et al., 1997), causing Pb to accumulate to more than 1% (w/w) of shoot dry biomass (Huang and Cunningham, 1996; Blaylock et al., 1997; Huang et al., 1997). For the in situ remediation of Pb-contaminated soils it appears that this chelate-assisted phytoextraction strategy (Salt et al., 1998) may be more effective than a strategy based on the natural ability of certain wild plant species for metal hyperaccumulation (Chaney, 1983; Baker et al., 1988).
For more than 40 years, synthetic chelates have been used to supply plants with micronutrients in both soil and hydroponics. Yet the mechanisms by which chelates enhance metal accumulation are still not well characterized (Wallace and Wallace, 1992), and what is known appears contradictory. For example, some evidence suggests that the Fe-chelate EDTA can be absorbed by plants and translocated to shoots (Weinstein et al., 1954; Hill-Cottingham and Llyod-Jones, 1961, 1965). However, Tiffin et al. (1960) concluded that Fe-chelates are excluded from root tissue, and this was supported by Chaney et al. (1972), who demonstrated that Fe is taken up by plants only after first being split from the Fe-chelate complex by the action of a specific plasma membrane-bound Fe-chelate reductase.
To optimize the process of chelate-assisted phytoextraction, it is important to understand the biological mechanisms responsible for this process. Because of the stimulatory role of chelate application in the uptake of Pb and other metals by plants, we have investigated the role of EDTA in Pb accumulation in plants. In this study we have demonstrated that the previously described EDTA-enhanced Pb accumulation in Indian mustard (Brassica juncea) is based on the ability of EDTA to chelate and transport Pb from soil into shoot tissue.
MATERIALS AND METHODS
Roots of Indian mustard (Brassica juncea [L.] Czern. var 426308) (Kumar et al., 1995) were grown under microbiologically controlled conditions as follows. Seeds were surface sterilized in 2.6% (w/v) bleach for 30 min, rinsed four times in autoclaved deionized water, and transferred onto sterile 1.2% (w/v) agar plates containing 3.0% (w/v) Suc. Plates were held vertically and the seeds allowed to germinate and grow in the dark at 22°C for 72 h. Etiolated seedlings that did not show microbial contamination on the agar plates were transferred individually into small glass vials (29 × 65 mm) containing 23 mL of sterile nutrient solution. Soft Styrofoam stoppers used to cap the vials were incised radially to provide support for the hypocotyls. The nutrient solution contained 0.7 mmol L−1 Ca2+, 1.5 mmol L−1 K+, 0.5 mmol L−1 Mg2+, 0.25 mmol L−1 NH4+, 2.9 mmol L−1 NO3−, 0.25 mmol L−1 H2PO4−, 0.5 mmol L−1 SO42−, 4.75 μmol L−1 ferric tartrate, 0.075 μmol L−1 Cu2+, 0.2 μmol L−1 Zn2+, 1.25 μmol L−1 Mn2+, 11.5 μmol L−1 H3BO3, and 0.025 μmol L−1 MoO3 at pH 6.0. Vials were agitated on an orbital shaker (Labline Instruments, Inc., Melrose Park, IL) at 60 rpm to provide aeration and mixing, and the nutrient solution was replaced weekly. Plants were cultivated for 11 d in a growth chamber with a 10-h light period, with light provided by fluorescent and incandescent lamps at a light intensity of 17,200 lux. All plants were maintained at day/night temperatures of 22/22°C and a constant humidity of 50%.
For the experiments plants were transferred under microbiologically controlled conditions to new vials containing 23 mL of nutrient solution supplemented with various concentrations of Pb and EDTA. Plants were exposed to the treatments for 48 h at room temperature under constant light (6700 lux) and with orbital shaking (60 rpm). To measure transpiration rates, water loss from vials containing solution but no plants was subtracted from the water loss from vials containing both plants and solution (Krämer et al., 1997). Plants treated for xylem sap collection were transferred under microbiologically controlled conditions to vials containing 10 mL of nutrient solution supplemented with Pb and EDTA. Filter-sterilized Pb(NO3)2 and K2EDTA were added to autoclaved nutrient solution, and autoclaved ferric tartrate was added to the nutrient solution just before the addition of EDTA and Pb.
