Abstract
Tumor-associated eosinophilia has been observed in numerous human cancers and several tumor models in animals, however, the details surrounding this eosinophilia remain largely undefined and anecdotal. We used a B16-F10 melanoma cell injection model to demonstrate that eosinophil infiltration of tumors occurred from the earliest palpable stages with significant accumulations only in the necrotic and capsule regions. Furthermore, the presence of diffuse extracellular matrix staining for eosinophil major basic protein was restricted to the necrotic areas of tumors indicating that eosinophil degranulation was limited to this region. Antibody-mediated depletion of CD4+ T cells and adoptive transfer of eosinophils suggested, respectively, that the accumulation of eosinophils is not associated with Th2-dependent immune responses and that recruitment is a dynamic ongoing process, occurring throughout tumor growth. Ex vivo migration studies have identified what appears to be a novel chemotactic factor(s) released by stressed/dying melanoma cells, suggesting that the accumulation of eosinophils in tumors occurs, in part, through a unique mechanism dependent on a signal(s) released from areas of necrosis. Collectively, these studies demonstrate that the infiltration of tumors by eosinophils is an early and persistent response that is spatial restricted. More importantly, these data also show that the mechanism(s) that elicits this host response occurs independent of immune surveillance, suggesting that eosinophils are part of an early inflammatory reaction at the site of tumorigenesis.
Keywords: Eosinophils, Tumor Immunology, Cancer, Mice, B16 Melanoma Cells
INTRODUCTION
Galen first noted the association between cancer and inflammation in his writings Opera Omnia over 2000 years ago [1]. During the succeeding millennia, the collective understanding of cancer-induced inflammatory responses evolved into a hypothesis first presented by Willis that the human body recognizes and mounts a defensive response against tumors [2]. A generation later, F.M. Burnet characterized these responses coining the term “immune surveillance” [3]. Since then, numerous studies have expanded the details of individual immune responses to tumors, including the recruitment of a variety of infiltrating lymphoid and myeloid cells. Moreover, many individual leukocyte subtypes have been investigated and in many cases the data suggest that they potentially participate in either promoting or retarding tumor progression (reviewed in [4–10]).
Despite an ever-increasing understanding of anti-tumor immune responses, several logistical problems have faced investigators studying the roles of leukocytes, preventing both a comprehensive understanding of the relevant immune responses and the development of immune-based strategies to combat malignancies. For example, many tumors evade immune surveillance or elicit only nominal immune responses [11–13]. Cancers also often suppress immune responses, quenching otherwise effective defense mechanisms [14–16]. In addition, leukocyte infiltrates often vary with tumor type and size, suggesting that immune responses are neither consistent nor static events [6, 17]. Investigations assessing these issues have led to the proposal that in addition to immune surveillance, host recognition of tumors also includes inflammatory responses [18]. Thus, in addition to specific immune-mediated responses, tumor sites are often centers of inflammatory reactions leading to the recruitment of proinflammatory leukocytes [5, 6, 9, 19, 20].
Eosinophils have been recognized in cellular infiltrates of tumors even in early histological studies of human cancers ([21–25]). Clinical observations have shown that the appearance of eosinophils in solid tumors is common and occurs in several tumor types, particularly those of epithelial origin (e.g., colon and breast tumors (reviewed in [21, 26])). In some studies, this infiltrate was suggested as a positive prognostic indicator of patient survival [27–31], however, the design of these studies casts doubts on this claim (e.g., the lack of statistical power) preventing a definitive link between tumor growth and the presence of eosinophils. Despite the prevailing belief that eosinophils participate in anti-tumor mechanisms, the role of these leukocytes in host defenses against tumors is at best equivocal. Tumor growth clearly occurs despite the presence of eosinophils, including tumors in animal model systems in which the malignant cells express eosinophil agonist factors (see for example [32]). The limited number of animal tumor models examined also fuels much of the controversy associated with eosinophils and tumor responses. For example, nearly all of the mouse studies examining eosinophils and eosinophil effector functions during tumorigenesis have used transfected cell lines modified to provoke defined immune responses in the recipient mice. In earlier studies IL-4 was expressed [33], and in a more recent attempt [34], melanoma cells were genetically modified to express a specific antigen (ovalbumin) to elicit Th2 inflammatory responses in tumor-bearing mice sensitized to this antigen. The contrived character of these transfected cell models limits their translation to human disease as it is unclear from any of these studies whether tumors are capable of recruiting eosinophils without the additional immune modulation of either the tumor cells or the recipient mice. Moreover, the kinetic and spatial details of this tumor associated eosinophilia in these models were often ignored because eosinophil-specific antibodies for histological detection were unavailable.
The current study defines the parameters surrounding the recruitment and accumulation of eosinophils in the classical, well defined B16 melanoma cell-derived tumor model. These studies utilized unmanipulated melanoma cells and wild type mice, demonstrating that eosinophil recruitment to tumors was an early host inflammatory response that occurred independent of Th2 immune responses. Interestingly, eosinophil accumulation occurred even in established tumors and, although the cause of this tumor associated eosinophilia remains unresolved, evidence is presented suggesting that the necrotic regions of tumors release a factor(s) that mediates eosinophil chemotaxis. Thus, the data presented demonstrate that eosinophil recruitment is spatially restricted to specific regions within tumors, occurs independent of immune surveillance mechanisms, and is likely an inflammatory response at the site of tumorigenesis promoting an early and persistent host recognition of solid tumors.
MATERIALS AND METHODS
Animals
Recipient mice in melanoma cell injection studies (i.e., C57BL/6J) were purchased from The Jackson Laboratory (Bar Harbor, ME). All procedures were conducted on female mice 8–16 weeks of age, maintained in ventilated microisolator cages housed within a specific pathogen-free animal facility surveyed by a mixed-bed sentinel mouse program. Protocols and studies involving animals were conducted in accordance with National Institutes of Health and Mayo Clinic Foundation institutional guidelines.
Generation of Solid Tumors
B16-F10 melanoma cells (ATCC, Manassas, VA) were cultured in DMEM medium, supplemented with 10% heat-inactivated fetal calf serum (FCS) and 1% Penicillin/Streptomycin, all purchased from Invitrogen, Carlsbad CA. Melanoma cells (5×105) were injected subcutaneously above the right shoulder area of syngeneic C57BL/6J female mice. The site of injection was monitored daily and the resulting solid tumors were allowed to grow either until they were palpable (day 10) or until the tumor weights averaged ~1 gram (day 16).
