Abstract
The terahertz region lies between the microwave and infrared regions of the electromagnetic spectrum such that it is strongly attenuated by water and very sensitive to water content. Terahertz radiation has very low photon energy and thus it does not pose any ionization hazard for biological tissues. Because of these characteristic properties, there has been an increasing interest in terahertz imaging and spectroscopy for biological applications within the last few years and more and more terahertz spectra are being reported, including spectroscopic studies of cancer. The presence of cancer often causes increased blood supply to affected tissues and a local increase in tissue water content may be observed: this acts as a natural contrast mechanism for terahertz imaging of cancer. Furthermore the structural changes that occur in affected tissues have also been shown to contribute to terahertz image contrast. This paper introduces terahertz technology and provides a short review of recent advances in terahertz imaging and spectroscopy techniques. In particular investigations relating to the potential of terahertz imaging and spectroscopy for cancer diagnosis will be highlighted.
Keywords: Terahertz, imaging, spectroscopy, cancer
Introduction to terahertz radiation
Terahertz (THz, 1THz=1012 Hz) radiation, also termed as THz waves, THz light, or T-rays, is situated in the frequency regime between optical and electronic techniques. This regime is typically defined as 0.1-10 THz and has become a new area for research in physics, chemistry, biology, materials science and medicine. Applications investigated hitherto range from using THz spectroscopic features to identify explosives (1), to checking for defects in airplanes (2) and to imaging breast cancer (3).
Cancer is the second most common cause of death in the US, exceeded only by heart disease. In the US, cancer accounts for nearly 1 of every 4 deaths (4). For many cancer types, early diagnosis could reduce mortality. Current diagnosis techniques include imaging such as X-ray imaging and MRI, and biopsy. Microscopic imaging methods are used in histopathology of biopsy samples to provide structural and functional information whereas X-ray imaging and MRI provide images of living tissues at the macroscopic level, but at much lower resolution and specificity. High-resolution techniques for in vivo subsurface imaging of tissue that would provide the best information for the early detection of disease are not available. One of the biggest unsolved problems in medical imaging is how to combine macroscopic and microscopic imaging advances so that the precise margins of cancers can be delineated (5). Many optical techniques are being investigated for this purpose and here we introduce THz imaging and spectroscopy to discuss about its potential to fill this niche.
The following unique features make THz very suitable for medical applications: (I) THz radiation has very low photon energy, which is insufficient to cause chemical damage to molecules, or knock particles out of atoms. Thus it will not cause harmful ionization in biological tissues; this makes it very attractive for medical applications. (II) THz radiation is very sensitive to polar substances, such as water and hydration state. For this reason, THz waves can provide a better contrast for soft tissues than x-rays. (III) THz-TDS techniques use coherent detection to record the THz wave's temporal electric fields, which means both the amplitude and phase of the THz wave can be obtained simultaneously. The temporal waveforms can be further Fourier transformed to give the spectra. This allows precise measurements of the refractive index and absorption coefficient of samples without resorting to the Kramers-Kronig relations. (IV) The energy of rotational and vibrational transitions of molecules lies in the THz region and intermolecular vibrations such as hydrogen bonds exhibit different spectral characteristics in the THz range. These unique spectral features can be used to distinguish between different materials or even isomers.
In this paper we focus on the recent THz research relating to cancer detection and diagnosis namely for skin cancer, breast cancer, cervical cancer and colon cancer. A brief introduction to the physical principles behind THz imaging and spectroscopy is also given.
Principles of THz imaging and spectroscopy
THz systems
The technology for generating and detecting THz radiation has advanced considerably over the past two decades. Several commercialized systems are available now (6)-(10) and THz systems have been set up by many groups all over the world. According to the laser source used, THz systems can be divided into two general classes: continuous wave (CW) and pulsed. It is also possible to generate THz radiation using oscillatory methods and this is often less expensive than using a laser based approach.