The Effect of EDTA on Shoot Pb Accumulation
Two-week-old Indian mustard seedlings were exposed to 0.5 mm Pb(NO3)2 and 0.01 to 2.5 mm K2EDTA in the nutrient solution, with a final pH adjusted to 4.0 with 1 n KOH. This was performed to approximate the pH optima for Pb accumulation from hydroponic solution by Indian mustard (Blaylock et al., 1997). After 48 h of exposure, shoot tissue was harvested from each of three replicate treatments (three plants per replicate were pooled) and oven dried at 80°C for 3 d. Dried plant material was digested at 180°C for 1.5 h in 5 mL of concentrated HNO3 and cooled to room temperature, and 1 mL of 30% H2O2 was added. Samples were then heated at 180°C for 20 min and cooled, and deionized water was added to a final volume of 12.5 mL. Pb concentrations were determined using a flame-atomic-absorption spectrometer (model 3110, Perkin-Elmer). The speciation of Pb and EDTA in the hydroponic solution was predicted using GEOCHEM-PC version 2.0 (Parker et al., 1995).
EDTA Accumulation in Shoots of Indian Mustard
Two-week-old Indian mustard seedlings were exposed to 1.5 mm K2EDTA (containing 0.015 μCi μmol−1 [14C]EDTA) in the nutrient solution at pH 4.0. After 48 h, the shoot tissue was harvested, frozen at −80°C, and lyophilized. Lyophilized material was ground to a powder and extracted with 1 mL of 50% (v/v) ethanol, heated at 80°C for 10 min, and centrifuged at 1550g for 20 min at room temperature, and the supernatant was removed. Successive extractions were performed by adding 1 mL of 50% (v/v) ethanol to the remaining pellet, and repeating the above extraction process. Supernatants from the first three extractions were pooled; supernatants from the fourth, fifth, and sixth extractions were analyzed separately for [14C]EDTA using a liquid-scintillation counter (model LS5000, Beckman). The 50% (v/v) ethanol-extracted pellet was digested for 5 d at 80°C with 1 mL of BTS-450 tissue-solubilizing solution (Beckman), and the [14C]EDTA in the solubilized pellet was determined using a liquid-scintillation counter. The efficiency of EDTA extraction was expressed as the amount of 14C extracted in 50% ethanol relative to the total tissue 14C. This extraction efficiency was later used to adjust the measured tissue EDTA concentrations to account for losses during the extraction procedure.
To determine the relationship between EDTA exposure and accumulation in shoots of Indian mustard, 2-week-old seedlings were exposed to 0.01 to 2.5 mm K2EDTA (containing 0.01–8.7 μCi μmol−1 [14C]EDTA) in the nutrient solution at pH 4.0. After 48 h, the shoot tissue was harvested and extracted three times with 50% (v/v) ethanol, as described above. Supernatants from all three extractions were pooled, dried in a Speed-Vac (model SVC200, Savant, Farmingdale, NY), and resuspended in 500 μL of deionized water. These samples were later analyzed by HPLC for EDTA (see below).
Time Course of Pb and EDTA Accumulation in Shoots
Two-week-old Indian mustard seedlings were treated with 0.5 mm Pb(NO3)2 and 1 mm K2EDTA in the nutrient solution at pH 4.0. Shoot tissue was harvested periodically during a 48-h period, frozen at −80°C, lyophilized, and ground to a powder. One-third of the powdered tissue was extracted in 50% (v/v) ethanol (see extraction protocol above), and the remaining tissue was digested with HNO3/H2O2 (see digestion protocol above). The digested materials were analyzed for Pb using a flame-atomic-absorption spectrometer, and the extracted material was analyzed by HPLC for total EDTA.
