Abstract
Drug-associated cues are believed to be important mediators of addiction and drug relapse. Although such cues may influence drug-seeking behavior through multiple routes, it is their putative incentive motivational properties – their ability to elicit “craving” – that interests many addiction researchers. The Pavlovian-to-instrumental transfer paradigm is commonly used to assay cue-evoked incentive motivation in situations involving natural rewards, but has not been widely applied to the study of drug self-administration. We used this paradigm to determine if cues paired with intravenous cocaine could promote performance of an independently trained task in which rats self-administered cocaine by completing a chain of two different lever press actions, a procedure used to parse behavior into cocaine-seeking (first action) and cocaine-taking (second action). Rats showed significant transfer, increasing task performance during cocaine-paired cues. This effect was observed for both seeking and taking actions, although a trend towards greater cocaine taking was observed, a result that is consistent with studies using natural rewards. Our results demonstrate that cocaine-paired cues can provoke the pursuit of cocaine through a Pavlovian motivational process. This phenomenon may provide a useful new tool for modeling drug relapse, particularly as a method for targeting the response-invigorating effects of stimulus-drug learning.
Keywords: Pavlovian instrumental transfer, cocaine, self-administration, seeking taking chain, addiction
It has been well established that drug-associated cues can exert a potent source of control over drug seeking behavior, leading to relapse even after a prolonged period of abstinence (Heather & Greeley, 1990; Rohsenow, Niaura, Childress, Abrams, & Monti, 1990). The cue-induced reinstatement paradigm is probably the most widely used method for animal studies targeting the excitatory influence of drug-paired cues on instrumental actions (Homberg, Raasø, Schoffelmeer, & de Vries, 2004; See, 2005; Shaham, Shalev, Lu, De Wit, & Stewart, 2003; Weiss et al., 2001). In such studies, response-contingent drug deliveries are typically accompanied by an extraneous cue. After significant training and extinction of the self-administration response in the absence of cue and drug delivery, the ability of the cue to reinstate the instrumental response is tested. This procedure typically generates a robust invigoration of drug seeking, and is commonly used to assess the impact of various treatments (e.g., brain lesion or inactivation, drug withdrawal period, pharmacological intervention) on cue-elicited drug seeking.
Despite the popularity of the cue-induced reinstatement paradigm, the behavioral processes that allow drug-paired cues to exert their influence over drug-seeking behavior have not been well characterized. It is generally assumed that this phenomenon depends on some form of interaction between Pavlovian and instrumental learning systems (Berridge & Robinson, 2003; Everitt, Dickinson, & Robbins, 2001; Everitt & Robbins, 2005; Weiss, 2005), with the cue acquiring its incentive motivational properties by virtue of its Pavlovian (stimulus-outcome) relationship with drug delivery. This account is supported by a large body of work showing that cues paired with natural rewards, like food, can facilitate instrumental reward-seeking behavior (Crombag, Galarce, & Holland, 2008; Dickinson, Smith, & Mirenowicz, 2000; Rescorla, 1994). However, the standard cue-induced reinstatement of drug seeking effect described above does not necessarily depend on a Pavlovian incentive motivational process. In such studies, the cue is paired with drug delivery in a response-contingent manner (e.g., response → (cue + drug)) (Cooper, Barnea-Ygael, Levy, Shaham, & Zangen, 2007; Fuchs, Evans, Parker, & See, 2004; Gál & Gyertyán, 2006; Kufahl et al., 2009; Lee, Milton, & Everitt, 2006; Meil & See, 1997; Zavala, Osredkar, Joyce, & Neisewander, 2008) or is used as a discriminative stimulus to explicitly signal that a particular response is active (e.g., cue: response → drug) (Bradberry, Barrett-Larimore, Jatlow, & Rubino, 2000; Weiss et al., 2001; Yun & Fields, 2003). Both procedures confound Pavlovian and instrumental contingencies. This is a major problem because the presence of the cue during instrumental training makes it possible for that stimulus to become directly associated with the drug- seeking action, providing alternative routes for response selection and elicitation. Furthermore, in many cases, cues are presented in a response-contingent manner at test, which does not distinguish their presumed ability to provoke and/or invigorate drug seeking actions from their ability to reinforce those actions through new learning (i.e., conditioned reinforcement)(Wyvell & Berridge, 2001).
The Pavlovian-to-Instrumental transfer (PIT) paradigm was developed specifically to target the incentive motivational effects of reward-paired cues on instrumental performance. In this case, the subject is given stimulus-reward and action-reward pairings in separate phases of the experiment in order to prevent associations from developing between the cue and instrumental action. The cue is then noncontingently presented during testing with the action available. Any increase in the performance of this action in the presence of the reward-paired cue must therefore be the result of the conditioned response-invigorating properties of that cue and not the product of a cue-response association or a conditioned reinforcement process. Despite its advantages over the conventional cue-induced reinstatement procedure, use of PIT has been largely restricted to studies of natural reward seeking actions, and the few studies that have used the PIT procedure to assay cue-elicited drug seeking have used orally administered alcohol as the rewarding outcome (Corbit & Janak, 2007; Glasner, Overmier, & Balleine, 2005; Milton et al., 2011). The reluctance to use this approach may stem from experienced or perceived difficulties in generating the PIT effect using conventional drug self-administration procedures (Kruzich, Congleton, & See, 2001). Indeed, Everitt and Robbins noted in their review (Everitt & Robbins, 2005) that:
…neither approach to a CS predictive of a drug, nor enhancement of drug seeking by the unexpected presentation of a drug-associated CS has been clearly demonstrated in laboratory studies of drug seeking or relapse, although both are readily seen in animals responding for natural rewards. It may be that the experimental conditions for demonstrating these phenomena in a drug seeking setting have not yet been optimized, but it may also be that the behavioral influence of CSs associated with drugs and natural reinforcers differ fundamentally in this regard (p.1482).