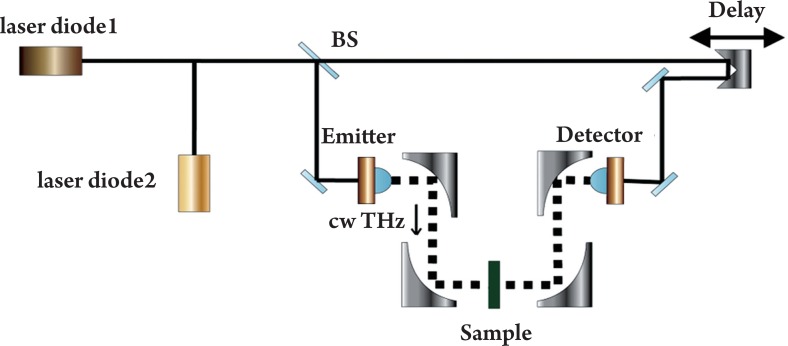
A typical CW system can produce a single fixed frequency or several discrete frequency outputs. Some of them can be tunable. Generation for CW THz radiation can be achieved by approaches such as photomixing (11), free-electron lasers (12) and quantum cascade lasers (13). Figure 1 illustrates a CW THz system that photomixes two CW lasers in a photoconductor as an example (14). The mixing of two above-bandgap (visible or near-infrared) wavelengths produces beating, which can modulate the conductance of a photoconductive switch at the THz difference frequency. The photomixing device is labeled “emitter” in Figure 1. Since the source spectrum of the CW system is narrow and sometimes only the intensity information is of interest, the data structures and post-processing are relatively simple. It is possible now to drive a whole CW system by laser diodes and thus it can be made compact and inexpensive. However due to the limited information that CW systems provide, they are sometimes confined to those applications where only features at some specific frequencies are of interest.
Figure 1. Schematic illustration of a CW THz imaging system in transmission geometry.
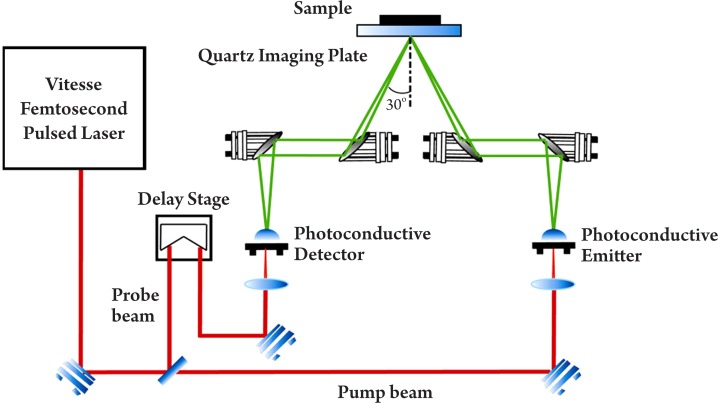
In pulsed systems, broadband emission up to several THz can be achieved. Currently, there are a number of ways to generate and detect pulsed THz radiation, such as ultrafast switching of photoconductive antennas (15), rectification of optical pulses in crystals (16), rapid screening of the surface field via photoexcitation of dense electron hole plasma in semiconductors (17) and carrier tunneling in coupled double quantum well structures (18). Among them, the most established approaches based on photoconductive antennas, where an expensive femtosecond laser is required and configured as shown in Figure 2. Unlike CW THz imaging system, coherent detection in pulsed THz imaging techniques can record THz waves in the time domain, including both the intensity and phase information, which can be further used to obtain more details of the target such as spectral and depth information (19). This key advantage lends coherent THz imaging to a wider range of applications.
Figure 2. Schematic illustration of a pulsed THz imaging system with reflection geometry.
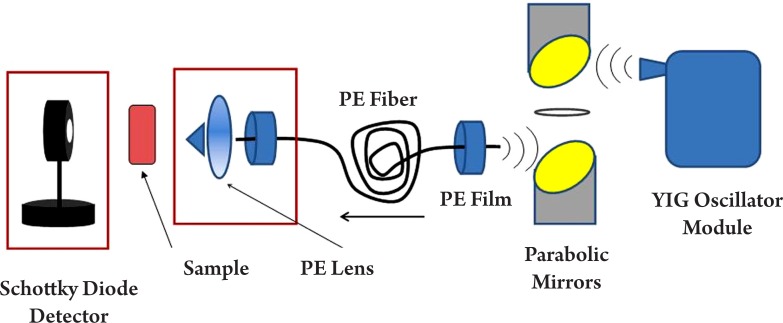
In Figure 3, the source is a frequency tunable THz oscillator module and the detector is a Schottky diode. The oscillator module consists of the following components: a YIG (Yttrium, Iron and Garnet) oscillator which can generate the base wave (at gigahertz level), an active doubler used to double the frequency (with some power loss), a variable attenuator used for modulation and signal level control, a power amplifier used to enlarge the beam power, a wideband isolator to filter the noise, a passive tripler to triple the frequency and a horn antenna to output the signal. The signal will then be collimated by a pair of parabolic mirrors and focused into the fiber with a low attenuation constant. At the fiber output end, a polyethylene lens focuses the THz light onto the sample. A Schottky diode acts as a receiver to detect the power of the transmitted through the sample. A Schottky diode is a type of semiconductor which has a metal-semiconductor barrier, it is fast switching (around nanosecond) and highly sensitive at the low frequency region for power detection.