HPLC Analysis of Total EDTA
Concentrated 50% ethanol plant extracts were centrifuged at 10,000g for 10 min and prepared for RP HPLC (5000 LC, Varian, Sunnyvale, CA) as follows. To each 100-μL sample of the plant extract, 400 μL of 6.47 mm iron(III) chloride (in 7.1 m acetic acid) and 500 μL of deionized water were added. The sample was filtered through a 0.45-μm nylon membrane spin-prep filter and 100 μL was injected onto an RP C18 column (60 Å, 8 μm, Dynamax, Rainin, Walnut Creek, CA). The column was run isocratically at 1 mL min−1 with 8 mm tetrabutyl ammonium hydroxide in 30 mm sodium acetate-acetic acid buffer (pH 4). EDTA was detected at 254 nm using a UV spectrophotometer (Spectroflow 783, Kratos Analytical, Hamilton, OH) according to the method of Bergers and DeGroot (1994). To validate the use of UV detection as a quantitative measure of EDTA, we also collected fractions (1 mL) of eluent from the HPLC column and measured the [14C]EDTA they contained using a liquid-scintillation counter.
Analysis of Xylem Exudate for the Presence of Pb Complexed to EDTA
Two-week-old Indian mustard seedlings were exposed to 0.5 mm Pb(NO3)2 (traced with 931.6 μCi mmol−1 210Pb) and 1 mm K2EDTA in the nutrient solution at pH 4.0. After 1 h, plants were decapitated and xylem exudate was collected during a 12-h period. Samples of the xylem exudate from 20 plants were pooled and a one-tenth dilution of the sap was analyzed by RP HPLC for the presence of Pb-EDTA (Buchberger et al., 1991). One hundred microliters of diluted sample was injected onto a RP C18 column and run isocratically at 1 mL/min with 27.4 mm hexadecyltrimethyl ammonium bromide in 0.7 mm KH2PO4 (pH 7.0) and 65% (v/v) acetonitrile. Standard Pb-EDTA was prepared by mixing equimolar solutions of K2EDTA and Pb(NO3)2. Metal-EDTA complexes were detected at 250 nm using a UV spectrophotometer (Kratos Analytical) after the postcolumn addition of 0.1 mm copper sulfate in 0.3 m acetic acid at 0.9 mL/min via a 10-μL mixer (The Lee Company, Westbrook, CT). Before peak detection, the column eluent and the added CuSO4 solution were allowed to react for 2 min in a delay loop. Postcolumn addition of CuSO4 was necessary to convert all metal-EDTA complexes to Cu-EDTA for efficient detection at 250 nm. Twenty fractions (1 mL) of eluent were collected and analyzed for 210Pb by liquid-scintillation counting.
RESULTS
Stimulation of Shoot Pb Accumulation by EDTA
Indian mustard plants were grown hydroponically and roots were maintained under microbiologically controlled conditions to prevent the biodegradation of EDTA by rhizobacteria. The hydroponic system was monitored for the presence of bacteria by periodically streaking samples of the nutrient solution onto agar plates. In addition, care was taken to eliminate bacterial contamination by screening seedlings as they germinated.
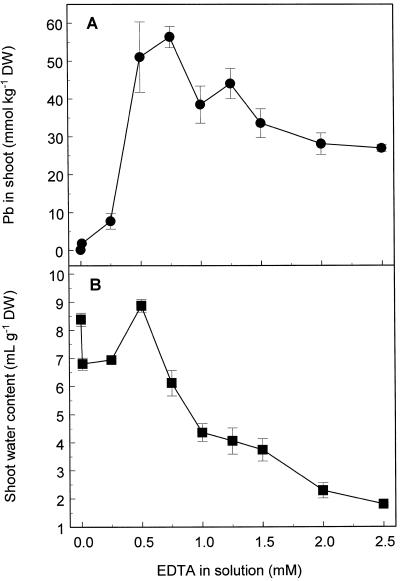
Treatment of Indian mustard with EDTA concentrations ranging from 0.1 to 2.5 mm in the presence of 0.5 mm Pb(NO3)2 caused enhanced shoot Pb accumulation (Fig. 1A). Above 0.25 mm EDTA in solution, shoot Pb accumulation increased rapidly, reaching a maximum Pb concentration of 56 mmol kg−1 dry weight at 0.75 mm EDTA. This represents a 400-fold increase in Pb accumulation compared with that in plants exposed to Pb in the absence of EDTA, and a 75-fold bioaccumulation of Pb from solution. At EDTA concentrations greater than 0.75 mm shoot Pb concentrations declined slowly.
Figure 1.
