Abstract
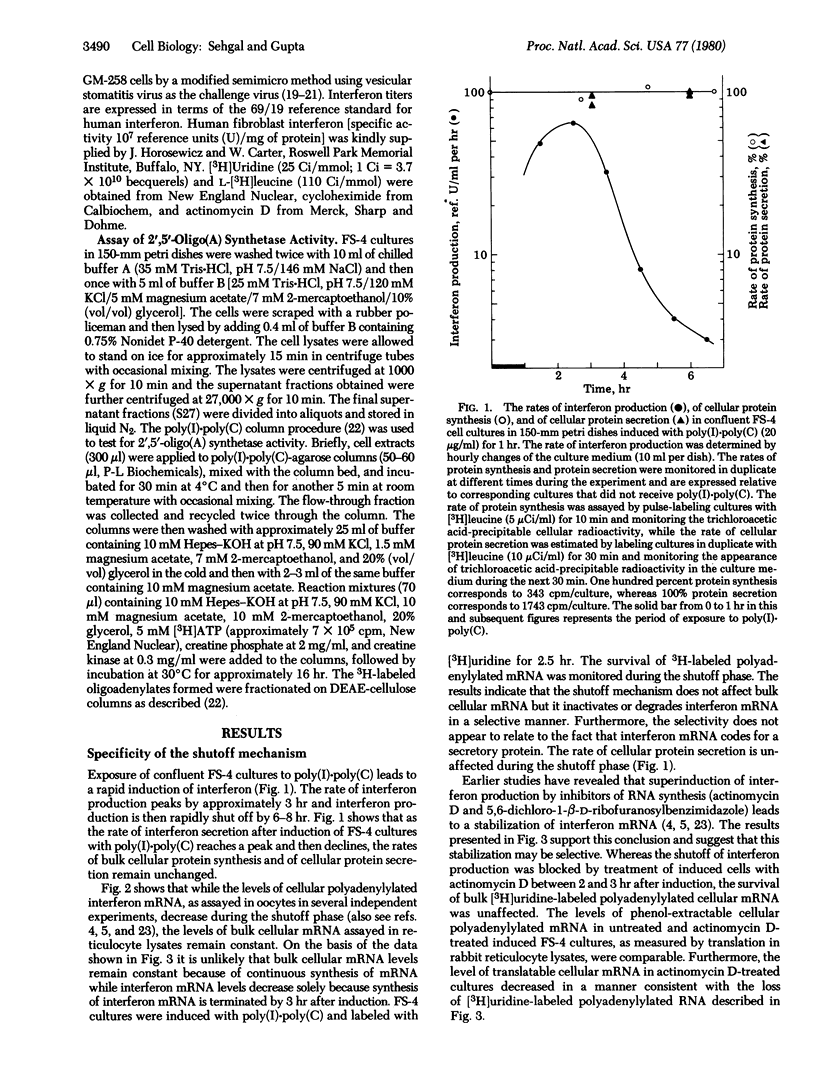
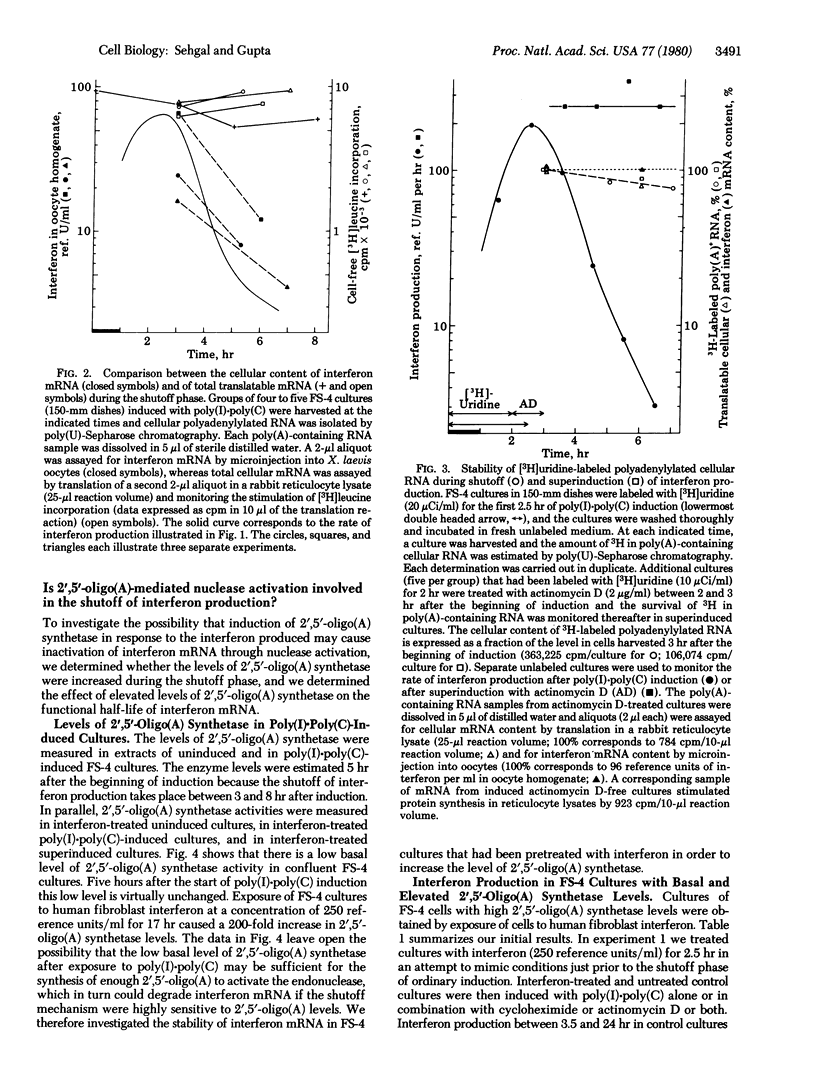
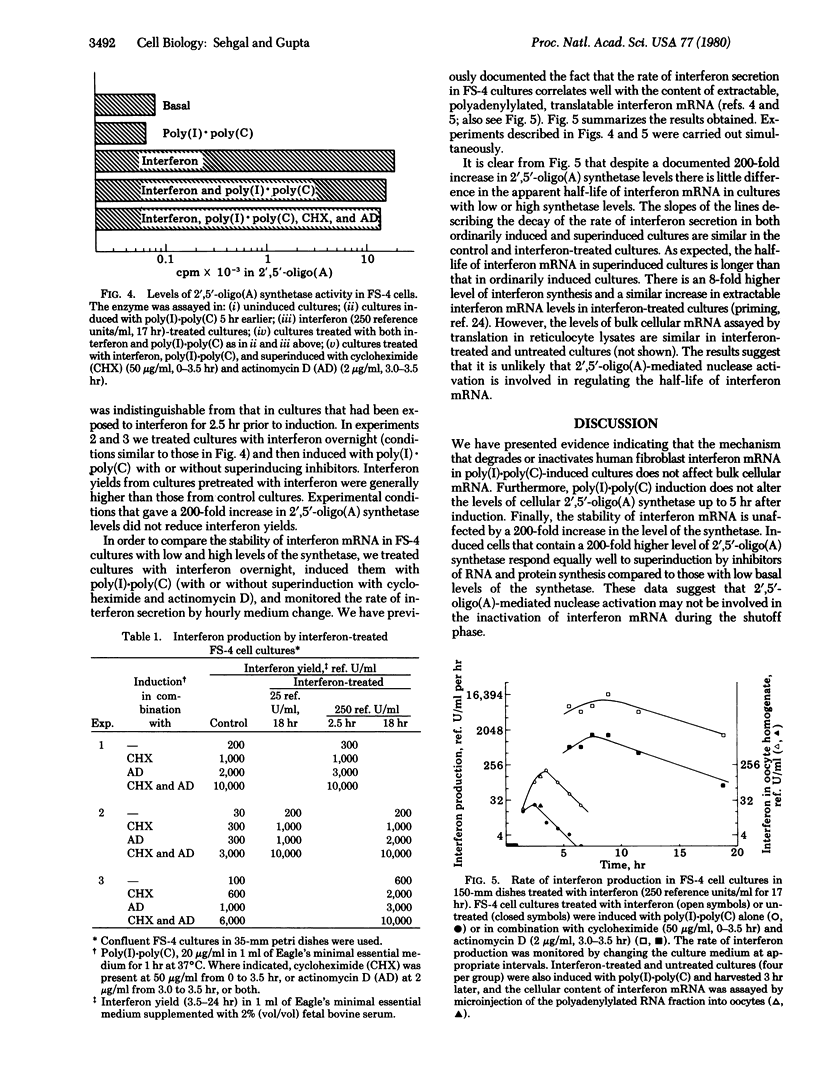
The inactivation of interferon mRNA during the shutoff phase of interferon production in poly(I)xpoly(C)-induced human fibroblast cultures is selective. We have determined that the shutoff of interferon production, which takes place from 3 to 8 hr after the beginning of induction, is not associated with an appreciable declined in the rate of bulk cellular protein synthesis or of cellular protein secretion. While the amount of translatable interferon mRNA declined markedly during the shutoff phase, the level of translatable bulk cellular mRNA and the stability of [3H]uridine-labeled mRNA were unaffected. Superinduction with actinomycin D selectively stabilized interferon mRNA with no apparent effect on the stability of bulk cellular mRNA. Furthermore, an activation of the 2',5'-oligo(A) synthetase/endonuclease system does not appear to be involved in the shutoff phenomenon. Uninduced FS-4 cells contained a low basal level of 2'5'-oligo(A) synthetase activity, which was unchanged in poly(I)xpoly(C)-induced cells during the shutoff phase. Treatment of FS-4 cells with interferon for 16-18 hr prior to induction increased the enzyme activity by approximately 200-fold. However, this did not inhibit interferon production after induction with poly(I)xpoly(C) alone or after superinduction with cycloheximide or actinomycin D or both. Furthermore, the rates of decay of interferon production were comparable in cells with either a basal or an increased level of 2',5'-oligo(A) synthetase. Thus a 200-fold increase in 2',5'-oligo(A) synthetase level did not affect either the stability of interferon mRNA or the efficacy of interferon superinduction by metabolic inhibitors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. L., Havell E. A., Vilcek J., Pestka S. Induction and decay of human fibroblast interferon mRNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4415–4419. doi: 10.1073/pnas.74.10.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Geoghegan T., Bergmann I., Brawerman G. Studies on the efficiency of translation and on the stability of actin messenger ribonucleic acid in mouse sarcoma ascites cells. Biochemistry. 1979 Jul 10;18(14):3153–3159. doi: 10.1021/bi00581a037. [DOI] [PubMed] [Google Scholar]
