Abstract
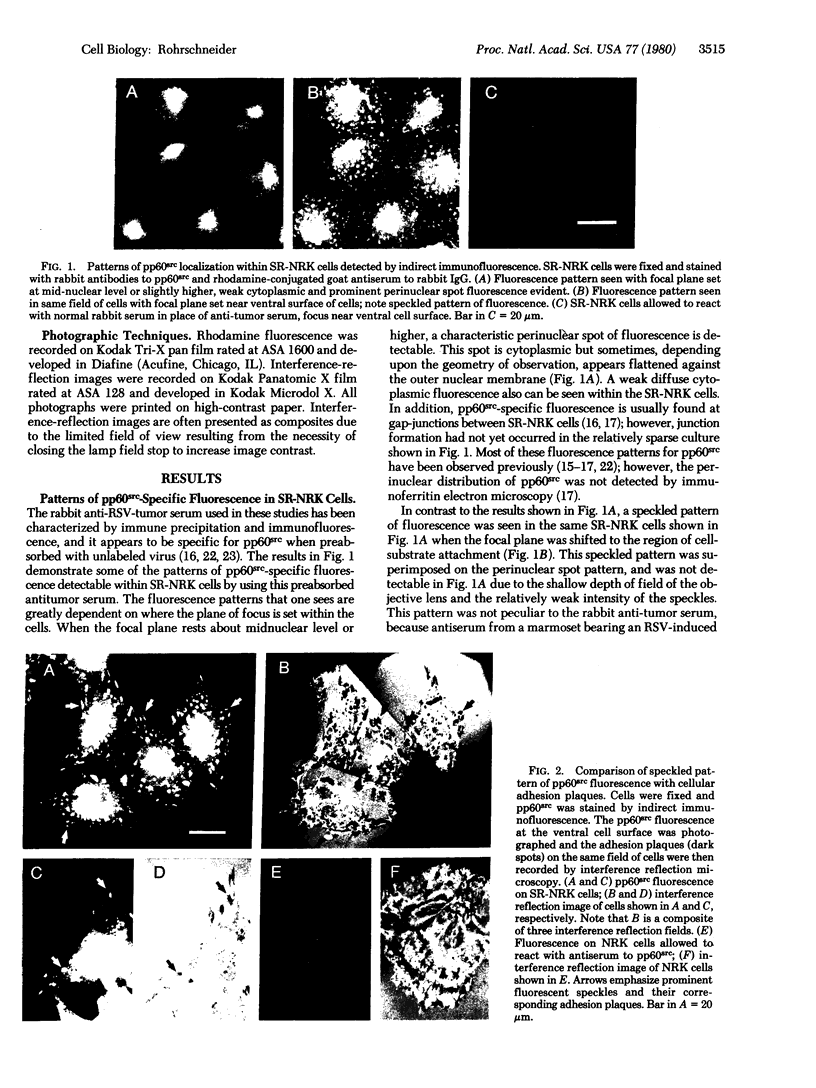
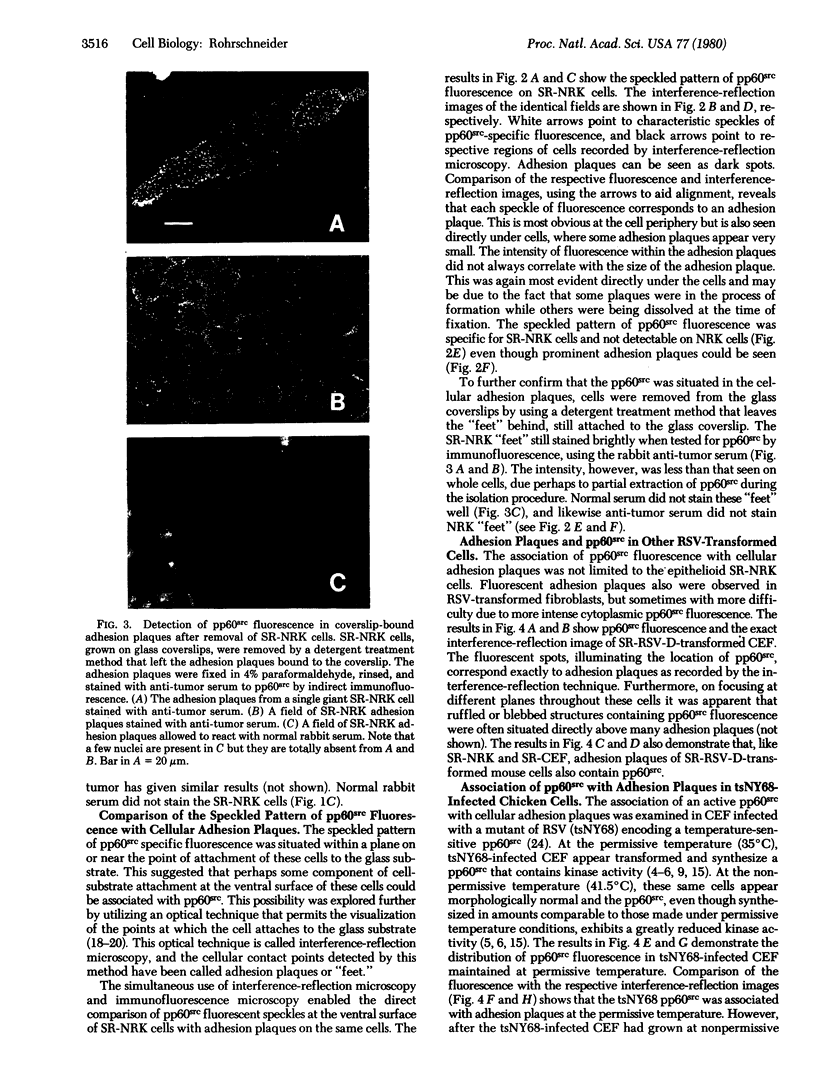
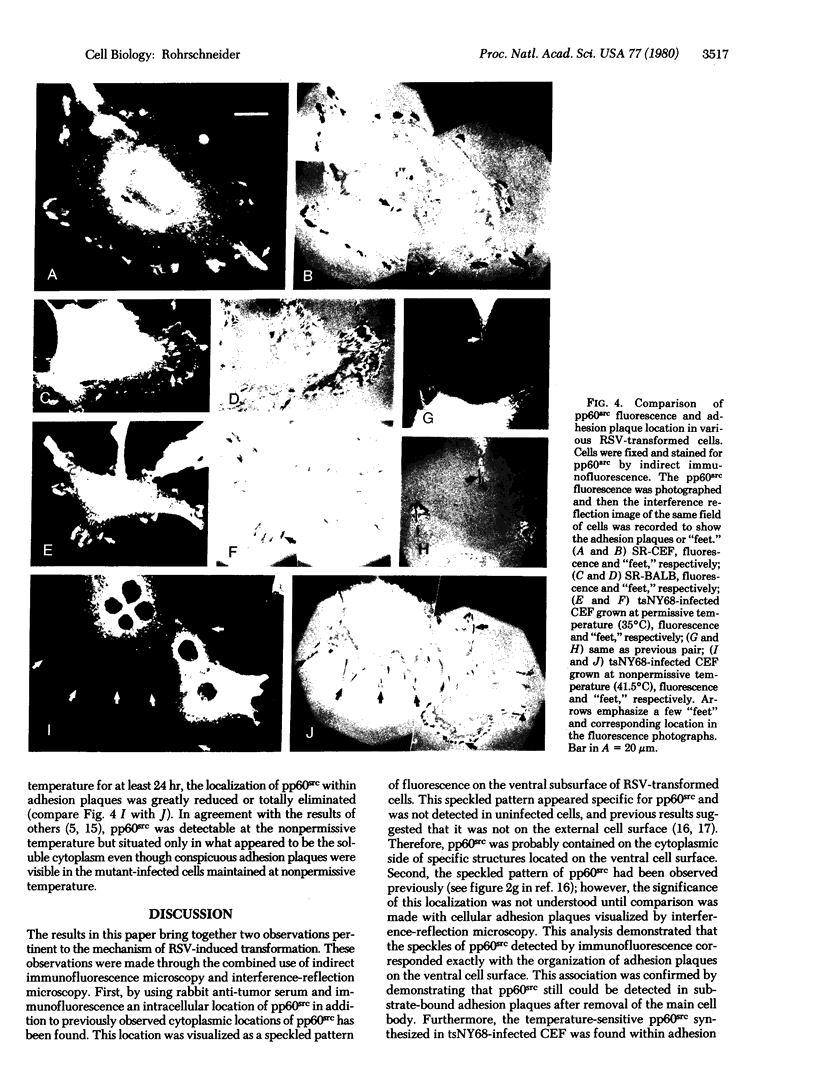
Another intracellular location of the Rous sarcoma virus (RSU) src gene product (pp60src) has been detected within RSV-transformed cells by indirect immunofluorescence. By using rabbit anti-tumor serum specific for pp60src, a speckled pattern of fluorescence was found on the ventral surface of RSV (Schmidt-Ruppin strain)-transformed normal rat kidney cells. Several tests indicated that this pattern was specific for pp60src. In addition, interference-reflection microscopy was used to visualize cellular adhesion plaques, which are the points at which cells attach to the substratum. Simultaneous immunofluorescence and interference-reflection microscopy indicated that the speckles of pp60src fluorescence corresponded exactly to the adhesion plaque structures. The presence of pp60src within the adhsion plaques was further demonstrated by indirect immunofluorescences on isolated adhesion plaques that remained bound to glass after removal of the cells. pp60src also was observed in adhesion plaques of RSV-tranformed chicken embryo fibroblasts (CEF) and mouse fibroblasts, as well as CEF infected with the temperature-sensitive RSV mutant tsNY68 and grown at permissive temperature. At nonpermissive temperature, pp60src was not detectable in adhesion plaques of the tsNY68-infected CEF. Adhesion plaques serve as focal points of microfilament bundle attachment, and thse results suggest that pp60src interacts directly with cellular cytoskeletal components.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Dunn G. A. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp Cell Res. 1975 Apr;92(1):57–62. doi: 10.1016/0014-4827(75)90636-9. [DOI] [PubMed] [Google Scholar]