Pb accumulation (A) and water content (B) of shoots of Indian mustard exposed to 0.5 mm Pb(NO3)2 and various K2EDTA concentrations for 48 h. Values represent the mean ± se of three replicate samples, and the data presented are from one experiment representative of three independent experiments. DW, Dry weight.
Changes in shoot-tissue water content, measured by subtracting the dry weight of the shoot tissue from its fresh weight, were used as a measure of phytotoxicity (Fig. 1B). Application of EDTA at concentrations greater than 0.5 mm was associated with significant water loss from the shoot tissue, which in severe cases resulted in visible drying of the tissue and formation of necrotic lesions. At an EDTA concentration of 0.5 mm, plants accumulated high quantities of Pb without apparent phytotoxicity.
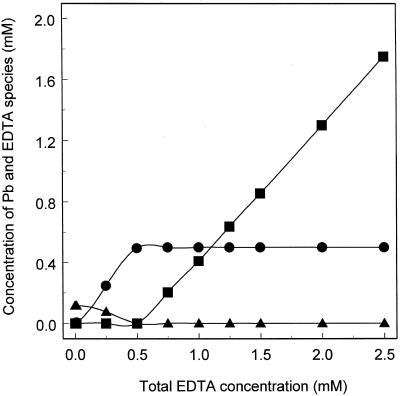
As shown in Figure 2, EDTA speciation in the hydroponic culture solution was predicted using GEOCHEM-PC. In the absence of EDTA, the majority of Pb was predicted to be insoluble; however, as EDTA concentrations increased, soluble Pb-EDTA was predicted to be formed, reaching a maximum concentration of 0.5 mm in the presence of 0.5 mm total EDTA. The concentration of free protonated EDTA was also predicated to increase rapidly above 0.5 mm total EDTA.
Figure 2.
The concentration of major soluble Pb and EDTA species in the hydroponic nutrient solution (as described in Methods) containing 0.5 mm Pb(NO3)2 and 0.01 to 2.5 mm K2EDTA, pH 4.0. Values were derived by modeling using GEOCHEM-PC version 2.0. •, Pb-EDTA; ▴, Pb2+; and ▪, protonated EDTA.
Detection and Accumulation of EDTA in Shoots
Different extractants, including water, 50% (v/v) methanol, and 50% (v/v) ethanol, were screened for their relative abilities to extract 14C from shoots of plants treated with 14C-labeled EDTA. Of the three extractants, 50% (v/v) ethanol proved to be the most efficient and was found to remove 91% of the total shoot 14C after three successive extractions. Three further 50% (v/v) ethanol extractions removed almost no additional 14C from the shoot tissue (data not shown). Based on this information, three ethanol extractions were used for the determination of total shoot EDTA concentrations. Shoot EDTA extraction efficiencies were not affected by exposure of plants to different EDTA concentrations in solution (data not shown).
To establish the validity of using HPLC as an assay for total EDTA extracted from shoot tissue, EDTA (traced with [14C]EDTA) was monitored by both UV absorption at 254 nm and liquid-scintillation counting. Approximately 98% of the 14C injected onto the HPLC column was recovered in the eluent. A distinct peak in chromatograms of shoot extracts, detected by UV and liquid-scintillation counting, was found to coelute with an authentic EDTA standard. Concentrations of EDTA in shoot extracts, determined by UV absorbance and liquid-scintillation counting, correlated linearly with an r2 value of 0.97. Based on this correlation, we routinely quantified EDTA by monitoring UV absorption at 254 nm.
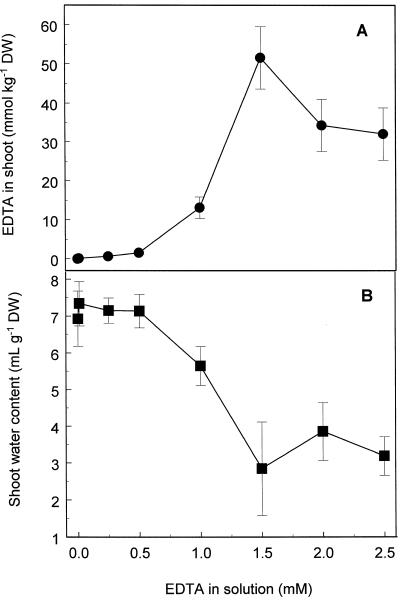
EDTA accumulation in shoots of Indian mustard increased significantly at EDTA concentrations greater than 0.5 mm in solution (Fig. 3A). A maximum shoot accumulation of 52 mmol EDTA kg−1 dry weight was observed after treatment of plants with 1.5 mm EDTA in solution. This represents a 35-fold higher concentration of EDTA in shoot tissue compared with that in the nutrient solution. Solution EDTA concentrations greater than 0.5 mm caused a large decrease in the water content of shoot tissue (Fig. 3B), and this was associated with tissue necrosis and drying at the highest EDTA concentrations.