The current study aims to establish an effective PIT procedure for studying the effects of cocaine-paired cues on instrumental intravenous cocaine seeking actions in rats.
Methods
Subjects
Male Long Evans rats, weighing on average 337g before surgery, were housed singly in a climate-controlled vivarium and were tested during the light phase of the light/dark cycle (lights on from 7am to 7pm). Food and tap water were provided ad libitum in the home cage throughout behavioral training and testing. All procedures were approved by the UCLA Institutional Animal Care and Use Committee, and were performedin accordance with National Research Council’s Guide for theCare and Use of Laboratory Animals. Five subjects were excluded from the experiment due to loss of catheter patency (N = 15).
Apparatus and Training
Rats were trained in eight identical Med Associates (East Fairfield, VT) operant chambers housed within sound- and light-resistant shells. The chambers contained two retractable levers that could be positioned on left and right side of one end wall. A 3-W, 24-V houselight was mounted on the top center of the opposite end wall provided illumination. The chambers were also equipped with a tone generator and a clicker. Microcomputers equipped with the MED-PC program (Med Associates) controlled the equipment and recorded lever presses.
Drugs
Cocaine hydrochloride (NIDA Drug Supply Program), dissolved in sterile saline (0.9% NaCl) and filtered-sterilized, was administered at 0.2mg/infusion over 4.35s using a Med Associates 100 pump during both instrumental and Pavlovian training.
Catheter surgery
Rats were deeply anesthetized with isoflurane (4–5% induction, 1.5–2.5% maintenance), and a silicon catheter (O.D. 0.63mm × I.D. 0.30mm × wall 0.17mm, CamCaths, Cambridgeshire, England) was placed into the right or left jugular vein. The catheter was advanced approximately 35 mm caudally to the right atrium. The proximal end was attached to a coiled length of wider bore tubing that exited through a mount inserted under the skin between the scapulae. Rats were given 5 days to recover from surgery and catheters were maintained with twice daily heparin injections (0.1 ml of 10 units/ml) for the duration of the experiment. The antibiotic, sulfamexazole (TMS), was placed in the drinking water (0.05%) for the duration of the experiment. Catheter patency was evaluated twice daily, before and after each self-administration session, by checking for backflow of blood in the flushing syringe. Any catheter of questionable patency was tested by evaluating the sedative effectiveness of 0.2ml of 1% propofol. Any subject not sedated was excluded. Cocaine was self-administered through polyethylene tubing threaded through a spring tether that was connected to a liquid swivel attached to a balance arm, allowing the animals free range of motion.
Pavlovian training
In the first conditioning session, which lasted approximately 2 hours, rats received 12 non-reinforced presentations of one of the two auditory stimuli (CS−; either a 3 kHz, 75dB tone or a 2Hz, 75dB click, 2-min duration) using a variable inter trial interval of 5 minutes (range: 3–7 minutes). In four daily subsequent 2-hour Pavlovian conditioning sessions, rats received 12 reinforced presentations of the alternate auditory stimulus (CS+), the onset of which signaled the delivery of a single infusion of cocaine (0.5 mg/kg/injection).
Instrumental training
Pavlovian training was followed by 10–12 days of instrumental training, during which the rats were allowed to self-administer cocaine by performing a two-action, seeking-taking chain (Balleine, 1995; Corbit & Balleine, 2003; Olmstead, Parkinson, Miles, Everitt, & Dickinson, 2000; Vanderschuren & Everitt, 2004). This behavioral paradigm requires the animal to press an initial, distal, lever to gain access to a second, proximal, lever, an action on which delivers the reward, and is designed to isolate the processes that control drug ‘seeking’ actions from those controlling drug ‘taking’ actions. For the current study, we used a modified version of the seeking-taking chain procedure employed by Corbit and Balleine (2003) to examine the effects of reward-paired cues on food seeking and taking. Rats initially received continuous reinforcement training with only one lever present (either the left or right lever), the ‘taking’ action. Sessions lasted until 20 outcomes had been earned, or until 2 hours had elapsed. Having reached criterion on the taking lever (earning 20 outcomes for two consecutive days) rats were trained on the full seeking-taking chain. The alternate lever, which served as the cocaine ‘seeking’ action, was inserted into the chamber at the beginning of each of these training sessions. A single press on this lever resulted in the insertion of the taking lever. Performing the taking response resulted in delivery of a cocaine infusion and immediate retraction of both the seeking and taking levers, followed by a 20-sec time out period. The seeking lever was then reinserted into the chamber, signaling that the seeking-taking contingency was once again active. As during taking lever training, these sessions were terminated after 20 outcomes had been earned or 2 hours had elapsed. Both components of the chain (the seeking-taking contingency and the taking-cocaine delivery contingency) were continuously reinforced for the first 2 sessions. The reinforcement schedule for each component was then shifted to random ratio (RR)-2 for 2 sessions, during which each response was reinforced with a probability of 0.5. The schedule was then shifted to RR-4 (p = 0.25) for both levers for the remainder of training until stable lever pressing was obtained (20 outcomes in 2 hours over 2 consecutive days). This ratio schedule further distinguishes between the seeking and taking components of the chain by weakening the temporal contiguity between the seeking action and the outcome delivery. We used the same reinforcement schedule on both levers to encourage the development of similar robust and persistent levels of responding on the seeking and taking levers, which should facilitate detection of the PIT effect (Corbit & Balleine, 2003). Importantly, no cues were used to signal cocaine infusions during instrumental training sessions.