Histology and Immunohistochemical Detection of Eosinophils
Mice were sacrificed and tumors harvested for histological analysis, fixing the tissue overnight at 4°C in 10% formalin prior to embedding in paraffin. Serial 4μm sections throughout the harvested tumors were either stained with hematoxylin and eosin (H&E) or assessed for the presence of eosinophils by immunohistochemistry using a rabbit polyclonal anti-mouse eosinophil major basic protein antiserum [35]. Sections stained with biotin-conjugated rabbit IgG (Sigma-Aldrich, St. Louis, MO) were included as an isotype control as described earlier [35]. Immunohistochemical staining was performed with the Vector Laboratories® VIP-peroxidase detection kit (Vector Laboratories, Inc., Burlingame, CA) using a modified version of the protocol supplied by the manufacturer. Briefly, all slide manipulations were done at room temperature. Deparaffinized slides were hydrated in 1X PBS prior to the quenching of endogenous peroxidase activity in the tissue sections through a 20-minute incubation in 0.6% H2O2/80% CH3OH. Quenched slides were washed in 1X PBS, digested with Pepsin (10 minutes), washed again with 1X PBS, and finally blocked with 1.5% normal goat serum (30 minutes). The rabbit polyclonal anti-mouse MBP antisera was used as a 1:1000 dilution in 1.5% normal goat serum and incubated with blocked slides for 60 minutes. Following three 5 minute rinses with 1X PBS/0.4% Tween-20, the slides were incubated with a biotinylated goat anti-rabbit secondary antibody, and MBP-specific antibody binding was visualized as a purple precipitate using the detection protocol outlined in the manufacturer’s instructions. The MBP-stained sections were counterstained with 0.1% methyl green in preparation for photomicroscopy, using Zeiss Axiotoplan microscope (Carl Zeiss, Obrkochen, Germany). The density of MBP-positive cells (i.e., eosinophils) within different regions of the tumors was quantified (cells/mm2) using the image analysis software program ImagePro Plus (Media Cybernetics, Silver Spring MD).
Eosinophil Adoptive Transfer and Confocal Microscopy
Adoptive transfers were performed using blood eosinophils isolated from IL-5 transgenic mice (NJ.1638 mice [36]) backcrossed on to C57BL/6J (n > 20 generations). Briefly, heparinized blood collected from several donors by cardiac puncture was layered onto a Percoll E gradient [60% Percoll E (ρ=1.084), 1X HBSS, 15mM Hepes (pH 7.4)] and centrifuged (45 minutes, 3000 rpm, 4°C). The eosinophil enriched interface was recovered and washed twice in PBS containing 2% FCS. Eosinophils were isolated using a magnetic cell separation system (MACS, Miltenyi Biotech, Auburn, California) through the elimination of contaminating B cells and T cells by positive selection with antibody conjugated magnetic beads specific for CD45-R (B220) and CD90 (Thy 1.2), respectively. Cytospin preparations revealed that the recovered eosinophils were a nearly homogeneous population (>98.5%, contaminating cells included 1% neutrophils and 0.5% monocytes) that displayed >99% viability via trypan blue exclusion.
The fluorescent marker, carboxylfluorescein diacetate (CFDA), was used to label purified peripheral blood mouse eosinophils as per the manufacturer’s instructions (Molecular Probes, Eugene, OR). CFDA-tagged eosinophils, 2 × 107 per animal, were transferred via the peritoneal cavity to tumor-bearing mice 24 hours prior to tumor harvest (i.e., day 15 of the melanoma cell injection protocol). Frozen serial 4μm sections were processed for confocal immunofluorescence microscopy using a coverplate system and a rat anti-mouse eosinophil associated ribonuclease (Ear) monoclonal antibody (clone: 32.2.3 [37]). Briefly, at room temperature sections were washed twice with 1X PBS, blocked with 1% normal goat serum for 30 minutes, treated with 1% Chromotrope 2R (Aldrich, Milwaukee, WI) for 30 minutes, and then rinsed twice with 1X PBS/0.4% Tween-20. Individual slides were incubated with primary rat anti-mouse Ear antibody (diluted 1:1500) for 1 hour at room temperature. Following incubation, the slides were washed two times with PBS, and an Alexa-568 conjugated goat anti-rat IgG secondary antibody (diluted 1:500; Molecular Probes, Junction City, OR) was added and incubated for 30 min at room temperature. Stained slides were washed twice with 1X PBS/0.4% Tween-20 prior to cover slipping with Immu-mount (Themo Electron Corporation, Pittsburgh, PA). CFDA and anti-Ear staining were evaluated using a Zeiss Laser Scanning Confocal Microscope (LSM 510; Zeiss, Thornwood, NY). Negative control stained sections revealed only nominal non-specific fluorescent staining of lung tissues.
Antibody Mediated Depletion of CD4+T cells
Anti-CD4 monoclonal antibody (GK1.5) was used to deplete CD4+ cells using a modification of a previously described protocol [38]. Briefly, GK1.5 was administered (i.p.) to mice (0.5mg/100μl) a week prior to the subcutaneous injection of B16-F10 melanoma cells as well as on the day of melanoma cell injection. Tumor-bearing mice subsequently received additional administrations of GK1.5 antibody every 7 days until tumor harvest. Control groups of mice were administered nonspecific rat IgG. The ablation of CD4+ cells from mice was confirmed by flow cytometric analysis of splenocytes isolated from tumor-bearing mice. Spleen samples were disassociated into single cells by passage through a 40μm mesh and repeated resuspension using a small pore pipette. Red blood cells were removed from the collected splenocytes with ammonium chloride lysis buffer and the recovered white cells were washed in HBSS containing 2% FCS. Flurochrome conjugated αCD4 (FITC) and αCD8 (PE) monoclonal antibodies were used for staining (BD Biosciences, San Jose, CA). Analysis was performed on a FACScan flow cytometer (BD Biosciences) with CellQuest Pro software (BD Biosciences) gated to exclude fewer than 0.1% of the control cells in the relevant region for lymphocytes. Splenocytes derived from a tumor-bearing animal receiving nonspecific rat IgG were used to set the gates.
Ex Vivo Transwell Assays of Eosinophil Chemotaxis Assay
Transwell chemotaxis assays were performed using eosinophils isolated and purified (>98%) from the peripheral blood of IL-5 transgenic mice ([36]) as described above. Eosinophil chemotaxis was determined via a transwell assay as previously described [39]. The eosinophil chemotactic character of media from sub-confluent cultures of mouse embryonic stem and B16-F10 melanoma cell cultures were tested as well as media from post-confluent B16-F10 melanoma cell cultures at defined times based on the percentage of dead cells present (i.e., cells grown beyond confluence in unchanged, nutrient depleted media). Eotaxin-1 and -2 (Pepro Tech, Rocky Hill, NJ) were used at three concentration levels (3nM, 10nM, and 30nM) as positive controls for migration while culture media alone was used to determine the assay baseline (i.e., negative control). Data are expressed as a migration index (MI), which is the number of cells that migrated in response to a chemotactic factor relative to the number of cells that migrated in response to media alone. Values presented are means ± SEM of duplicate determinations conducted on three separate occasions.