Figure 3. Schematic illustration of a THz system with transmission geometry using a frequency tuneable oscillator as the THz source and a Schottky diode for detection. PE = polyethylene.
Molecular interactions in the THz regime
Many of the intricate interactions on a molecular level rely on changes in biomolecular conformation of the basic units of proteins such as α helices and β sheets. In recent years, dynamic signatures of the THz frequency vibrations in RNA and DNA strands have been characterized (20),(21). Furthermore, studies of water molecule interactions with proteins have attracted significant research interest (22). In a protein-water network, the protein's structure and dynamics are affected by the surrounding water which is called biological water, or hydration water. Hydrogen bonds, which are weak attractive forces, form between the hydrated water molecules and the side chains of protein. These affect the dynamic relaxation properties of protein and enable distinction between the hydration water layer and bulk water. The effects of the hydrogen bonds associated with the intermolecular information are able to be detected using THz spectroscopy. THz spectra contain information about intermolecular modes as well as intra-molecular bonds and thus usually carry more structural information than vibrations in the mid-infrared spectral region which tend to be dominated by intra-molecular vibrations.
Unique advantages and challenges for biomedical applications
The energy level of 1 THz is only about 4.14 meV (which is much less than the energy of X-rays 0.12 to 120 keV), it therefore does not pose an ionization hazard as in X-ray radiation. Research into safe levels of exposure has also been carried out through studies on keratinocytes (23) and blood leukocytes (24),(25), neither of which has revealed any detectable alterations. This non-ionizing nature is a crucial property that lends THz techniques to medical applications.
The fundamental period of THz-frequency electromagnetic radiation is around 1ps, and so it is uniquely suited to investigate biological systems with mechanisms at picosecond timescales. The energy levels of THz light are very low, therefore damage to cells or tissue should be limited to generalized thermal effects, i.e., strong resonant absorption seems unlikely. To test this hypothesis, Wilmink et al. in (26) examined the cellular response of human mammalian cells exposed to high-power THz radiation. They exposed human dermal fibroblasts to THz radiation (2.52 THz, 84.8 mWcm−2) for intervals up to 80 mins. After the exposure, the temperature had increased by about 3 °C to 39.8 °C. Therefore to isolate the effects of heat from those effects specific to THz radiation, as a control, another set of cells were heated to be at 39.8 °C for 80 min. Cell viability data showed that there was no significant difference between the cells exposed to THz radiation and the control cells. This suggests that only thermal effects were at play. Further investigations were also performed to test for more subtle differences such as gene expression and cell death mechanisms and these are reviewed in reference (27).
From a spectroscopy standpoint, biologically important collective modes of proteins vibrate at THz frequencies, in addition, frustrated rotations and collective modes cause polar liquids (such as water) to absorb at THz frequencies. Many organic substances have characteristic absorption spectra in this frequency range (28),(29), enabling research into THz spectroscopy for biomedical applications.
THz wavelengths have a diffraction limited spot size consistent with the resolution of a 1990's vintage laser printer (1.22 λ0=170 µm at 2.160 THz or 150 dots/in). At 1 THz, the resolution could be as good as a decent computer monitor (70 dots/in). Submillimeter-wavelength means that THz signals pass through tissue with only Mie or Tyndall scattering (proportional to f2) rather than much stronger Rayleigh scattering (proportional to f4) that dominates in the IR and optical since cell size is less than the wavelength.
Since most tissues are immersed in, dominated by, or preserved in polar liquids, the exceptionally high absorption losses at THz frequencies make penetration through biological materials of any substantial thickness infeasible. However, the same high absorption coefficient that limits penetration in tissue also promotes extreme contrast between substances with lesser or higher degrees of water content which can help to show distinctive contrast in medical imaging.