Pavlovian-to-instrumental transfer testing
After the self-administration criterion was reached, the rats underwent the first of two PIT tests to assess the impact of the cocaine-predictive cues on their performance of the seeking-taking chain. PIT studies using food reward have shown that the expression of this effect is particularly sensitive to the conditions present at test (Holmes, Marchand, & Coutureau, 2010). The response-outcome contingency at test appears to be a particularly important factor; the transfer effect tends to be considerably more robust when subjects are tested in extinction (i.e., in the absence of response-contingent reward). Therefore, in the first test, separate groups of subjects were tested under extinction or rewarded conditions to determine if this factor influences the expression of PIT on a cocaine seeking-taking chain of actions.
To lower response rates and thereby facilitate detection of the excitatory effects of the CS+, both groups began the test with 5 minutes of extinction. This was directly followed by 4 non-contingent trials in which two auditory cues (CS+ and CS−) were strictly alternated (tone, click), with each CS period being followed by an ITI. For the rewarded group (n = 8), the seeking component of the chain was in place throughout the test, such that responding on the seeking lever resulted in insertion of the taking lever according to a RR-4 schedule. Responding on the taking lever resulted in retraction of that lever according to a RR-4 schedule, but did not result in cocaine delivery during the extinction period of the test. Following the extinction period, both components of the chain were fully intact, allowing rats to earn cocaine on a RR-4 schedule. This phase of the test was nearly identical to instrumental training sessions except for presentations of CS+ and CS−. To encourage low pre-CS response rates, scheduled cue deliveries were delayed until rats withheld responding on either lever for a period of at least 60 seconds, without any relationship to the cocaine delivery itself. This ensured an ITI of at least one minute. For the extinction group (n = 7), we were particularly interested in comparing the influence of the cues across the seeking and taking levers. Therefore, in this condition, rats were given access to both levers for the duration of the transfer test in the absence of either the seeking → taking or taking→cocaine contingency. Cue presentations were separated by an ITI of 2 min. All lever presses were recorded during this session but no reinforcement was delivered. All rats (from both groups) were subsequently given three daily Pavlovian CS+ extinction sessions, which consisted of 12 CS+ presentations in the absence of outcome delivery, with an average ITI of three minutes (range 1–5 min), followed by an instrumental retraining session. They were then administered a second test performed under extinction conditions with both levers available throughout. Cue presentations began 10 minutes after the start of the session, following the procedure of the extinction group in the first test, but with a fixed 6-minute ITI to minimize the carryover of post-cue responding into the next pre-cue period.
Data analysis and statistics
To assess the influence of the cues on lever press performance, we subtracted the number of lever presses occurring in the minute before the cue onset (i.e., Pre-CS baseline) from the number of lever presses performed during each of the next three minutes, which included the two-minute CS delivery and a one-minute post-CS period. We included the first minute of post-CS responding in this analysis because previous studies have shown that the excitatory impact of reward-paired cues on lever pressing can persist beyond the initial CS delivery period (Lovibond, 1983). For Test 1, statistical analysis of these data was conducted using a mixed ANOVA with CS (CS+ vs. CS−), action (Seeking vs. Taking) and period (CS minute 1, CS minute 2, post-CS minute 1) serving as within-subjects factors and group (rewarded or extinction test conditions) serving as a between-subjects factor. Test 2 was analyzed with the same mixed ANOVA, except with no between-subjects factor.
Results
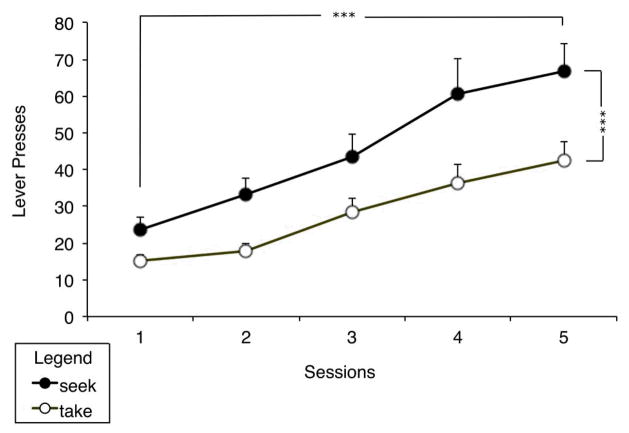
Although there was no direct measure of learning for Pavlovian conditioning, all rats displayed characteristic behavioral effects of cocaine administration (e.g., increased locomotor behavior, rearing, stereotypy) after each training session. They were then trained to self-administer cocaine by performing a seeking-taking chain of actions. Over the course of training, rats showed a significant increase in both seeking and taking lever presses, with significantly more lever pressing on the seeking lever (see Figure 1). A repeated-measures ANOVA detected a significant main effect of day (F (1,14) = 35.284, p < 0.001), action (F (1,14) = 31.438, p < 0.001) and a significant interaction of day and action (F (1,14) = 5.601, p < 0.05). The difference in rate across actions is likely due to persistent responding on the seeking lever, which remained in the chamber during periods when the second (taking) component of the chain was active.
