Abstract
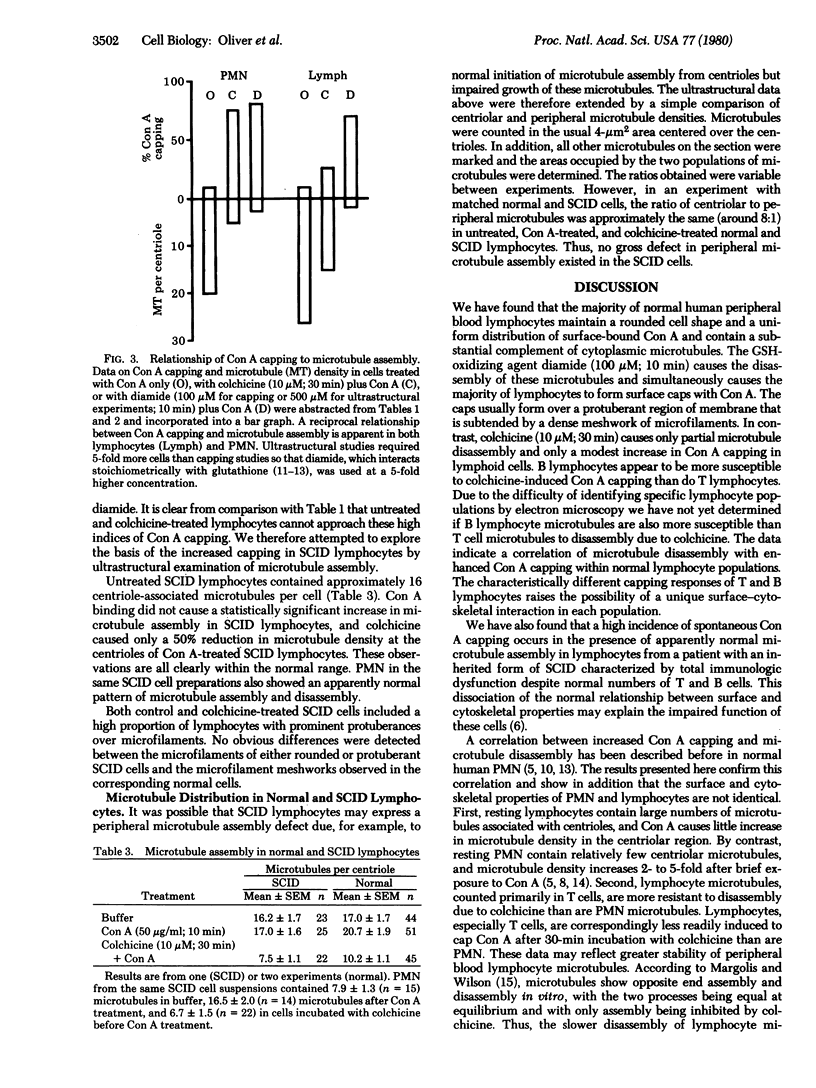
We have analyzed the assembly of microtubules and the distribution of concanavalin A(Con A)-receptor complexes in the same populations of human peripheral blood T and B lymphocytes. We hoped to resolve the prolonged controversy over the relationship of microtubules to Con A cap formation in lymphocytes and to explain the abnormally high spontaneous and colchicine-induced Con A capping that was observed recently in lymphocytes from a patient with an inherited form of severe combined immunodeficiency disease (SCID) characterized by total immunologic dysfunction despite normal numbers and distribution of T and B cells. The data establish that (i) microtubule disassembly is correlated with enhanced Con A cap formation on normal human lymphocytes; (ii) T and B cells differ significantly from each other and from circulating polymorphonuclear leukocytes with respect to their capping responses after exposure to colchicine; and (iii) there is an abnormal relationship of microtubule assembly to surface topography in the functionally defective SCID cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini D. F., Berlin R. D., Oliver J. M. The mechanism of concanavalin A cap formation in leukocytes. J Cell Sci. 1977 Aug;26:57–75. doi: 10.1242/jcs.26.1.57. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Analogous ultrastructure and surface properties during capping and phagocytosis in leukocytes. J Cell Biol. 1978 Jun;77(3):789–804. doi: 10.1083/jcb.77.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M., Walter R. J. Surface functions during Mitosis I: phagocytosis, pinocytosis and mobility of surface-bound Con A. Cell. 1978 Oct;15(2):327–341. doi: 10.1016/0092-8674(78)90002-8. [DOI] [PubMed] [Google Scholar]
- De Brabander M. J., Van de Veire R. M., Aerts F. E., Borgers M., Janssen P. A. The effects of methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl) carbamate, (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res. 1976 Mar;36(3):905–916. [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand E. W., Oliver J. M., Schuurman R. K., Matheson D. S., Dosch H. M. Abnormal lymphocyte capping in a patient with severe combined immunodeficiency disease. N Engl J Med. 1979 Dec 6;301(23):1245–1249. doi: 10.1056/NEJM197912063012301. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Soberman R., Goldstein I., Weissmann G. Concanavalin A induces microtubule assembly and specific granule discharge in human polymorphonuclear leukocytes. J Cell Biol. 1976 Mar;68(3):781–787. doi: 10.1083/jcb.68.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Mellon M. G., Rebhun L. I. Sulfhydryls and the in vitro polymerization of tubulin. J Cell Biol. 1976 Jul;70(1):226–238. doi: 10.1083/jcb.70.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath J., Rebhun J. I. Effects of caffeine and other methylxanthines on the development and metabolism of sea urchin eggs. Involvement of NADP and glutathione. J Cell Biol. 1976 Mar;68(3):440–450. doi: 10.1083/jcb.68.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Albertini D. F., Berlin R. D. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976 Dec;71(3):921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Berlin R. D. Microtubules, microfilaments and the regulation of membrane functions. Symp Soc Exp Biol. 1979;33:277–298. [PubMed] [Google Scholar]
- Oliver J. M. Concanavalin A cap formation on human polymorphonuclear leukocytes induced by R17934, a new antitumor drug that interferes with microtubule assembly. J Reticuloendothel Soc. 1976 Jun;19(6):389–395. [PubMed] [Google Scholar]
- Oliver J. M., Spielberg S. P., Pearson C. B., Schulman J. D. Microtubule assembly and function in normal and glutathione synthetase-deficient polymorphonuclear leukocytes. J Immunol. 1978 Apr;120(4):1181–1186. [PubMed] [Google Scholar]
- Rudd C. E., Rogers K. A., Brown D. L., Kaplan J. G. Microtubules, colchicine, and lymphocyte blastogenesis. Can J Biochem. 1979 Jun;57(6):673–683. doi: 10.1139/o79-085. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]