Figure 3.
EDTA accumulation (A) and water content (B) of shoots of Indian mustard exposed to various K2EDTA concentrations for 48 h. Values represent the mean ± se of three replicate samples, and the data presented are from one experiment representative of three independent experiments. DW, Dry weight.
Time Course of Shoot Accumulation of Pb and EDTA
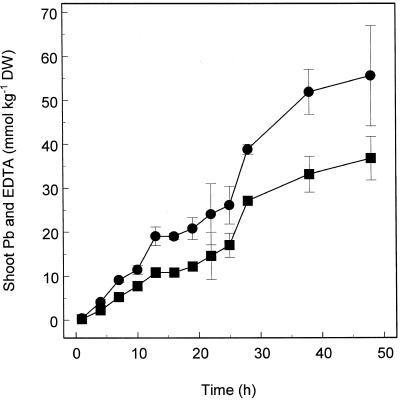
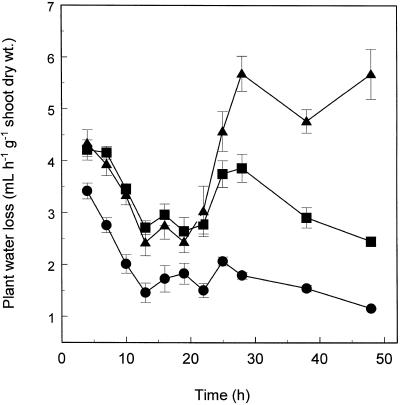
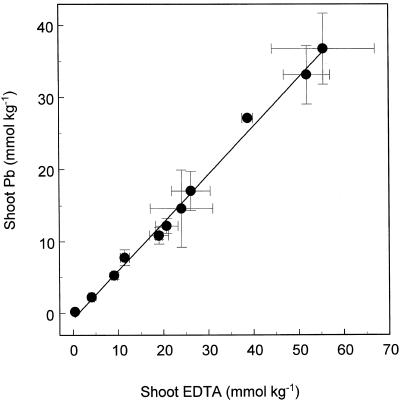
The rates of EDTA and Pb accumulation in shoots of Indian mustard were essentially linear during a 48-h period in plants exposed to 0.5 mm Pb(NO3)2 and 1.5 mm EDTA in hydroponic solution (Fig. 4). It is possible that the slight reduction in accumulation rates of Pb and EDTA, observed between 13 and 22 h, was caused by the corresponding reduction in transpiration rates associated with the night period (Fig. 5). Accumulation of Pb in shoots of Indian mustard was tightly correlated with accumulation of EDTA (Fig. 6), with an accumulation ratio of 1:0.67 (EDTA:Pb).
Figure 4.
Time course of the shoot accumulation of Pb (▪) and EDTA (•) in Indian mustard plants exposed to Pb(NO3)2 (0.5 mm) and K2EDTA (1 mm). Values represent the mean ± se of three replicate samples each composed of three individual plants. DW, Dry weight.
Figure 5.
Time course of water-loss rates from shoots of Indian mustard exposed to 0.5 mm Pb(NO3)2 and 1 mm K2EDTA (•), 0.5 mm Pb(NO3)2 alone (▪), or of unexposed control plants (▴). Values represent the mean ± se of three replicate samples each composed of three individual plants. dry wt., Dry weight.
Figure 6.
A correlation between Pb and EDTA accumulated in shoots of Indian mustard exposed to Pb(NO3)2 (0.5 mm) and K2EDTA (1 mm). Values represent the mean ± se of three replicate samples each composed of three individual plants.