Figure 1.
Acquisition of the seeking-taking chain, shown as average lever presses over the session for the last five sessions of training. Means + SEM. *** p < 0.001.
After Pavlovian and instrumental training, we tested the tendency for cocaine-paired cues to motivate lever-pressing behavior in the absence or presence of response-contingent cocaine deliveries. Pre-CS baselines are reported in Table 1. Surprisingly, a mixed ANOVA conducted on the pre-CS baselines revealed a significant main effect of CS (F (1,13) = 6.19, p < 0.05), and a significant action by group interaction (F (1,13) = 5.89, p < 0.05), with no other significant main effects or interactions (largest F value: F (1,13) = 4.26, p > 0.05). These results indicate higher baselines for the CS− than the CS+, and more responding in the baseline period on the taking lever for the extinction group. Since these cues were delivered in strict alternation, the relatively elevated baseline response rate going into CS− trials may reflect carry over of the excitatory influence of recent CS+ deliveries.
Table 1.
Test 1. Pre-CS baselines and extinction responding
| Group | Lever | CS+ | CS− |
|---|---|---|---|
| Extinction Group | Seeking lever | 1.43±0.69 | 3.14±0.83 |
| Taking lever | 3.71± 2.17 | 8.57±2.99 | |
| Rewarded Group | Seeking lever | 1.75±1.08 | 2.63±0.89 |
| Taking lever | 0.625±0.38 | 3.13±1.37 |
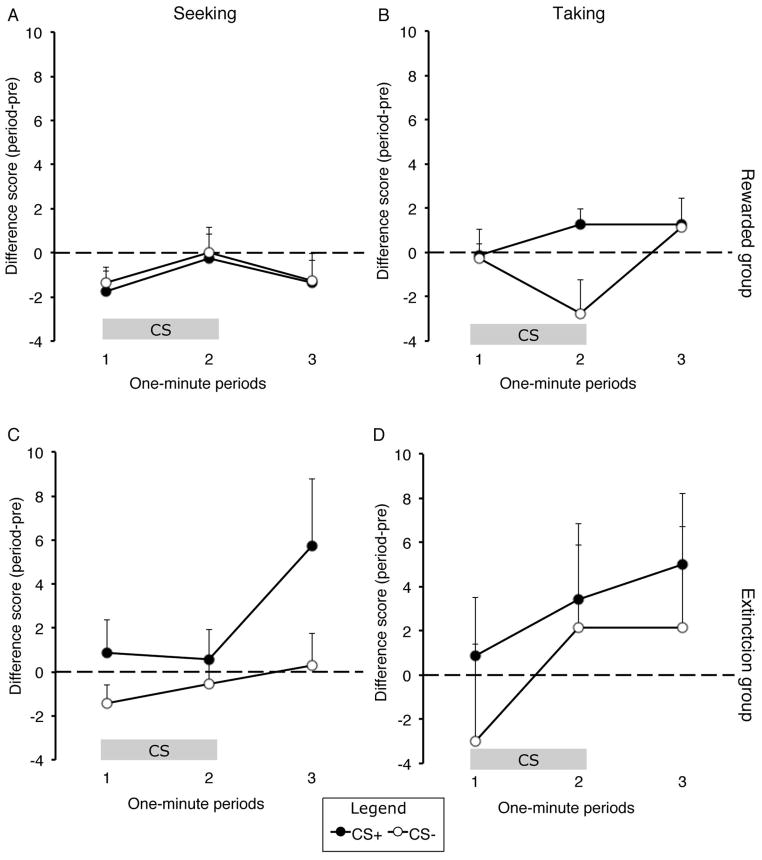
The results of the first PIT test are presented in Figure 2. Although the findings from this initial round of testing were not particularly clear, some features of these data are worth noting. First, it appears that rats tested in extinction show a slight increase in lever pressing during cue presentations, an effect that was at least numerically greater during CS+ trials and was most prominent once the cues were terminated. Second, for rats that were rewarded with cocaine at test, there was little indication that the CS+ was capable of invigorating cocaine seeking or taking behavior. An ANOVA performed on these data found no effect of CS (F (1,13) = 1.958, p > 0.05) or action (F (1,13) = 1.396, p > 0.05), nor was there a significant interaction between these variables (F(1,13) = 0.177, p > 0.05), or between these variables and test group (F = 0.03, p > 0.05). However, though not specific to action or CS, there was a significant effect of period (F (2,26) = 4.907, p < 0.05) and a period by group interaction (F (2,26) = 4.365, p < 0.05), indicating that the groups differed in the rate at which they lever pressed during and immediately after the cue presentations. To explore this effect further we conducted separate CS x Action x Period ANOVAs for each test group. Both groups demonstrated a significant main effect of period (F (2,12) = 4.23, p < 0.05 – extinction group, F (2,14) = 3.90, p < 0.05 – rewarded group), with no other significant main effect or interactions (largest F value: F (2,14)=2.741, p > 0.05).