Imaging and spectroscopy
THz pulsed imaging actually can be viewed as an extension of the THz time-domain spectroscopy (THz-TDS) method. In addition to providing valuable spectral information, 2D images can be obtained with THz-TDS by spatial scanning of either the THz beam or the object itself. In this way, geometrical images of the sample can be produced to reveal its inner structures (30). Thus, it is possible to provide three-dimensional views into a layered structure.
When a THz pulse is incident on such a target, a train of pulses will be reflected back from the various interfaces. For each individual pulse in the detected signal, the amplitude and timing are different and can be measured precisely. The principle of time of flight technique is to estimate the depth information of the internal dielectric profiles of the target through the time that is required to travel over a certain distance. This permits looking into the inside of optically opaque material and it has been used for THz 3D imaging. Among the earliest demonstrations of THz 3D imaging, Mittleman et al. imaged a conventional floppy disk (31). In their work the various parts inside the disk were identified and the discontinuous refractive index profile was derived. This method was further extended into THz reflection computed tomography (32),(33), where the target was rotated to provide back reflection from different angles. In a similar way to X-ray CT imaging, the filtered back projection algorithm was applied to reconstruct the edge map of the target's cross-section. With advances in interactive publishing, Wallace et al. highlight the ability of 3-D THz imaging in a number of applications (34). For example they were able to resolve two layers of drugs beneath the protective coating of a pharmaceutical tablet.
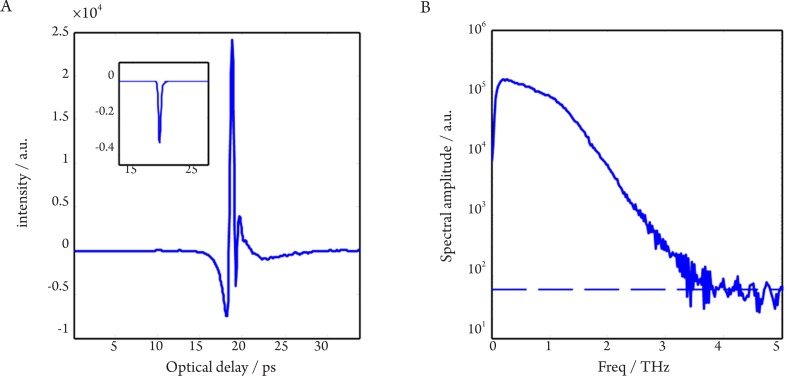
THz spectroscopy is typically done with a single point measurement (with transmission geometry in most cases) of a homogenous sample and the resulting THz electric field can be recorded as a function of time. Thus it can be Fourier transformed to offer meaningful spectroscopic information due to the broadband nature of pulsed THz radiation, shown in Figure 4. Although the spectral resolution is not as good as that with narrowband techniques, coherent detection of THz-TDS can provide both high sensitivity and time-resolved phase information (35). This spectroscopic technique is primarily used to probe material properties and it is helpful to see where it lies in the electromagnetic spectrum in relation to atomic and molecular transitions.
Figure 4. A: Temporal waveform of a sample (drawn by raw data with the inset representing its corresponding processed data after decovolution with a reference measurement); B: The corresponding spectrum of the raw data with the noise floor (indicated by dashed line).
THz spectroscopy is complementary to THz imaging and is primarily used to determine optical properties in the frequency domain. Since THz pulses are created and detected using short pulsed visible lasers with pulse widths varying from ∼ 200 fs down to ∼ 10 fs, it is now possible to make time resolved far-infrared studies with sub-picosecond temporal resolution (36). This was not achievable with conventional far-infrared studies. An important aspect of THz time-domain spectroscopy is that both the phase and amplitude of the spectral components of the pulse are determined. The amplitude and phase are directly related to the absorption coefficient and refractive index of a sample and thus the complex permittivity is obtained without requiring Kramers-Kronig analysis. Furthermore, another advantage of THz spectroscopy is that it is able to non-destructively detect differences because it uses radiation of sufficiently long wavelength and low energy that does not induce any phase changes or photochemical reactions to living organisms.