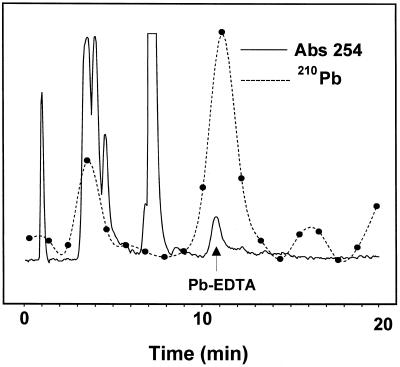
Identification of Pb-EDTA Complex in Xylem Exudate
Xylem exudate collected from Indian mustard plants exposed to 0.5 mm Pb(NO3)2 (traced with 100 μCi mmol−1 210Pb) and 1.0 mm K2EDTA were analyzed for the presence of a Pb-EDTA complex by HPLC. Analysis of xylem exudate showed the presence of a distinct UV and 210Pb-containing peak, with the same retention time (10.75 min) as an authentic Pb-EDTA standard (Fig. 7).
Figure 7.
HPLC profile of xylem sap collected from Indian mustard plants exposed to 0.5 mm Pb(NO3)2 (traced with 931.6 μCi mmol−1 210Pb) and 1 mm K2EDTA. The arrow represents the elution time of standard Pb-EDTA.
Effect of Pb and EDTA on Plant Water Loss through Transpiration
As expected, rates of water loss from Indian mustard followed a diurnal rhythm (Fig. 5). Rates of water loss declined from the start of the experiment (0 h), reaching a minimum after the onset of the dark period (10 h). After the onset of the light period (24 h), transpiration increased to a maximum and declined again, reaching a minimum at the onset of the next dark period. Addition of Pb and EDTA (0.5 mm Pb(NO3)2, 1.0 mm K2EDTA) caused an immediate decline in transpiration relative to the control. Transpiration reached a minimum at the onset of the dark period, and did not recover after the onset of the light period (Fig. 5). After 48 h of exposure, transpiration in Pb-EDTA-exposed plants was reduced by 80% relative to the control. Exposure to Pb alone also reduced transpiration relative to control plants, but only after approximately 25 h of exposure. This inhibition resulted in a 55% reduction in the transpiration of Pb-exposed plants after 48 h (Fig. 5).
DISCUSSION
EDTA appears to chelate Pb outside of the plant, then the soluble Pb-EDTA complex is transported through the plant, via the xylem, and accumulates in the leaves. This is supported by our findings that Pb-EDTA is the major transported form of Pb in Pb- and EDTA-exposed plants (Fig. 7). Also, Pb accumulation in shoots is correlated with formation of soluble Pb-EDTA in the hydroponic solution (Figs. 1A and 2) and the accumulation of EDTA in shoots (Fig. 6).
The kinetics of Pb and EDTA accumulation were found to be biphasic (Figs. 1A and 3A), suggesting that a threshold concentration of EDTA is required to “induce” the accumulation of high concentrations of EDTA or Pb-EDTA in shoots. This has also been observed for chelated Fe uptake. Jeffreys and Wallace (1968) showed that application of a threshold concentration of 1 mm Fe-N,N′-ethylenebis(2-[2-hydroxyphenyl]-glycine) (EDDHA) induced the rapid accumulation of the red-colored Fe-EDDHA in shoots of several different plant species. The physiological basis of this induction of chelate uptake and accumulation is unknown. However, we speculate that at these threshold concentrations, synthetic chelates including EDTA destroy the physiological barrier(s) in roots that normally function to control uptake and translocation of solutes. The plasma membrane surrounding root cells is thought to play a major role in forming this barrier. Both Zn2+ and Ca2+ ions are involved in stabilizing plasma membranes (Pasternak, 1987, 1988; Kaszuba and Hunt, 1990). Therefore, synthetic chelates may induce metal-chelate uptake and accumulation by removal of stabilizing Zn2+ and Ca2+ from the plasma membrane. This scenario would lead to the rapid equilibration of hydroponic or soil solution with the xylem sap. Once in the xylem, solutes such as Pb-EDTA would follow the transpiration stream and accumulate to a high concentration in shoots.