- Ash J. F., Vogt P. K., Singer S. J. Reversion from transformed to normal phenotype by inhibition of protein synthesis in rat kidney cells infected with a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3603–3607. doi: 10.1073/pnas.73.10.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Steinbaugh P. J., Erikson R. L. Characterization of the avian sarcoma virus protein p60src. Virology. 1978 Nov;91(1):130–140. doi: 10.1016/0042-6822(78)90361-6. [DOI] [PubMed] [Google Scholar]
- CURTIS A. S. THE MECHANISM OF ADHESION OF CELLS TO GLASS. A STUDY BY INTERFERENCE REFLECTION MICROSCOPY. J Cell Biol. 1964 Feb;20:199–215. doi: 10.1083/jcb.20.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Collett M. S., Erikson R. L. In vitro synthesis of a functional avian sarcoma virus transforming-gene product. Nature. 1978 Aug 31;274(5674):919–921. doi: 10.1038/274919a0. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Collett M. S., Erikson E., Purchio A. F. Evidence that the avian sarcoma virus transforming gene product is a cyclic AMP-independent protein kinase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6260–6264. doi: 10.1073/pnas.76.12.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Heath J. P., Dunn G. A. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J Cell Sci. 1978 Feb;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- Henderson D., Weber K. Three-dimensional organization of microfilaments and microtubules in the cytoskeleton. Immunoperoxidase labelling and stereo-electron microscopy of detergent-extracted cells. Exp Cell Res. 1979 Dec;124(2):301–316. doi: 10.1016/0014-4827(79)90206-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. Relationships between fibronectin (LETS protein) and actin. Cell. 1978 Nov;15(3):875–886. doi: 10.1016/0092-8674(78)90272-6. [DOI] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976 Jun;21(1):129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Engeser H., Greenberg M. E., O'Farrell M., Gall W. E., Edelman G. M. Characterization of the protein kinase activity of avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5028–5032. doi: 10.1073/pnas.76.10.