Figure 2.
Results of the first Pavlovian-to-instrumental transfer test. Difference scores for each minute of the CS and the first minute of the post-CS period, displayed separately for action (seeking or taking) and group (extinction or rewarded). A–B, results for the rewarded group for the seeking lever (A) and taking lever (B). C–D, results for the extinction group for the seeking lever (C) and taking lever (D). Means + SEM.
In light of these results, we conducted a second test using procedures likely to facilitate the expression of PIT. First, given the clear lack of effect in the rewarded group, all rats were tested under extinction conditions in Test 2. Second, we increased the initial extinction period from 5 to 10 minutes to further suppress pre-CS response rates and avoid a potential behavioral “ceiling” or upper limit on responding. Third, we increased the interval between trials to 6 minutes to minimize carryover effects. Fourth, we gave rats 3 sessions of extinction of the CS+ before Test 2. Though counterintuitive, recent evidence suggests that such extinction of the CS+ can enhance the PIT effect, presumably by weakening competing conditioned responses (Holmes et al., 2010).
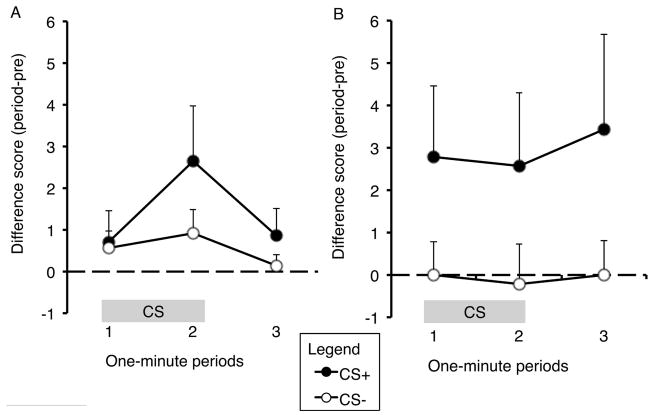
Pre-CS baselines for Test 2 are reported in Table 2. There was a significant main effect of action (F (1,13) = 7.05, p < 0.05), but no other significant effects (largest F value: F (1,13) = 2.17, p > 0.05), reflecting more pre-CS responding on the taking lever than the seeking lever. Figure 3 presents the results of the Test 2. As is clear from these data, rats displayed a stimulus-specific increase in lever pressing to the CS+, a pattern indicative of PIT. A repeated-measures ANOVA showed a significant main effect of CS (F (1,13) = 6.752, p < 0.05), indicating that the enhancement in lever pressing was greater for the CS+ than for the CS−. There was no effect of action (F (1,13) = 0.324, p > 0.05) or period (F (1,13) = 0.208, p > 0.05), nor were there any significant interactions between any of these factors (largest F value: F (1,13) = 2.25, p > 0.05). Although these analyses failed to identify action-specificity in the PIT effect, inspection of the data in Figure 3 suggests that the CS+ produced a more pronounced and longer-lasting enhancement in performance of the taking action than of the seeking action, consistent with previous food PIT studies (Corbit & Balleine, 2003). Indeed, when we confined our analysis to one or the other action, we found a significant effect of CS for the taking lever (F (1,13) = 5.795, p <0.05), but found no such effect for the seeking lever (F (1,13) = 1.68, p > 0.05).
Table 2.
Test 2. Pre-CS baselines
| Lever | CS+ | CS− |
|---|---|---|
| Seeking lever | 0.43±0.29 | 0.29±0.13 |
| Taking lever | 1.14±0.43 | 1.86±0.53 |
Figure 3.
Results of the second Pavlovian-to-instrumental transfer test. Difference scores for each minute of the CS and the first minute of the post-CS period, separately plotted for seeking (A) and taking (B) levers. Means + SEM.
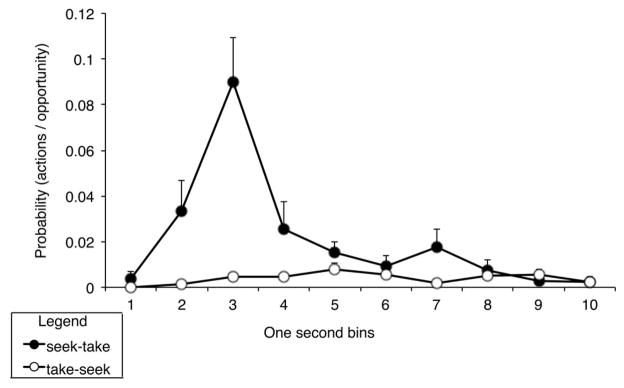
In view of the trend towards a difference in the degree of transfer on the seeking and taking levers, an additional analysis of transfer test performance was conducted to determine if the rats performed the chain task during the test as trained, i.e. shifting from the seeking lever to the taking lever rather than moving in the opposite direction. To quantify these shifts in performance we followed Balleine and colleagues’ example (Corbit & Balleine, 2003) in assessing the probability that the taking lever action was performed in each of the 10 seconds that followed performance of the seeking action, and generated a similar probability distribution for seeking presses that occurred in the 10 seconds that followed each taking action. These data, which are shown in Figure 4, clearly demonstrate that the rats were more likely to shift from seeking-to-taking, which was the order of actions reinforced by cocaine delivery, than from taking-to-seeking. This shift in performance appeared to be most frequent during approximately the first five seconds in the post-seeking period. A repeated measures ANOVA using action order (Seek-Take vs. Take-Seek) and time bin (1–10) as factors confirmed this analysis, revealing a significant main effect of action order (F (1,14) = 22.121, p<0.001), a significant main effect of bin (F (9,126) = 8.176, p<0.001), and a significant interaction between action order and bin (F (9,126) = 8.029, p<0.001).