Cancer studies
Tissue characterization
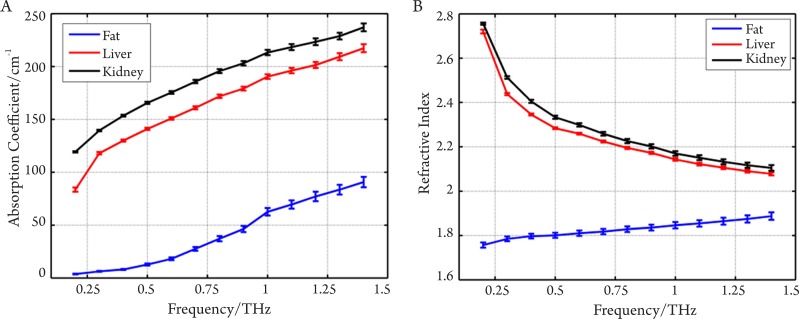
THz imaging has shown potential for in vivo and ex vivo identification of abnormalities, hydration, and sub-dermal probing. Only a small number of measurements have been made to date, and systematic investigations to catalog absorption coefficients, refractive indices and contrast mechanisms are just beginning to accumulate. Measurements on the absorption and refractive index of biological materials in the THz region go back at least to 1976 (37). Several research groups have investigated excised and fixed tissue samples, either alcohol perfused (38), formalin fixed (39)-(43), or freeze dried and wax mounted (44), looking for inherent contrast to define unique modalities. One of the first applications on human ex vivo wet tissue involved imaging of excised basal cell carcinoma (45),(46). In vivo work has focused on the skin (47) and accessible external surfaces of the body for measuring hydration (48) and tumor infiltration (46). A catalogue of unfixed tissue properties (including blood constituents) was compiled by the University of Leeds, U.K. (40) for frequencies between 0.5-1.5 THz using a pulsed time-domain system. Difficulties in extrapolating measurements on excised tissue to in vivo results are numerous and include for example uptake of saline from the sample storage environment, changes in hydration level during measurement, temperature-dependent loss, measurement chamber interactions, and scattering effects. In our previous work (49), we have performed reflection geometry spectroscopy to investigate the properties of several types of healthy organ tissues, including liver, kidney, heart muscle, leg muscle, pancreas and abdominal fat tissues using THz pulsed imaging. The frequency dependent refractive index and the absorption coefficient of the tissues are shown in Figure 5. All the results are the mean values of the ten samples and error bars represent the 95% confidence intervals. We found clear differences between the tissue properties, particularly the absorption coefficient. Since fatty tissue largely consists of hydrocarbon chains and relatively few polar molecules, the absorption coefficient and refractive index of the fatty tissue are much lower that those of the kidney and liver tissues.
Figure 5. A: Mean absorption coefficients of kidney, liver and abdominal fat; B: Mean refractive indices of all the tissue samples.
Error bars represent 95% confidence intervals. Having looked at how healthy tissues can be differentiated using THz spectroscopy, in the next sections we look at investigations involving cancer tissue samples.
Skin cancer-Basal Cell Carcinoma
One potential application of THz imaging is the diagnosis of skin cancer. Work by Wallace et al. has demonstrated the potential to use THz imaging to determine regions of skin cancer non-invasively using a reflection geometry THz imaging system (50). The first ex vivo measurements revealing the ability of THz imaging to detect skin cancer were of basal cell carcinoma (BCC) (51).