Statistical analysis
Unless otherwise noted all data presented are mean values of the indicated groups (±SEM). Statistical analysis was performed on parametric data using Student’s t-test with differences between means considered significant when p < 0.05.
RESULTS
Subcutaneous injection of melanoma cells leads to solid tumors with characteristic regions of viable cells, areas of necrosis, and a surrounding acellular capsule/stromal layer
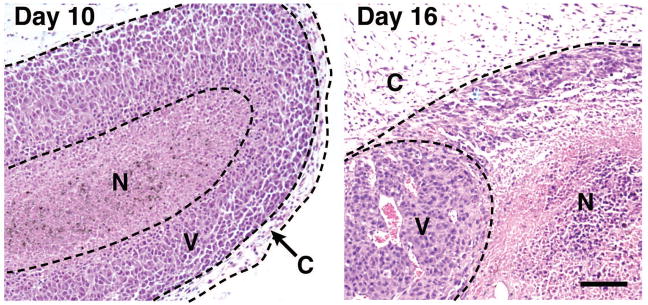
Subcutaneous injection of B16-F10 melanoma cells into syngeneic C57BL/6J mice (n = 10 animals/group) resulted in well-developed solid tumors as early as day 10 post-injection with an average weight of 0.106 ± 0.03g which increased dramatically by day 16, resulting in an average weight of 1.02 ± 0.17g. Histological examinations of these tumors (Figure 1) revealed progressive growth with distinct regions of necrosis within viable regions. The small size of 10-day tumors was associated with a disproportionate amount of viable tissue relative to areas of necrosis whereas the larger 16-day-old tumors displayed extensive areas of necrosis. All tumors, irrespective of size, were surrounded by a largely fibrous acellular region (i.e., capsule) separating the tumor from surrounding host tissue.
Figure 1. B16-F10 melanoma cell-derived solid tumors have a defined internal structure that occurs even in the smallest palpable growths.
Subcutaneous injection of 5 × 105 B16-F10 melanoma cells (day 0) resulted in the growth of solid tumors that were palpable by day 10 and >1 gram in mass by day 16. Day 10 and day 16 tumors each revealed a distinct yet similar internal morphology consisting of necrotic (N) and viable (V) areas surrounded by a fibrotic capsule region (C). Scale bar = 100μm.
Eosinophil recruitment is an early response to tumor growth, leading to the differential accumulation of eosinophils in the capsule and necrotic areas of solid tumors
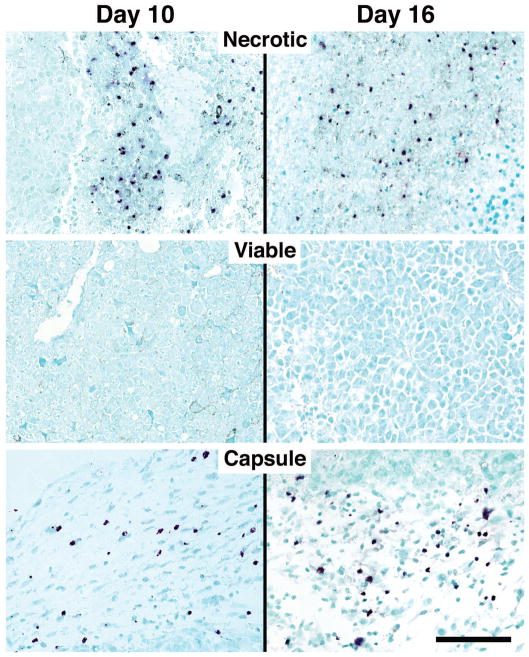
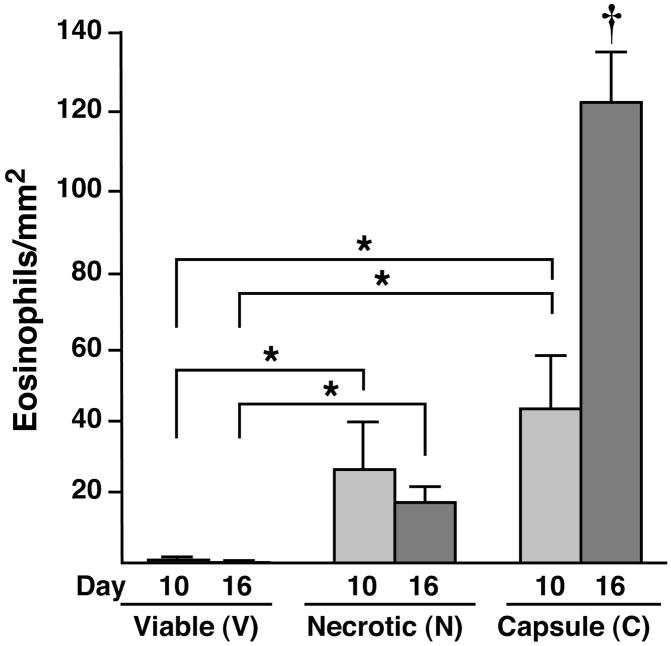
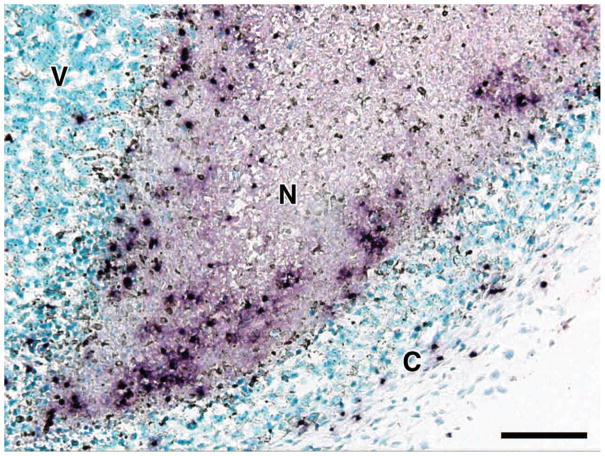
Serial sections of tumors were subjected to immunohistochemistry with an eosinophil-specific rabbit polyclonal anti-mouse MBP antisera to identify infiltrating eosinophils and to quantify the extent and localization of this infiltrate. Despite their size, even the smallest palpable tumors (i.e., day 10) displayed a robust eosinophil infiltrate, which differentially accumulated in the necrotic and capsule areas of the tumor with few, if any, eosinophils observed in the viable areas of the tumors examined (Figure 2). This pattern remained unchanged as the tumors grew in size and was observed in tumors at day 16. Interestingly, the tumor-associated eosinophilia in these animals occurred without any additional effects on eosinophilopoiesis such that the peripheral eosinophil counts of tumor-bearing mice were unchanged relative to tumor-free controls (i.e., 3.15%±0.64 vs. 3.59%±0.58, respectively). The density of eosinophils present within specific regions of the tumors (i.e., eosinophils/mm2) demonstrated that a significant eosinophilia occurred within both necrotic and capsule regions as compared to areas of viable tumor cells (Figure 3). Although no significant difference in eosinophil concentration in the necrotic versus capsule regions was observed at day 10, the density of eosinophils was three-fold greater in the capsules of 16-day tumors as compared to the necrotic regions. In addition to the identification of infiltrating eosinophils, the anti-MBP antisera revealed diffuse extracellular matrix staining within the necrotic areas of the day-10 and -16 tumors reflective of degranulation (Figure 4). It is also noteworthy that this eosinophil degranulation occurred in all tumors examined and was not observed in any other regions of the tumors.