Figure 4.
Probability of transitioning from the seeking to the taking lever vs. transitioning from the taking to the seeking lever. Probabilities are calculated by dividing the total number of transitions in each 1-second bin by the total number of 1st action lever presses in the session. A transition was operationally defined as the first response on the 2nd action lever within 10 seconds after a response on the 1st action lever. Means + SEM.
Discussion
To our knowledge, this study represents the first demonstration that environmental cues paired with intravenous cocaine administration acquire the ability to provoke and invigorate cocaine self-administration in rats specifically through a Pavlovian-to-instrumental transfer process. As such, the data support the hypothesis that drug-paired cues can invigorate drug-related activities by inducing a state of incentive motivation or “craving”.
The vast majority of studies examining the influence of drug-paired cues on drug-seeking behavior have used the cue-induced reinstatement paradigm. (Homberg et al., 2004; Shaham et al., 2003; Weiss et al., 2001). However, as noted in the Introduction, this approach confounds a number of distinct action selection strategies that may contribute to drug relapse. For instance, the cue may enter into a direct association with the self-administration response, allowing a habit to control reinstatement performance (Balleine & Ostlund, 2007; Ostlund & Balleine, 2008). By exposing subjects to the cue-drug and action-drug relationships in separate training phases, the PIT procedure makes it possible to isolate the influence of Pavlovian learning on drug self-administration, a behavioral process that has been assigned a fundamental role in mediating incentive motivation (Berridge & Robinson, 2003; Dickinson et al., 2000) and compulsive drug-seeking behavior (Robbins & Everitt, 2002). Furthermore, since the PIT effect is elicited by unexpected (response-independent) presentations of a reward-paired cue, it cannot be explained by that stimulus’s ability to increase behavior through conditioned reinforcement, unlike certain versions of the reinstatement procedure (Kruzich et al., 2001).
Our findings also shed light on some of the factors controlling the expression of drug-motivated PIT. For instance, in Test 1 rats that were given response-contingent cocaine reward at test failed to show any evidence of response invigoration during presentations of the cocaine-paired cue, indicating not only that rewarded conditions are not necessary to produce PIT, but that receiving the drug at test may in fact disrupt the expression of this effect, consistent with similar studies using food self-administration tasks (Azrin & Hake, 1969; Dickinson et al., 2000; Lovibond, 1981, 1983; Rescorla, 1994). It is possible that the lack of cue-elicited responding in the rewarded group was due to the rats’ tendency to control their drug intake. For instance, rats tend to self-administer cocaine to maintain a preferred level of drug in their bloodstream (Suto & Wise, 2011; Tsibulsky & Norman, 1999, 2001). Thus, it is possible that a short-term satiety for cocaine was responsible for attenuating the PIT effect. Indeed, studies using food-motivated PIT have established that this effect can be abolished by sating rats on food prior to the test session (Balleine, 1994; Corbit, Janak, & Balleine, 2007).
As with food-motivated PIT studies, we found that testing rats in extinction facilitated the expression of cue-evoked behavior. For instance, in Test 1, the rats tested in extinction showed a significant increase in responding during and immediately after CS+ presentations. Surprisingly, however, these rats also showed a somewhat similar increase in responding to the CS−, a stimulus that was never paired with cocaine. This pattern of results could reflect a nonspecific effect of these stimulus presentations (e.g., disinhibition or arousal) (Brimer, 1970), or it could have resulted from a cue discrimination impairment, perhaps brought about by the repeated administration of cocaine. However, we chose to test an alternative hypothesis: that expression of PIT to the CS+ was at least partially being masked by that cue’s tendency to evoke incompatible conditioned responses, including locomotor activity (Ma, Maier, Ahrens, Schallert, & Duvauchelle, 2010). For food motivated tasks, it is known that response competition between conditioned orienting and approach behaviors can interfere with the expression of PIT (Baxter & Zamble, 1980; Delamater & Oakeshott, 2007; Lovibond, 1983; Overmier, Payne, Brackbill, Linder, & Lawry, 1979), and that extinguishing the CS+ will eliminate the competing response and allow the full excitatory impact of that cue to emerge (Holmes et al., 2010) without eliminating the transfer effect (Delamater, 1996). Therefore, we extinguished the CS+ over three sessions before giving the rats a second PIT test. Consistent with the response competition account, we found that rats selectively increased their rate of lever pressing to the CS+ during this round of testing, relative to baseline periods and CS− trials.