- Chatterjee B., Hopkins J., Dutchak D., Roy A. K. Superinduction of alpha 2u globulin by actinomycin D: evidence for drug-mediated increase in alpha 2u mRNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1833–1837. doi: 10.1073/pnas.76.4.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Enger M. D., Rall L. B., Hildebrand C. E. Thionein gene expression in Cd++-variants of the CHO cell: correlation of thionein synthesis rates with translatable mRNA levels during induction, deinduction, and superinduction. Nucleic Acids Res. 1979 Sep 11;7(1):271–288. doi: 10.1093/nar/7.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARREN L. D., HOWELL R. R., TOMKINS G. M., CROCCO R. M. A PARADOXICAL EFFECT OF ACTINOMYCIN D: THE MECHANISM OF REGULATION OF ENZYME SYNTHESIS BY HYDROCORTISONE. Proc Natl Acad Sci U S A. 1964 Oct;52:1121–1129. doi: 10.1073/pnas.52.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Wood J., Meurs E., Montagnier L. Increased nuclease activity in cells treated with pppA2'p5'A2'p5' A. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3261–3265. doi: 10.1073/pnas.76.7.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Hovanessian A. G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977 Aug 11;268(5620):540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenhaupt K., Lingrel J. B. Synthesis and turnover of globin mRNA in murine erythroleukemia cells induced with hemin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5173–5177. doi: 10.1073/pnas.76.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Baglioni C. Mechanism for discrimination between viral and host mRNA in interferon-treated cells. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2600–2604. doi: 10.1073/pnas.76.6.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Dobberstein B., Tamm I. Interferon messenger RNA content of human fibroblasts during induction, shutoff, and superinduction of interferon production. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3409–3413. doi: 10.1073/pnas.74.8.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Lyles D. S., Tamm I. Superinduction of human fibroblast interferon production: further evidence for increased stability of interferon mRNA. Virology. 1978 Aug;89(1):186–198. doi: 10.1016/0042-6822(78)90051-x. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I. An evaluation of messenger RNA competition in the shutoff of human interferon production. Proc Natl Acad Sci U S A. 1976 May;73(5):1621–1625. doi: 10.1073/pnas.73.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I. Halogenated benzimidazole ribosides, Novel inhibitors of RNA synthesis. Biochem Pharmacol. 1978;27(21):2475–2485. doi: 10.1016/0006-2952(78)90313-1. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I. Two mechanisms contribute to the superinduction of poly(I).poly(C)-induced human fibroblast interferon production. Virology. 1979 Jan 15;92(1):240–244. doi: 10.1016/0042-6822(79)90230-7. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Enhancement of human interferon production by neutral red and chloroquine: analysis of inhibition of protein degradation and macromolecular synthesis. J Exp Med. 1975 Nov 1;142(5):1283–1300. doi: 10.1084/jem.142.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Human interferon production: superinduction by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Science. 1975 Oct 17;190(4211):282–284. doi: 10.1126/science.1179208. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Regulation of human interferon production. I. Superinduction by 5, 6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Virology. 1976 Apr;70(2):532–541. doi: 10.1016/0042-6822(76)90294-4. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Dower W. J., Schimke R. T., Brown R. E., Kerr I. M. 2-5A synthetase: assay, distribution and variation with growth or hormone status. Nature. 1979 Mar 29;278(5703):471–473. doi: 10.1038/278471a0. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H., Schneider E. L., Tischfield J., Epstein C. J., Ruddle F. H. Human chromosome 21 dosage: effect on the expression of the interferon induced antiviral state. Science. 1974 Oct 4;186(4158):61–63. doi: 10.1126/science.186.4158.61. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Levinson B. B., Baxter J. D., Dethlefsen L. Further evidence for posttranscriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1972 Sep 6;239(88):9–14. doi: 10.1038/newbio239009a0. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A. Stabilization of interferon messenger RNA activity by treatment of cells with metabolic inhibitors and lowering of the incubation temperature. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3909–3913. doi: 10.1073/pnas.70.12.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Rossman T. G., Varacalli F. Differential effects of actinomycin D and puromycin on the release of interferon induced by double stranded RNA. Nature. 1969 May 17;222(5194):682–683. doi: 10.1038/222682a0. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Kerr I. M. Inhibition of protein synthesis by 2'-5' linked adenine oligonucleotides in intact cells. Nature. 1978 Nov 2;276(5683):88–90. doi: 10.1038/276088a0. [DOI] [PubMed] [Google Scholar]