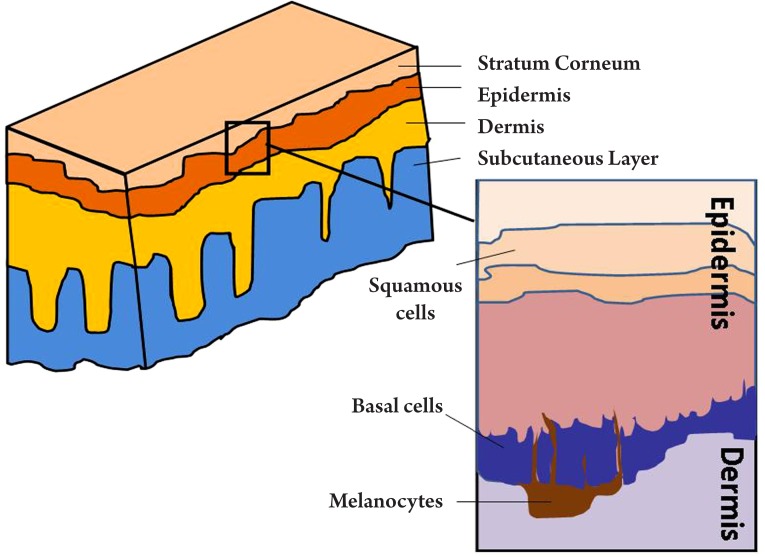
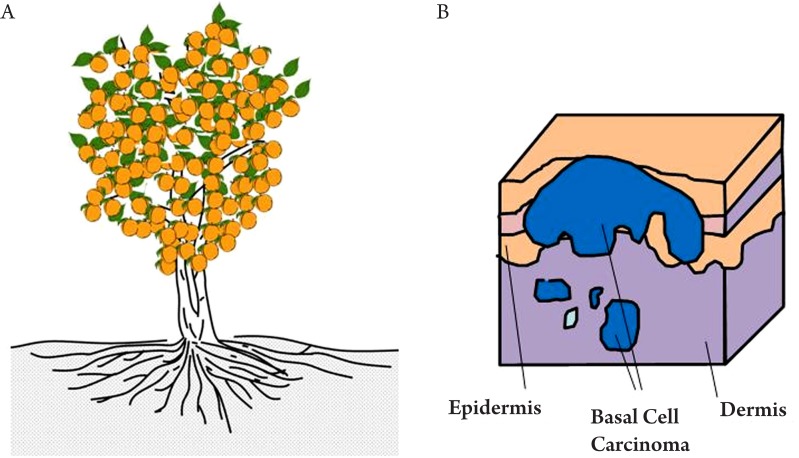
As illustrated in Figure 6, human skin consists of three principle layers, stratum corneum, epidermis, dermis and subcutaneous layer. The basal layer lies against the dermis, along which are most basal cells. Studies have shown that too much UV exposure to the basal layer may damage the DNA, thus causing the basal cells to divide without control. These uncontrollable cells can form a tumour, which is called basal cell carcinoma (BCC). BCC is one of the most prevailing form of skin cancer among the white populations and in the US alone, over one million cases are reported annually (52). Early diagnosis is vital in the treatment of BCC. The geometry of BCC is analogous to a tree root that spreads out just beneath the surface of the soil (Figure 7A). Only the tree trunk and branches are seen from above the surface, but the roots are also part of the tree. Similarly, with a BCC, only the some of the tumour will be visible on the skin (Figure 7B), and the lateral extent of the tumour beneath the skin's surface is not visible, but is a key part of the tumour and must be removed if the BCC is to stop growing.
Figure 6. Schematic diagrams of the layers in human skin, with the locations of melanocytes, basal cells and squamous cells highlighted.
Figure 7. Tree root analogy to basal cell carcinoma.
A: An orange tree with roots spreading out laterally underground; B: Basal cell carcinoma spreading out laterally beneath the skin's surface.
Previous research has found that some tumours have increased water content compared to normal tissues (53),(54). As water has strong absorptions in the THz range, the diseased and normal skin have different THz properties, namely the refractive index and absorption coefficient of diseased skin are both lower than those of normal tissue (55).
Research has also been done by S. Joseph et al. to identify intrinsic biomarkers for non-melanoma skin cancer (basal cell and squamous cell skin cancers) and their absorption frequencies using CW THz imaging. In their study, they measured chemicals such as water, tyrosine, tryptophan, melanin, collagen and urocanic acid with a CW system at 1.56 THz. To ensure that those chemicals' absorption frequencies are still observable in liquid water environment, their water suspensions were also measured. Their results found that in addition to water, tryptophan is a good biomarker for skin tumor diagnosis. Specifically, they found that tryptophan has resonant absorptions at 1.42 and 1.84 THz.