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain D. A., Maness P. F., Edelman G. M. Assay for early cytoplasmic effects of the src gene product of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2750–2754. doi: 10.1073/pnas.75.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Wolken K. Electronmicroscope investigations of the underside of cells in culture. Exp Cell Res. 1973 Mar 30;78(1):1–14. doi: 10.1016/0014-4827(73)90031-1. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R., Eisenman R. N., Leitch C. R. Identification of a Rous sarcoma virus transformation-related protein in normal avian and mammalian cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4479–4483. doi: 10.1073/pnas.76.9.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R. Immunofluorescence on avian sarcoma virus-transformed cells: localization of the src gene product. Cell. 1979 Jan;16(1):11–24. doi: 10.1016/0092-8674(79)90183-1. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Product of in vitro translation of the Rous sarcoma virus src gene has protein kinase activity. J Virol. 1979 Apr;30(1):311–318. doi: 10.1128/jvi.30.1.311-318.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H. The gene order of avian RNA tumor viruses derived from biochemical analyses of deletion mutants and viral recombinants. Annu Rev Microbiol. 1978;32:561–592. doi: 10.1146/annurev.mi.32.100178.003021. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Hale A. H., Losasso L. Decreased adherence to the substrate in Rous sarcoma virus-transformed chicken embryo fibroblasts. Cell. 1977 Jan;10(1):45–51. doi: 10.1016/0092-8674(77)90138-6. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada K. M., Yamada S. S., Pouysségur J., Pastan I. Microfilament bundles and cell shape are related to adhesiveness to substratum and are dissociable from growth control in cultured fibroblasts. Cell. 1977 Mar;10(3):375–380. doi: 10.1016/0092-8674(77)90024-1. [DOI] [PubMed] [Google Scholar]