Abstract
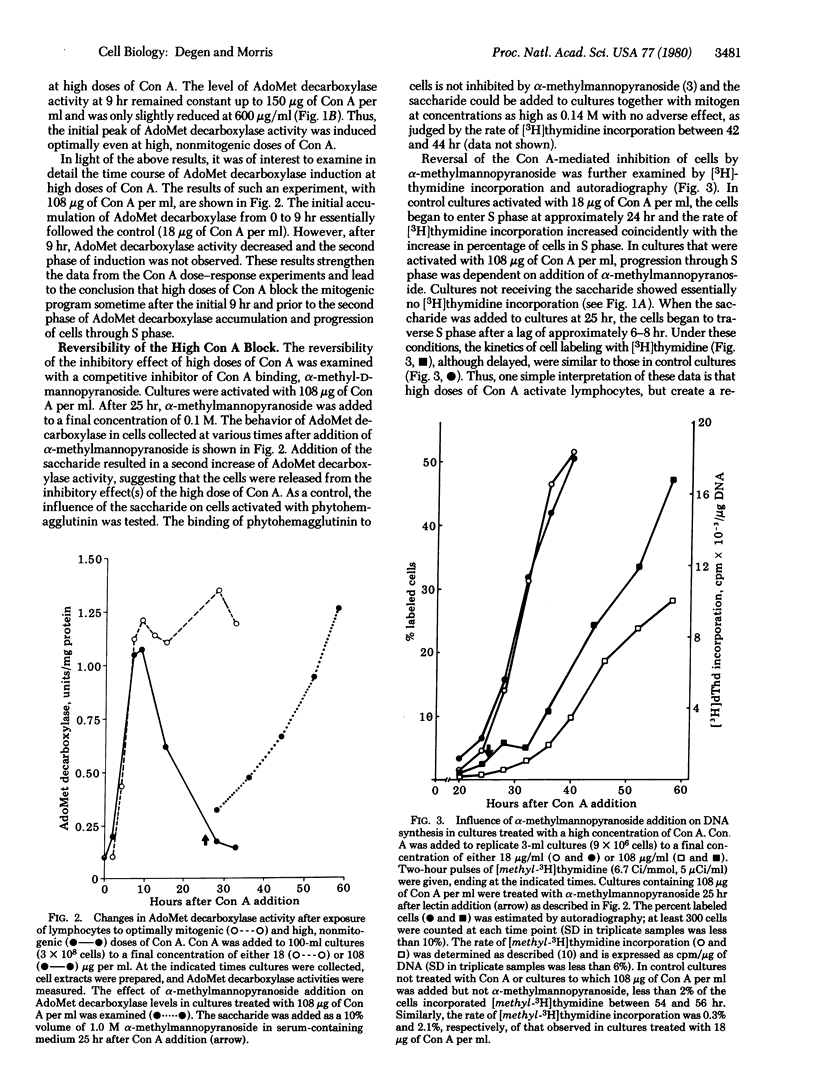
Lymphocyte mitogenesis is generally assessed by measuring the incorporation of [3H]thymidine into DNA. By this criterion, small lymphocytes, which are activated by relatively low doses of concanavalin A, are either unresponsive to or inhibited by higher concentrations. Because lymphocytes begin to synthesize DNA about 24 hr after addition of mitogen, the response is far removed temporally from the initial stimulus. We have chosen to use the induction of S-adenosylmethionine decarboxylase (S-adenosyl-L-methionine carboxy-lyase, EC 4.1.1.50) to assess early activation events in bovine lymphocytes. Adenosylmethionine decarboxylase induction is bimodal, with an initial phase beginning 3 hr after addition of concanavalin A and a second wave coinciding with the onset of DNA synthesis. The initial accumulation of the decarboxylase (0-9 hr) in cultures treated with “nonmitogenic” levels of concanavalin A (108 μg/ml) was similar to that observed in cultures stimulated with optimally mitogenic doses (18 μg/ml). The early induction of ornithine decarboxylase (L-ornithine carboxy-lyase, EC 4.1.1.17) was also similar under these two culture conditions. However, the second phase of adenosylmethionine decarboxylase accumulation, the induction of thymidine kinase (ATP: thymidine 5′-phosphotransferase, EC 2.7.1.21), and DNA replication were blocked at the higher concentrations of concanavalin A. The inhibition of late events by high doses of concanavalin A was reversible. Cells treated with α-methyl-D-mannopyranoside 25 hr after addition of a high dose of lectin responded with a second period of adenosylmethionine decarboxylase accumulation, induction of thymidine kinase, and progression through S phase. These results suggest that initial lymphocyte activation occurs normally at high doses of concanavalin A, but that the cells are reversibly blocked prior to induction of “late” enzymes and progression through S phase.