Pb is known to be effective at displacing various cationic metals from roots (Harrison et al., 1979), suggesting that Pb may also play a role in destabilizing the physiological barrier to solute movement in roots. This is supported by the observation that 0.5 mm Pb lowers the concentration of EDTA required to induce uptake from 1.5 to 0.5 mm (Figs. 1A and 3A). Also supporting this is the observation that high concentrations of Pb alone appear to be capable of inducing Pb accumulation in Indian mustard (Kumar et al., 1995).
Shoot EDTA accumulation, however, does not always appear to be related to physiological stress. At a low EDTA concentration (10 μm) and neutral pH (pH 6.0), we observed the accumulation of 0.1 ± 0.02 (se) mmol EDTA kg−1 dry weight in shoots of Indian mustard during 48 h. This suggests that EDTA, even when present at low concentrations, is accumulated by plants. The potential for metal-chelate accumulation should be considered when metal-chelate-buffered solutions are used to study metal uptake and nutritional requirements in plants.
The phytotoxicity of EDTA treatment was quantified by monitoring shoot desiccation. Generally, increasing concentrations of EDTA (Figs. 1B and 3B) caused significant reductions in shoot water content. An interesting exception to this, however, was treatment with equimolar Pb and EDTA. Under these conditions, no phytotoxicity was observed (Fig. 1B), even though maximum Pb accumulation was achieved (Fig. 1A). This suggests that EDTA-induced foliar necrosis may be attributable to the presence of free protonated EDTA in leaves. We predict that uncoordinated EDTA would be available to bind various essential divalent cations, including Fe2+, Zn2+, and Cu2+, disrupting the biochemistry of the leaf cells and ultimately causing cell death. This is supported by the finding that toxicity symptoms in Indian mustard exposed to Pb and EDTA are strongly correlated with the presence of free protonated EDTA in solution (Figs. 1B and 2). This toxicity is also reflected in a reduction in the rate of water loss from shoots (Fig. 5), demonstrating that increased transpiration is not the mechanism through which EDTA exerts its influence on Pb accumulation.
Mass flow of water through the xylem to the shoots is required for Cd accumulation in Indian mustard (Salt et al., 1995), and we would expect the same to be true for Pb, suggesting that if rates of water loss from plants exposed to Pb-EDTA could be maintained at control levels, Pb accumulation rates could be enhanced. To achieve this, plants would need to be able to tolerate the toxic effects of high concentrations of EDTA. This may be achieved by applying EDTA to soils at rates that minimize the availability of free chelate.
Our data on the transport of chelated metals in plants not only advance our understanding of the role of metal chelates in plant nutrition, but also point to potential areas of improvement for the development of chelate-assisted phytoextraction. These improvements should enhance Pb accumulation and ultimately allow more effective remediation of Pb-contaminated sites.
ACKNOWLEDGMENTS
We thank Dr. Robert Smith for his ideas and contributions to discussions and Dr. Ute Krämer for her help with GEOCHEM-PC. Special thanks to Robin Torquatti for her support. A.D.V. was a George H. Cook honors undergraduate scholar.
Abbreviation:
- RP
reversed-phase
Footnotes
This research was supported by grants from the U.S. Department of Agriculture (no. 96-35102-3838 to D.E.S.) and Phytotech, Inc., to I.R.