Although the CS extinction procedure is likely to have played a role in facilitating the expression of PIT, procedural differences between the two tests may have also contributed to our ability to detect a significant, CS+ specific PIT effect in Test 2. First, the initial extinction phase of the test was increased from 5 to 10 minutes to further suppress baseline response rates during Test 2 in an attempt to avoid a potential “ceiling effect” that may have countered cue-induced increases in responding during Test 1. It should be noted, however, that a significant (albeit nonspecific) elevation in responding was detected in Test 1, indicating that an absolute upper limit on lever pressing did not prevent rats from increasing their rate of responding following the cue deliveries. We also lengthened the ITI to minimize carryover of cue-evoked behavior into the baseline period of subsequent trials and further suppress baseline responding.
Another important factor controlling the expression of PIT appears to be the position of the target action in the chain of events leading up to reward delivery. The current study used a seeking-taking chain designed to distinguish between those actions required to seek out or pursue cocaine and those involved in cocaine taking or consumption. Previous studies using a food-rewarded seeking-taking task have established that food-paired cues have a greater influence over the performance of the taking response (Balleine, 1995; Corbit & Balleine, 2003). Based on such findings, it has been argued that distinct motivational processes control these two types of actions; whereas reward seeking is guided by value estimates for specific behavioral (instrumental) goals, reward taking is dependent on Pavlovian incentive motivation generated by environmental cues (Balleine, 1995; Corbit & Balleine, 2003). We also found some evidence of taking-specific PIT in the second test session, suggesting symmetry between drug- and food-motivated PIT, and indicating that a fundamental feature of reward-paired cues is their ability to motivate actions that are associated with imminent reward delivery or consumption. However, this should not be taken as evidence that reward-paired cues have no effect on reward-seeking behavior. Just as in the previous reports that PIT is specific to food taking behavior, the current study found a stronger PIT effect on the taking lever in a test in which both levers were continuously available. During training, this situation predicted that the taking lever was active, making the reward-seeking action obsolete. So it is possible that the cocaine-paired cue would have had a stronger impact on cocaine seeking if that action were tested in isolation. However, it should also be noted that although the taking lever was, in this sense, more predictive of reward delivery, our rats distributed their actions across the two levers at test just as if the full chain contingency was in effect, performing the taking action shortly after they performed the seeking action, but not the other way around. Therefore their behavior at test was not confined to a simple strategy of focusing their performance on the taking lever.
This study confirms that PIT can be generated in rodents performing a cocaine self-administration task. Studies of food-motivated PIT have established that dopamine signaling plays a particularly important role in mediating the response-invigorating effects of reward-paired cues (Dickinson et al., 2000; Lex & Hauber, 2008; Ostlund & Maidment, 2012; Wassum, Ostlund, Balleine, & Maidment, 2011). Furthermore, studies using the PIT paradigm have shown that repeated psychostimulant sensitization could potentiate the cue-evoked food seeking behavior (Saddoris, Stamatakis, & Carelli, 2011; Wyvell & Berridge, 2001), providing support for the incentive sensitization theory of addiction (Robinson & Berridge, 1993, 2000, 2001, 2008). It will be of interest to see if dopamine plays a similar role in cocaine-motivated PIT and whether this phenomenon can be modulated by treatments that sensitize the dopamine system, like repeated drug pre-exposure. Future studies should also examine whether other drugs of abuse, such as opioids or nicotine, can support PIT. Establishing these effects will make it possible to advance our understanding of the behavioral and neural processes underlying cue-motivated drug-seeking behavior.
Acknowledgments
This research was supported by Grants DA09359 and DA05010 from NIDA to NTM, Grant DA029035 to SBO and training fellowship T32-DA024635 to KHL.
References
- Azrin NH, Hake DF. Positive conditioned suppression: conditioned suppression using positive reinforcers as the unconditioned stimuli. J Exp Anal Behav. 1969;12(1):167–173. doi: 10.1901/jeab.1969.12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B. Asymmetrical interactions between thirst and hunger in Pavlovian-instrumental transfer. Q J Exp Psychol B. 1994;47(2):211–231. [PubMed] [Google Scholar]
- Balleine B. Motivation control of heterogenous instrumental chains. Journal of Experimental Psychology. 1995;21:203–217. [Google Scholar]
- Balleine BW, Ostlund SB. Still at the choice-point: action selection and initiation in instrumental conditioning. Ann N Y Acad Sci. 2007;1104:147–171. doi: 10.1196/annals.1390.006. [DOI] [PubMed] [Google Scholar]
- Baxter DJ, Zamble E. Reinforcer and response specificity in appetitive transfer of control. Learning & Behavior. 1980;10(2):201–210. [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bradberry C, Barrett-Larimore R, Jatlow P, Rubino S. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20(10):3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimer C. Disinhibition of an operant response. Learning and Motivation. 1970;1:346–371. [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology (Berl) 2007;194(1):117–125. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit L, Balleine B. Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. J Exp Psychol Anim Behav Process. 2003;29(2):99–106. doi: 10.1037/0097-7403.29.2.99. [DOI] [PubMed] [Google Scholar]
- Corbit L, Janak P. Ethanol-associated cues produce general pavlovian-instrumental transfer. Alcohol Clin Exp Res. 2007;31(5):766–774. doi: 10.1111/j.1530-0277.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- Corbit L, Janak P, Balleine B. General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci. 2007;26(11):3141–3149. doi: 10.1111/j.1460-9568.2007.05934.x. [DOI] [PubMed] [Google Scholar]