Figure 2. Eosinophils are recruited to B16-F10 melanoma cell-derived tumors and localize within defined regions.
Immunohistochemistry using an eosinophil-specific polyclonal rabbit anti-mouse MBP antisera identified resident populations of infiltrating eosinophils (dark purple staining cells) in the necrotic and capsule, but not viable, regions of day 10 and day 16 tumors. Scale bar = 100μm.
Figure 3. Eosinophils differentially accumulate within the necrotic and capsule regions of B16-F10 melanoma cell-derived tumors.
Quantitative assessments of eosinophil density (i.e., eosinophils/mm2) were derived from serial sections of entire tumors. Eosinophils were counted within the necrotic, viable, and capsule regions from 4μm sections taken every 100μm through each tumor (n=10 mice/group) and expressed as a function of the region’s area. The data presented represent mean averages ± SEM. All evaluations were performed in duplicate as independent observer-blinded assessments. *P<0.05; †, P<0.05 relative to all other groups.
Figure 4. Eosinophil accumulation within the necrotic areas of tumors is accompanied by degranulation and the release of eosinophil secondary granule proteins.
Extracellular matrix deposition of MBP (i.e., degranulation) was detected in the necrotic regions of the B16-F10 melanoma cell-derived tumors by immunohistochemistry with an eosinophil-specific polyclonal rabbit anti-mouse MBP antisera (i.e., diffuse reddish-purple extracellular matrix staining). N, necrotic; V, viable; C, capsule. Scale bar = 50μm.
Recruitment of eosinophils is an active ongoing process occurring throughout tumor growth
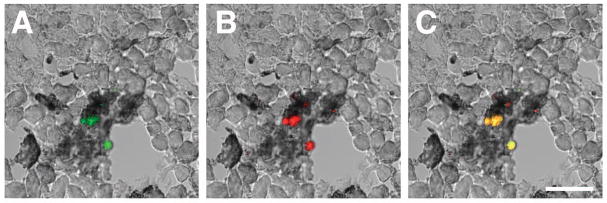
The increasing density of eosinophils in regions of day-16 compared to day-10 tumors suggested that eosinophils are continually recruited throughout tumor growth. This hypothesis was tested by adoptively transferring (i.p.) CFDA-labeled eosinophils (green) into tumor bearing mice to determine if recruitment was a dynamic event occurring even in established tumors (Figure 5). The identification of CFDA-labeled cells as eosinophils was achieved by co-staining the sections with the eosinophil-specific rat monoclonal anti-mouse Ear antisera (orange), overlapping the two images to display exogenous eosinophils as yellow. The confocal photomicrographs in Figure 5 demonstrate that transfer of eosinophils into tumor bearing mice 24 hours prior to tumor harvest (i.e., day 15 of the melanoma cell injection protocol) resulted in the accumulation of exogenously-derived leukocytes in the necrotic areas of the tumor.
Figure 5. Adoptive transfer of eosinophils into mice with established tumors demonstrates that eosinophil recruitment to necrotic areas is an active process.
CFDA-tagged peripheral blood eosinophils were transferred (i.p.) to tumor bearing mice 24 hours prior to recovery of 16 day old tumors. Labeled cells were identified in the necrotic areas of tumors assessing sections for (A) the presence of cells with the fluorescent CFDA tag (green) that also stained positive with (B) a rat monoclonal anti-mouse Ear antibody (orange). The co-localization of both the fluorescent tag and the antibody stain (i.e., adoptively transferred eosinophils) is shown as yellow in panel (C). Scale bar = 25μm.
The accumulation of eosinophils in solid tumors occurred independent of CD4+ T cell activities
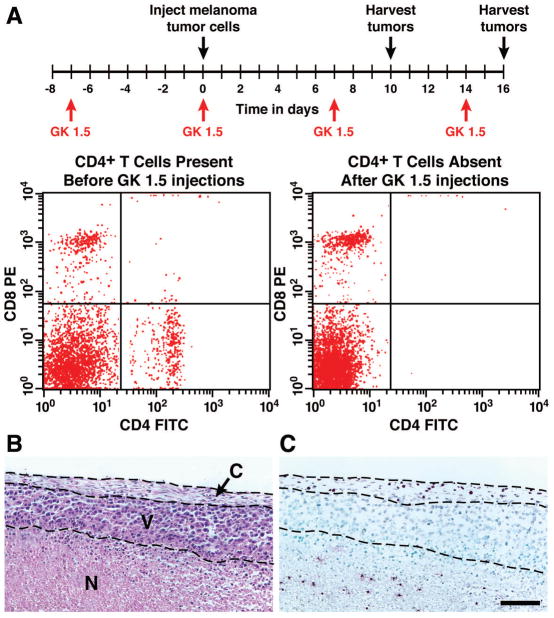
The presence of eosinophils within the B16-F10 melanoma cell-derived tumors raises the possibility that tissue accumulation of these leukocytes occurs as a consequence of Th2-mediated events orchestrated by CD4+ T cells. The dependence of tumor-associated eosinophil infiltration on CD4+ T cells was investigated by depletion of these T cells using an anti-CD4+ antibody (GK1.5 [38]). FACS assessment of splenocytes for the presence of CD4+ and CD8+ T cells revealed the complete ablation of CD4+ T cells following treatment with GK1.5 with no change in CD8+ cell numbers (Figure 6(A)). Eosinophils (i.e., MBP+ cells) in both the necrotic and capsule regions of tumors from GK1.5 treated mice were present in numbers equivalent to similar regions of tumors derived from control antibody-treated animals (Figure 6(B, C)), demonstrating that eosinophil infiltration of tumors occurred independent of CD4+T cells.