LITERATURE CITED
- Baker AJM, Brooks R, Reeves R. Growing for gold… and copper… and zinc. New Sci. 1988;10:44–48. [Google Scholar]
- Bergers PJM, DeGroot AC. The analysis of EDTA in water by HPLC. Water Res. 1994;28:639–642. [Google Scholar]
- Blaylock MJ, Salt DE, Dushenkov S, Zakarova O, Gussman C, Kapulnik Y, Ensley BD, Raskin I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ Sci Technol. 1997;31:860–865. [Google Scholar]
- Buchberger W, Haddad PR, Alexander PW. Separation of metal complexes of ethylenediaminetetraacetic acid in environmental water samples by ion chromatography with UV and potentiometric detection. J Chromatogr. 1991;558:181–186. doi: 10.1016/s0021-9673(01)93028-6. [DOI] [PubMed] [Google Scholar]
- Chaney RL (1983) Plant uptake of inorganic waste constituents. In JE Parr, PB Marsh, JM Kla, eds, Land Treatment of Hazardous Wastes. Noyes Data, Park Ridge, NJ, pp 50–76
- Chaney RL, Brown JC, Tiffin LO. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 1972;50:208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Lepp NW, Phipps DA. Uptake of copper by excised roots. II. Copper desorption from the free space. Z Pflanzenphysiol. 1979;94:27–34. [Google Scholar]
- Hill-Cottingham DG, Llyod-Jones CP. Absorption and breakdown of iron-ethylenediamine tetraacetic acid by tomato plants. Nature. 1961;189:312. [Google Scholar]
- Hill-Cottingham DG, Llyod-Jones CP. The behaviour of iron chelating agents with plants. J Exp Bot. 1965;16:233–242. [Google Scholar]
- Huang JW, Chen J, Berti WB, Cunningham SD. Phytoremediation of lead-contaminated soils: role of synthetic chelates in lead phytoextraction. Environ Sci Technol. 1997;31:800–805. [Google Scholar]
- Huang JW, Cunningham SD. Lead phytoextraction: species variation in lead uptake and translocation. New Phytol. 1996;145:75–84. [Google Scholar]
- Jeffreys RA, Wallace A. Detection of iron ethylenediamine di(O-hydroxyphenylacetate) in plant tissue. Agron J. 1968;60:613–616. [Google Scholar]
- Jørgensen SE. Removal of heavy metals from compost and soil by ecotechnological methods. Ecological Engineering. 1993;2:89–100. [Google Scholar]
- Kaszuba M, Hunt GRA. Protection against membrane damage: a H-NMR investigation of the effect of Zn2+ and Ca2+ on the permeability of phospholipid vesicles. J Inorg Biochem. 1990;40:217–225. doi: 10.1016/0162-0134(90)80055-3. [DOI] [PubMed] [Google Scholar]
- Krämer U, Smith RD, Wenzel WW, Raskin I, Salt DE. The role of metal transport and tolerance in nickel hyperaccumulation by Thlaspi goesingense Hálácsy. Plant Physiol. 1997;115:1641–1650. doi: 10.1104/pp.115.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschwitz JI. Iron compounds. In: Kroschwitz JI, editor. Kirk-Othmer Encyclopedia of Chemical Technology, Ed 4, Vol 14. New York: John Wiley & Sons; 1995. pp. 887–888. [Google Scholar]
- Kumar PBNA, Dushenkov V, Motto H, Raskin I. Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol. 1995;29:1232–1238. doi: 10.1021/es00005a014. [DOI] [PubMed] [Google Scholar]
- Means JL, Crerar DA. Migration of radioactive wastes: radionuclide mobilization by complexing agents. Science. 1978;200:1477–1481. doi: 10.1126/science.200.4349.1477. [DOI] [PubMed] [Google Scholar]
- Parker DR, Chaney RL, Norvell WA (1995) GEOCHEM-PC: a chemical speciation program for IBM and compatible personal computers. In Chemical Equilibrium and Reaction Models. Special Publication 42. Soil Science Society of America, Madison, WI, pp 163–200
- Pasternak CA. A novel form of host defense: membrane protection by Ca2+ and Zn2+ Biosci Rep. 1987;7:81–91. doi: 10.1007/BF01121871. [DOI] [PubMed] [Google Scholar]
- Pasternak CA. A novel role of Ca2+ and Zn2+: protection of cells against membrane damage. Biosci Rep. 1988;8:578–583. doi: 10.1007/BF01117337. [DOI] [PubMed] [Google Scholar]
- Salt DE, Prince RC, Pickering IJ, Raskin I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995;109:427–433. doi: 10.1104/pp.109.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Smith R, Raskin I. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Tiffin LO, Brown JC, Krauss RW. Differential absorption of metal chelate components by plant roots. Plant Physiol. 1960;35:362–367. doi: 10.1104/pp.35.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A, Wallace GA. Some of the problems concerning iron nutrition of plants after four decades of synthetic chelating agents. J Plant Nutr. 1992;15:1487–1508. [Google Scholar]
- Weinstein LH, Robbins WR, Perkins HF. Chelating agents and plant nutrition. Science. 1954;120:41–43. doi: 10.1126/science.120.3106.41. [DOI] [PubMed] [Google Scholar]