Keywords: mitogenesis, S-adenosylmethionine decarboxylase, ornithine decarboxylase, thymidine kinase, DNA synthesis
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edelman G. M., Cunningham B. A., Reeke G. N., Jr, Becker J. W., Waxdal M. J., Wang J. L. The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. Polyamine accumulation during lymphocyte transformation and its relation to the synthesis, processing, and accumulation of ribonucleic acid. Biochemistry. 1973 Oct 23;12(22):4479–4487. doi: 10.1021/bi00746a028. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. S-adenosyl-L-methionine decarboxylase during lymphocyte transformation: decreased degradation in the presence of a specific inhibitor. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1020–1025. doi: 10.1016/0006-291x(73)91039-5. [DOI] [PubMed] [Google Scholar]
- Fuller D. J., Gerner E. W., Russell D. H. Polyamine biosynthesis and accumulation during the G1 to S phase transition. J Cell Physiol. 1977 Oct;93(1):81–88. doi: 10.1002/jcp.1040930111. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives D. H., Durham J. P., Tucker V. S. Rapid determination of nucleoside kinase and nucleotidase activities with tritium-labeled substrates. Anal Biochem. 1969 Apr 4;28(1):192–205. doi: 10.1016/0003-2697(69)90170-5. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Polyamine synthesis during lymphocyte activation. Induction of ornithine decarboxylase and S-adenosyl methionine decarboxylase. Exp Cell Res. 1973 Mar 15;77(1):428–436. doi: 10.1016/0014-4827(73)90597-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loeb L. A., Ewald J. L., Agarwal S. S. DNA polymerase and DNA replication during lymphocyte transformation. Cancer Res. 1970 Oct;30(10):2514–2520. [PubMed] [Google Scholar]
- McClain D. A., Edelman G. M. Analysis of the stimulation-inhibition paradox exhibited by lymphocytes exposed to concanavalin A. J Exp Med. 1976 Dec 1;144(6):1494–1508. doi: 10.1084/jem.144.6.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. A biosynthetic ornithine decarboxylase in Escherichia coli. Biochem Biophys Res Commun. 1965 Sep 22;20(6):697–702. doi: 10.1016/0006-291x(65)90072-0. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., CIECIURA S. J., ROBINSON A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J Exp Med. 1958 Dec 1;108(6):945–956. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro L., Bernengo M. G. Thymidine kinase, deoxycytidine kinase and deoxycytidylate deaminase activities in phytohaemagglutinin stimulated human lymphocytes. Exp Cell Res. 1971 Oct;68(2):283–290. doi: 10.1016/0014-4827(71)90152-2. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Tyrsted G., Munch-Petersen B. Early effects of phytohemagglutinin on induction of DNA polymerase, thymidine kinase, deoxyribonucleoside triphosphate pools and DNA synthesis in human lymphocytes. Nucleic Acids Res. 1977 Aug;4(8):2713–2723. doi: 10.1093/nar/4.8.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., McClain D. A., Edelman G. M. Modulation of lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1975 May;72(5):1917–1921. doi: 10.1073/pnas.72.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]