Figure 6. Eosinophil accumulation in solid tumors occurred independent of CD4+ T cells.
(A) Study protocol of antibody (GK1.5) depletion of CD4+ cells. Anti-CD4 mAb was administered (i.p.) 7 days prior to injection of B16-F10 melanoma cells. Additional administrations were given every 7 days throughout the protocol. FACS analyses of splenocytes on day 16, assessing for the presence of CD8 (y-axis) and CD4 (x-axis) cells, showed that CD4+ cells are uniquely absent. (B) A representative hematoxylin-eosin stained section showed the necrotic, viable, and capsule portions of a day 16 tumor from a mouse depleted of CD4+ cells and a serial section (C) revealed the presence of eosinophils by immunohistochemistry using a rabbit polyclonal anti-mouse eosinophil major basic protein antisera (dark purple staining cells). Despite the loss of CD4+ cells, eosinophils were present in both necrotic and capsule regions of these tumors, showing that this infiltration occurred independent of this T cell subtype. N, necrotic; V, viable; C, capsule. Scale bar = 100μm.
Conditioned media derived from post-confluent cultures of B16-F10 melanoma cells contains a factor(s) chemotactic for eosinophils
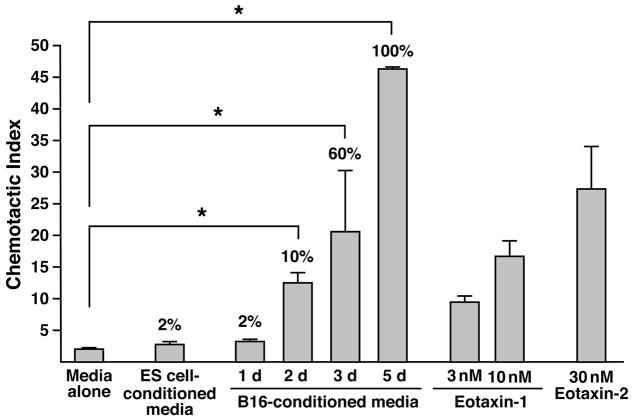
We performed ex vivo chemotaxis studies with isolated eosinophils and conditioned culture media from B16-F10 melanoma cells to determine if recruitment and accumulation of eosinophils within tumors were consequences of factors released by the melanoma cells themselves. Conditioned media (CM) derived from exponentially growing melanoma cells displayed no eosinophil chemotactic ability. In addition, CM from another actively dividing cell line, mouse embryonic stem cells, also failed to show any eosinophil chemotactic character. In contrast, CM from post-confluent B16-F10 cultures possessed eosinophil chemotactic activities in proportion to the level of cell death observed in the culture. Specifically, day 5 post-confluent cultures, which were composed of 100% dead cells, displayed a chemotactic activity that exceeded either eotaxin-1 or -2, both physiologically-relevant chemokines with eosinophil agonist activities (Figure 7). The release of this eosinophil chemotactic factor(s) was not limited to melanoma cells as post-confluent cultures from a variety of transformed (e.g., K1735 [40], Lewis lung carcinoma cells [41], and CMT-93 [42]) and non-transformed (e.g., primary embryonic fibroblasts) cell lines also displayed chemotactic abilities. In addition, although the identity of this eosinophil chemoattractant remains unknown, ELISA assays of the CM from post-confluent B16-F10 cultures failed to detect the presence of the prominent eosinophil chemotactic CCR3 ligands (i.e., eotaxin-1 or -2) and migration studies assessing small molecule mediators with the potential of eliciting eosinophil chemotaxis (e.g., adenosine [43], uric acid ([44]), and cyclophillins [45]) also failed to implicate these potential candidate pathways (data not shown).
Figure 7. Eosinophil migration in response to conditioned media is limited to stressed and/or dying B16-F10 melanoma cell cultures.
Eosinophil migration in response to conditioned media from dividing, as well as post-confluent cultures, was assessed using a transwell insert assay system. The eosinophil agonist chemokines, eotaxin-1 and eotaxin-2 were used at several concentrations as positive controls for migration; the data are presented as a chemotactic index normalized to the eosinophil chemotactic response to media alone; this baseline migration was consistently 1% of total input cells. Conditioned media from dividing cultures of either B16-F10 melanoma cells or mouse embryonic stem (ES) cells were unable to elicit eosinophil chemotaxis. In contrast, the conditioned media from cultures of B16-F10 melanoma cells (expressed as days (d) post-confluence) displayed significant eosinophil chemotactic activity that increased dramatically with both the time post-confluence and the concomitant decrease in culture viability. The values noted above each histogram indicate the percentage of cell death observed prior to recovery of conditioned culture media (as determined by trypan blue exclusion and/or loss of adherence). These data suggest that eosinophil accumulation in tumors is not a consequence of a factor(s) secreted by growing melanoma cells but instead, may result from the unique release of a factor(s) by stressed/dying cells within necrotic areas of tumors. * P<0.05.
DISCUSSION
The onset and growth of cancers often appear to be a consequence of a tumor’s ability to avoid recognition by the host immune system and/or elicit immunosuppression (reviewed in [46–48]). The lack of tumor-induced immune responses is clearly problematic for the host, limiting effective immune-based defense mechanisms with which to eliminate and/or attenuate tumor growth. However, in contrast to the lack of immune-mediated responses, tumor-mediated changes at the site of growth may lead to the recruitment and accumulation of proinflammatory leukocytes [17–19]. Thus, while tumors are not necessarily immunogenic, the sites of growth often elicit an inflammatory response that may represent an early hostrecognition mechanism of cancer.
The observations presented in this study suggest that the recruitment and accumulation of eosinophils to tumors is part of a site-specific early host recognition response. The eosinophil infiltration of B16-F10 melanoma cell-derived tumors occurred in all tumors examined without a concomitant induction of a marrow or peripheral blood eosinophilia beyond hemostatic baseline levels (i.e., without induced systemic immune responses). This eosinophil infiltration also occurred following the subcutaneous injection of two other tumorigenic lines (Lewis Lung and CMT-93, data not shown), suggesting that the eosinophil infiltrate is a ubiquitous host response to solid tumor growth. In addition, this robust resident eosinophilia occurred even in the earliest palpable tumors. Significantly, the eosinophil tumor accumulation occurred without any additional immunomodulating events as the injected melanoma cells were not manipulated to express either a unique antigen (e.g., ovalbumin [34]) or an eosinophil agonist cytokine/chemokine [25, 33, 49]. Moreover, the recipient wild type mice were not allergen sensitized/challenged to manipulate peripheral eosinophil numbers or their state of activation [34].