- Crombag H, Galarce E, Holland P. Pavlovian influences on goal-directed behavior in mice: the role of cue-reinforcer relations. Learn Mem. 2008;15(5):299–303. doi: 10.1101/lm.762508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR. Effects of several extinction treatments upon the integrity of Pavlovian stimulus-outcome associations. Learning&Behavior. 1996;24(4):437–449. [Google Scholar]
- Delamater AR, Oakeshott S. Learning about multiple attributes of reward in Pavlovian conditioning. Ann N Y Acad Sci. 2007;1104:1–20. doi: 10.1196/annals.1390.008. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci. 2000;114(3):468–483. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- Everitt B, Dickinson A, Robbins T. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36(2–3):129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt B, Robbins T. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24(29):6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner SV, Overmier JB, Balleine BW. The role of Pavlovian cues in alcohol seeking in dependent and nondependent rats. J Stud Alcohol. 2005;66(1):53–61. doi: 10.15288/jsa.2005.66.53. [DOI] [PubMed] [Google Scholar]
- Gál K, Gyertyán I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81(1):63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Heather N, Greeley J. Cue exposure in the treatment of drug dependence: the potential of a new method for preventing relapse. Drug Alcohol Rev. 1990;9(2):155–168. doi: 10.1080/09595239000185211. [DOI] [PubMed] [Google Scholar]
- Holmes N, Marchand A, Coutureau E. Pavlovian to instrumental transfer: a neurobehavioural perspective. Neurosci Biobehav Rev. 2010;34(8):1277–1295. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Raasø HS, Schoffelmeer AN, de Vries TJ. Individual differences in sensitivity to factors provoking reinstatement of cocaine-seeking behavior. Behav Brain Res. 2004;152(1):157–161. doi: 10.1016/j.bbr.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Congleton KM, See RE. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav Neurosci. 2001;115(5):1086–1092. doi: 10.1037//0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- Kufahl P, Zavala A, Singh A, Thiel K, Dickey E, Joyce J, Neisewander J. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63(10):823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26(22):5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem. 2008;15(7):483–491. doi: 10.1101/lm.978708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF. Appetitive Pavlovian-instrumental interactions: effects of inter-stimulus interval and baseline reinforcement conditions. Q J Exp Psychol B. 1981;33(Pt 4):257–269. doi: 10.1080/14640748108400811. [DOI] [PubMed] [Google Scholar]
- Lovibond PF. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J Exp Psychol Anim Behav Process. 1983;9(3):225–247. [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212(1):109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87(2):139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Milton A, Schramm M, Wawrzynski M, Gore F, Oikonomou-Mpegeti F, Wang N, Samuel D, Economidou D, Everitt B. Antagonism at NMDA receptors, but not β-adrenergic receptors, disrupts the reconsolidation of pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacology. 2011;219(3):751–761. doi: 10.1007/s00213-011-2399-9. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Parkinson JA, Miles FJ, Everitt BJ, Dickinson A. Cocaine-seeking by rats: regulation, reinforcement and activation. Psychopharmacology (Berl) 2000;152(2):123–131. doi: 10.1007/s002130000498. [DOI] [PubMed] [Google Scholar]
- Ostlund S, Balleine B. On habits and addiction: An associative analysis of compulsive drug seeking. Drug Discov Today Dis Models. 2008;5(4):235–245. doi: 10.1016/j.ddmod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT. Dopamine receptor blockade attenuates the general incentive motivational effects of noncontingently delivered rewards and reward-paired cues without affecting their ability to bias action selection. Neuropsychopharmacology. 2012;37(2):508–519. doi: 10.1038/npp.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmier JB, Payne RJ, Brackbill RM, Linder B, Lawry JA. On the mechanism of the post-asymptotic CR decrement phenomenon. Acta Neurobiol Exp (Wars) 1979;39(6):603–620. [PubMed] [Google Scholar]
- Rescorla R. Control of instrumental performance by Pavlovian and instrumental stimuli. J Exp Psychol Anim Behav Process. 1994;20(1):44–50. [PubMed] [Google Scholar]
- Robbins T, Everitt B. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78(3):625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict. 1990;25(7A–8A):957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Stamatakis A, Carelli RM. Neural correlates of Pavlovian-to-instrumental transfer in the nucleus accumbens shell are selectively potentiated following cocaine self-administration. Eur J Neurosci. 2011;33(12):2274–2287. doi: 10.1111/j.1460-9568.2011.07683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526(1–3):140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Suto N, Wise R. Satiating Effects of Cocaine Are Controlled by Dopamine Actions in the Nucleus Accumbens Core. The Journal of Neuroscience. 2011;31:17917–17922. doi: 10.1523/JNEUROSCI.1903-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839(1):85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold during maintained cocaine self-administration in outbred mice. Neuroreport. 2001;12(2):325–328. doi: 10.1097/00001756-200102120-00029. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305(5686):1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 2011;18(7):475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5(1):9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25(3):361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21(19):7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121(3):747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- Zavala A, Osredkar T, Joyce J, Neisewander J. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008;62(6):421–431. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]