Several lines of evidence suggest that the B16-F10 melanoma cell-derived tumor itself and/or a host inflammatory response at the site of tumor growth is eliciting the accumulation of eosinophils. That is, the eosinophil infiltrate is not the result of acquired immunity or Th2-driven responses which are part of larger host tumor surveillance mechanisms: (i) The infiltration of tumors by eosinophils has been demonstrated in mice deficient of most acquired immune responses (e.g., in athymic nude mice [49]). (ii) B16 melanoma cells are syngeneic with the C57BL/6J recipient mice and do not elicit lymphocyte-mediated MHC-associated immune responses [50], (iii) Targeted depletion of all CD4+ cell types from recipient mice did not prevent the development of a tumor-associated eosinophilia, (iv) Tumor growth did not induce an increase in eosinophilopoiesis, leading to an increase in circulating eosinophil numbers. In addition, the accumulation of eosinophils wasn’t simply an initial inflammatory response to tumor injection/establishment. Eosinophil adoptive transfer showed that eosinophils accumulate even in established tumors, suggesting that their recruitment is an active site-specific event that occurs independently of T cell-mediated responses.
The observation that dead/dying, but not actively dividing, melanoma cells release one or more factors capable of mediating eosinophil chemotaxis suggests that the areas of necrosis, and not the actively dividing viable portions of the tumor, may be the source of the factor(s) that result in the recruitment and accumulation of eosinophils. A cursory examination of several small molecule mediators released by stressed/dying cells failed to elicit eosinophil chemotaxis, however, other potential candidates remain to be examined that may contribute this chemotactic response, including various arachidonic acid metabolites suggested to have eosinophil agonist activities (e.g., cysteinyl-leukotrienes [51], 5-oxo-eicosanoids [52], and lipid mediators such as PAF [53]). Moreover, the observation that multiple cell types (both transformed and non-transformed) also elicit this response suggests that this may be a more generalized mechanism mediated by a ubiquitous factor (e.g., HMGB-1 [54]) that has a broader importance for eosinophil trafficking beyond recruitment to tumors. In addition, the ability of necrotic regions to induce eosinophil recruitment suggests that eosinophils are not trafficking to tumors as a secondary consequence of factors released by previously recruited leukocytes (i.e., inflammatory cells recruited prior to eosinophils).
Presumably, eosinophil recruitment occurs by migration from outside of the tumor through the capsule and viable regions as these tumors display little evidence of vascularization that would permit movement of eosinophils directly to the necrotic regions from circulation. In this model, the steady-state levels of accumulating eosinophils in each region of the tumor occurred as a consequence of a specific trafficking mechanism: Necrotic regions: Eosinophils accumulate in the necrotic regions first and foremost because this is the destination of the vectorial movement of these cells. Although, the functionality of this accumulation remains unresolved, the ability of eosinophils to release copious amounts of vasoactive leukotrienes [55] and potentially promote localized angiogenesis [56, 57] suggest that this eosinophilia may represent a physiologic response to localized hypoxia [58]. This relationship between eosinophils and necrotic regions would also create a positive feedback-loop that may explain the increased eosinophil accumulation occurring as tumors become larger. That is, eosinophils recruited to necrotic regions of tumors may expand these areas of necrosis through destructive effector functions (e.g., release of toxic cationic proteins such as MBP) and increase the release of a chemotactic factor(s), which, in turn, leads to the recruitment of yet more eosinophils. Viable regions: The small steady-state levels of resident eosinophils in the viable regions of the tumors may simply reflect the rapid transit of eosinophils or alternatively the absence of a significant resident population reflects the lack of stabilizing signals prolonging eosinophil survival in these regions. Capsule regions: The presence of a robust resident population of eosinophils in the capsule regions likely reflects the partial trapping of eosinophils that are continually infiltrating from outside of the tumor as they attempt to move toward the necrotic regions (i.e., source of chemotactic factor(s)). Alternatively, as the growing tumor crowds and physically perturbs the surrounding host tissue, the induced stress on these normal cells may lead to the release of remodeling signals causing an initial influx of eosinophils to the tumor site prior to their subsequent response to the more localized chemotactic signals released by necrotic regions. This paradigm provides an explanation for our observation that eosinophil effector functions such as degranulation occur in the necrotic and not the capsule areas of tumors. The steady-state population of eosinophils in the capsule would not be expected to be necessarily activated or “functional” as these cells would be present only because of an inability to traverse this region efficiently or because of chemotactic signals released by the normal cells surrounding the growing tumor. In contrast, the necrotic regions of tumors are the sites toward which the eosinophils are moving because of a functional demand for eosinophil-mediated activities (i.e., the accumulation of eosinophils in this region is not a random event leading to the accumulation of “bystander” cells). Therefore, unlike other regions associated with the tumors, the necrotic areas promote eosinophil activation and the release of toxic cationic granule proteins (i.e., degranulation). Furthermore, eosinophil degranulation in the necrotic regions likely contributes to an overall loss of intact eosinophils from these regions, suggesting a mechanism leading to the lower steady-state levels observed in regions of necrosis relative to the capsule regions.
Regardless of the cause of accumulation or the mechanisms by which eosinophils traffic to tumors, a salient question remains: “What are the consequences of this eosinophil infiltration?” Specifically, are eosinophils destructive cytotoxic effector cells limiting tumor growth as part of a host surveillance mechanism or do the infiltrating eosinophils facilitate tumor growth by remodeling and immunoregulation of the tumor microenvironment? That is, do eosinophils promote the necrotic areas of tumors which, in turn, limits the rate of tumor growth or are eosinophils recruited to tumors as a consequence of induced host inflammatory/tissue remodeling responses. The absolute number of eosinophils in the necrotic areas of tumors, although significant, is relatively small (e.g., compared to macrophages [59] ) which may limit the cytotoxic (i.e., destructive) effects potentially mediated by these granulocytes. In contrast, eosinophils are capable of elaborating numerous cytokines and growth factors that have agonist activities on remodeling events and immune responses (reviewed in [60]) and have been linked to wound healing [61], each consistent with hypotheses linking the induced recruitment to the necrotic areas of tumors to larger tissue remodeling mechanisms.
The difficulties defining the role of these granulocytes in cancers occur because of both the nominal character of the eosinophil infiltrate and the lack of studies of sufficient statistical power demonstrating a link between eosinophils and modulations of tumor growth (see for example [22, 28, 31, 62]). Irrespective of these difficulties, tumors arise and grow despite the presence of an eosinophil infiltrate and correlations with tumor growth have tended not to be linear (e.g., [63] vs. [34] vs. [25]). Moreover, exceptions to the rule exist with apparent dissociations between the presence of eosinophils (and/or the lack thereof) and rates of tumor onset/growth (e.g., [64]). In addition, all of the mouse studies attempting to causatively link the presence of eosinophils and modulations of tumor growth utilized either genetically engineered the tumor cells [33, 49, 64, 65] and/or immunized recipient mice to recognize the tumor cells [34] thus promoting the tumor as a target of Th2 inflammatory responses (i.e., an induced immune response vs. an elicited inflammatory response). Unfortunately, the narrow character of the models used as well as potential of pleiotropic effects mediated by the induction of contrived immune responses limits the usefulness of these approaches. However, the demonstration here that eosinophil infiltration is spatially restricted even in the smallest tumors and occurs independent of acquired immune responses suggests that this recruitment is part of an early host recognition of unique regional heterogeneities at the sites of tumorigenesis. More importantly, the understanding of the circumstances surrounding this tumor associated eosinophil infiltrate provides a unique opportunity to define relevant effector functions that may represent novel therapeutic options to modulation tumor onset/growth.
Acknowledgments
The work presented was supported by the Mayo Foundation and a grant from the NCI to JJL (CA112442-1S). Additional support was provided by NIH training grants to SAC (AR08545 and AI07047-23) and SIO (HL07897-07). We wish to thank Dr. Pinku Mukherjee for her assistance with the ex vivo chemotaxis studies as well as the invaluable contributions of numerous individuals not listed as authors. We also wish to thank the tireless efforts of the Mayo Clinic Arizona Core facilities (Laboratory Animal Resources Core (LARC): Dr. Ron Marler; Histology: Lisa Barbarisi; Immunology: Tammy Brehm-Gibson; Medical Graphic Arts: Marv Ruona; Research Library Services: Joseph Esposito). Mr. Tim Jensen and Dr. Helene Rosenberg provided insightful comments and critical review of early versions of this manuscript. In addition, we wish to express our gratitude to the Lee Laboratories administrative staff (Linda Mardel, Margaret (Peg) McGarry, and Jennifer Ford) without whom we could not function as an integrated group.
References
- 1.Galen . Opera omnia. In: Kühn KG, Assmann FW, editors. Medicorum Graecorum opera quae exstant. 1–20. C. Cnobloch; Lipsiae: 1821. p. 20. in 22. [Google Scholar]
- 2.Willis RA. The spread of tumours in the human body. Butterworth; 1952. [Google Scholar]
- 3.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 4.Oldham RK. Natural killer cells: artifact to reality: an odyssey in biology. Cancer Metastasis Rev. 1983;2:323–36. doi: 10.1007/BF00048565. [DOI] [PubMed] [Google Scholar]
- 5.Khavari P. Cytotoxic cellular mediators of the immune response to neoplasia: a review. YaleJ Biol Med. 1987;60:409–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Verbik DJ. Immune cells and cytokines: their role in cancer immunotherapy. Review. International Journal of Oncology. 1995:205. doi: 10.3892/ijo.7.2.205. [DOI] [PubMed] [Google Scholar]
- 7.Herberman R. Role of Natural Killer cells and T cells in immune surveillance. Leuk Res. 2000;24:775. doi: 10.1016/s0145-2126(00)00058-8. [DOI] [PubMed] [Google Scholar]
- 8.Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol Med Today. 2000;6:324–9. doi: 10.1016/s1357-4310(00)01756-1. [DOI] [PubMed] [Google Scholar]
- 9.Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001;11:97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- 10.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochsenbein AF. Immunological ignorance of solid tumors. Springer Semin Immunopathol. 2005;27:19–35. doi: 10.1007/s00281-004-0192-0. [DOI] [PubMed] [Google Scholar]
- 12.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–39. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53:844–54. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boles KS, Barchet W, Diacovo T, Cella M, Colonna M. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood. 2005;106:779–86. doi: 10.1182/blood-2005-02-0817. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 17.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 18.Lozupone F, Luciani F, Venditti M, Rivoltini L, Pupa S, Parmiani G, Belardelli F, Fais S. Murine granulocytes control human tumor growth in SCID mice. Int J Cancer. 2000;87:569–73. doi: 10.1002/1097-0215(20000815)87:4<569::aid-ijc17>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Wislez M, Philippe C, Antoine M, Rabbe N, Moreau J, Bellocq A, Mayaud C, Milleron B, Soler P, Cadranel J. Upregulation of bronchioloalveolar carcinoma-derived C-X-C chemokines by tumor infiltrating inflammatory cells. Inflamm Res. 2004;53:4–12. doi: 10.1007/s00011-003-1215-3. [DOI] [PubMed] [Google Scholar]
- 20.Munitz A, Levi-Schaffer F. Eosinophils: ‘new’ roles for ‘old’ cells. Allergy. 2004;59:268–75. doi: 10.1111/j.1398-9995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. [Review] Journal of Clinical Pathology. 1981;34:1343–8. doi: 10.1136/jcp.34.12.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986;58:1321–7. doi: 10.1002/1097-0142(19860915)58:6<1321::aid-cncr2820580623>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Samoszuk MK, Espinoza FP. Deposition of autofluorescent eosinophil granules in pathologic bone marrow biopsies. Blood. 1987;70:597–9. [PubMed] [Google Scholar]
- 24.Ohashi Y, Ishibashi S, Suzuki T, Shineha R, Moriya T, Satomi S, Sasano H. Significance of tumor associated tissue eosinophilia and other inflammatory cell infiltrate in early esophageal squamous cell carcinoma. Anticancer Res. 2000;20:3025–30. [PubMed] [Google Scholar]
- 25.Samoszuk M, Deng T, Hamamura MJ, Su MY, Asbrock N, Nalcioglu O. Increased blood clotting, microvascular density, and inflammation in eotaxin-secreting tumors implanted into mice. Am J Pathol. 2004;165:449–56. doi: 10.1016/S0002-9440(10)63310-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samoszuk M. Eosinophils and human cancer. Histol Histopathol. 1997;12:807–12. [PubMed] [Google Scholar]
- 27.Ownby HE, Roi LD, Isenberg RR, Brennan MJ. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer. 1983;52:126–30. doi: 10.1002/1097-0142(19830701)52:1<126::aid-cncr2820520123>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith MM, Belchis DA, Cresson DH, Merritt WD, 3rd, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg. 1992;106:27–33. doi: 10.1177/019459989210600124. [DOI] [PubMed] [Google Scholar]
- 29.Dalal BI, Das KC, Dutta TK, Malakar K. Local and systemic eosinophilia in patients with carcinoma of the uterine cervix undergoing radiation therapy: correlation with radiation response. Clin Oncol (R Coll Radiol) 1992;4:18–21. doi: 10.1016/s0936-6555(05)80766-6. [DOI] [PubMed] [Google Scholar]
- 30.Bethwaite PB, Holloway LJ, Yeong ML, Thornton A. Effect of tumour associated tissue eosinophilia on survival of women with stage IB carcinoma of the uterine cervix. Journal of Clinical Pathology. 1993;46:1016–20. doi: 10.1136/jcp.46.11.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487–95. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Kruger-Krasagakes S, Li W, Richter G, Diamantstein T, Blankenstein T. Eosinophils infiltrating interleukin-5 gene-transfected tumors do not suppress tumor growth. Eur J Immunol. 1993;23:992–5. doi: 10.1002/eji.1830230438. [DOI] [PubMed] [Google Scholar]
- 33.Tepper RI, Pattengale PK, Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989;57:503–12. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- 34.Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, Parish C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: An eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, Leikauf GD, Lee NA. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. Journal of Experimental Medicine. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–1344. [PubMed] [Google Scholar]
- 37.Cormier SA, Yuan S, Crosby JR, Protheroe CA, Dimina DM, Hines EM, Lee NA, Lee JJ. T(H)2-mediated pulmonary inflammation leads to the differential expression of ribonuclease genes by alveolar macrophages. Am J Respir Cell Mol Biol. 2002;27:678–87. doi: 10.1165/rcmb.4882. [DOI] [PubMed] [Google Scholar]
- 38.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–93. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 39.Borchers MT, Ansay TL, DeSalle R, Daugherty BL, Shen HH, Metzger M, Lee NA, Lee JJ. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. Journal of Leukocyte Biology. 2002;71:1033–1041. [PubMed] [Google Scholar]
- 40.Schackert G, Fidler IJ. Development of in vivo models for studies of brain metastasis. Int J Cancer. 1988;41:589–94. doi: 10.1002/ijc.2910410419. [DOI] [PubMed] [Google Scholar]
- 41.Bertram JS, Janik P. Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture. Cancer Lett. 1980;11:63–73. doi: 10.1016/0304-3835(80)90130-5. [DOI] [PubMed] [Google Scholar]
- 42.Franks LM, Hemmings VJ. A cell line from an induced carcinoma of mouse rectum. J Pathol. 1978;124:35–8. doi: 10.1002/path.1711240108. [DOI] [PubMed] [Google Scholar]
- 43.Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, Chan TS, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol. 2004;173:1380–9. doi: 10.4049/jimmunol.173.2.1380. [DOI] [PubMed] [Google Scholar]
- 44.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 45.Stenfeldt AL, Wenneras C. Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunology. 2004;112:605–614. doi: 10.1111/j.1365-2567.2004.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kowalczyk DW. Tumors and the danger model. Acta Biochim Pol. 2002;49:295–302. [PubMed] [Google Scholar]
- 47.Parish CR. Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol. 2003;81:106–13. doi: 10.1046/j.0818-9641.2003.01151.x. [DOI] [PubMed] [Google Scholar]
- 48.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–51. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 50.Fidler IJ, Gersten DM, Budmen MB. Characterization in vivo and in vitro of tumor cells selected for resistance to syngeneic lymphocyte-mediated cytotoxicity. Cancer Res. 1976;36:3160–5. [PubMed] [Google Scholar]
- 51.Saito H, Morikawa H, Howie K, Crawford L, Baatjes AJ, Denburg E, Cyr MM, Denburg JA. Effects of a cysteinyl leukotriene receptor antagonist on eosinophil recruitment in experimental allergic rhinitis. Immunology. 2004;113:246–252. doi: 10.1111/j.1365-2567.2004.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powell WS, Chung D, Gravel S. 5-oxo-6,8,11,14-eicosatetraenoic acid is a potentstimulator of human eosinophil migration. J Immunol. 1995;154:4123–4132. [PubMed] [Google Scholar]
- 53.Teixeira MM, Giembycz MA, Lindsay MA, Hellewell PG. Pertussis toxin shows distinct early signalling events in platelet- activating factor-, leukotriene B4-, and C5a-induced eosinophil homotypic aggregation in vitro and recruitment in vivo. Blood. 1997;89:4566–73. [PubMed] [Google Scholar]
- 54.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–9. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henderson WR, Harley JB, Fauci AS. Arachidonic acid metabolism in normal and hypereosinophilic syndrome human eosinophils: generation of leukotrienes B4, C4, D4 and 15-lipoxygenase products. Immunology. 1984;51:679–86. [PMC free article] [PubMed] [Google Scholar]
- 56.Temkin V, Aingorn H, Puxeddu I, Goldshmidt O, Zcharia E, Gleich GJ, Vlodavsky I, Levi-Schaffer F. Eosinophil major basic protein: first identified natural heparanase-inhibiting protein. J Allergy Clin Immunol. 2004;113:703–9. doi: 10.1016/j.jaci.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 57.Samoszuk M, Lin F, Rim P, Strathearn G. New marker for blood vessels in human ovarian and endometrial cancers. Clin Cancer Res. 1996;2:1867–71. [PubMed] [Google Scholar]
- 58.Hudson G, Smith NC, Wilson RS, Yoffey JM. Eosinophil granulocytes and hypoxia. Nature. 1967;213:818–9. doi: 10.1038/213818a0. [DOI] [PubMed] [Google Scholar]
- 59.Samoszuk M. Eosinophils and human cancer. Histol Histopathol. 1997;12:807–12. [PubMed] [Google Scholar]
- 60.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clinical and Experimental Allergy. 2005;35:986–94. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 61.Yang J, Torio A, Donoff RB, Gallagher GT, Egan R, Weller PF, Wong DT. Depletion of eosinophil infiltration by anti-IL-5 monoclonal antibody (TRFK-5) accelerates open skin wound epithelial closure. Am J Pathol. 1997;151:813–9. [PMC free article] [PubMed] [Google Scholar]
- 62.Pretlow TP, Keith EF, Cryar AK, Bartolucci AA, Pitts AM, Pretlow TG, 2nd, Kimball PM, Boohaker EA. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Research. 1983;43:2997–3000. [PubMed] [Google Scholar]
- 63.Wong DT, Bowen SM, Elovic A, Gallagher GT, Weller PF. Eosinophil ablation and tumor development. Oral Oncol. 1999;35:496–501. doi: 10.1016/s1368-8375(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 64.Noffz G, Qin Z, Kopf M, Blankenstein T. Neutrophils but not eosinophils are involved in growth suppression of IL-4-secreting tumors. J Immunol. 1998;160:345–50. [PubMed] [Google Scholar]
- 65.Tepper RI, Mule JJ. Experimental and clinical studies of cytokine gene-modified tumor cells. [Review] Human Gene Therapy. 1994;5:153–64. doi: 10.1089/hum.1994.5.2-153. [DOI] [PubMed] [Google Scholar]