Breast tumors
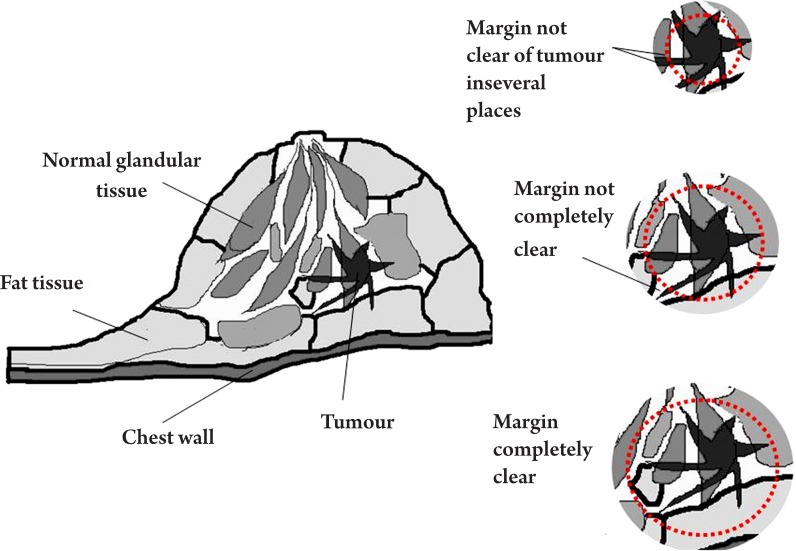
Breast-conserving surgery is also an area of medicine which may benefit from THz imaging. Fitzgerald et al. conducted ex vivo studies of breast cancer to investigate the potential of THz imaging to aid the removal of breast cancer intra-operatively (56). In particular, they studied the feasibility of THz pulsed imaging to map the tumour margins. The margins in this context refer to the thickness of healthy tissue that is next to the excised tumour. To be sure that the entire tumour has been removed, a surgeon will check that there is a sufficient margin of healthy tissue surrounding the tumour. This point is illustrated in Figure 8. Guidelines for margin thickness vary from 1.5 cm to 2.0 cm according to the conventional surgery (57).
Figure 8. Schematic diagram of a human breast containing tumour.
To be sure that the entire tumour is removed, a surgeon would need to remove sufficient tissue such that the margin does not contain any tumour.
To investigate the potential of THz imaging to check the margins, Fitzgerald et al. imaged freshly excised human breast tissue containing tumour. Good correlation was found for the area and shape of tumour in the THz images compared with that of histology. They have also performed a spectroscopy study comparing the THz optical properties (absorption coefficient and refractive index) of the excised normal breast tissue and breast tumour. Both the absorption coefficient and refractive index were higher for tissue that contained tumour and this is a very positive indication that THz imaging could be used to detect margins of tumour and provide complementary information to techniques such as infrared and optical imaging, thermography, electrical impedance, and magnetic resonance imaging (58).
More recently, successful detection of early breast cancer in vivo has been demonstrated by using a mouse mode (59). Cancer cells were injected into the skin part of the mouse along with fatty tissues. The reason for using fatty tissues was to imitate the situation for breast cancer in humans in which the breast tumor is surrounded by fatty tissues. The absorption of breast tissues increases with frequency and so this study was conducted using radiation at 108 GHz (generated by a YIG Oscillator with an output power of 3 mW and detected using a Schottky diode with sensitivity of 10−10 W/Hz.). The mouse was imaged every 3∼4 days in transmission geometry and cancer volumes as small as 0.02 mm3 were detected in vivo at room temperature.
Cervical cancer and Colon cancer
THz technology continues to improve and THz endoscopes have recently been demonstrated in (60),(61). This has triggered research in other types of cancer that are less accessible including cervical cancer and colon cancer.
Lymph node metastasis is closely related to poor prognosis and decreased survival rate of cervical cancer and conventional imaging methods such as CT, MRI, and PET only have 52%, 38%, 54% sensitivity respectively in node-based comparisons. Thus there is a need for a better method for detection of micro-metastatic lymph nodes. In a study by Jung et al. (62) lymph node samples were embedded in paraffin before the THz measurement. The reflected peak amplitudes of the THz wave were smaller for the metastatic than for the non-metastatic portions of lymph nodes and good delineation of the metastatic tissue was achieved.
THz studies of freshly excised colon cancer by Reese et al. using THz pulsed imaging (63) found good contrast between the normal and the diseased colon tissue. Additionally, Wahaia et al. measured colon tissue samples fixed in formalin with water present as well as samples embedded in paraffin (water is eliminated) (64) and found that there was still good contrast, suggesting that water is not the only factor contributing to the contrast between cancerous and normal tissues. This is consistent with results of a liver cirrhosis study by the Pickwell-MacPherson group in which it was concluded that components other than water account for upto 66% of the difference in absorption coefficient between healthy and cirrhotic liver tissue (65).
Improving image contrast: gold nano-particle labeling and high refractive index meta-materials
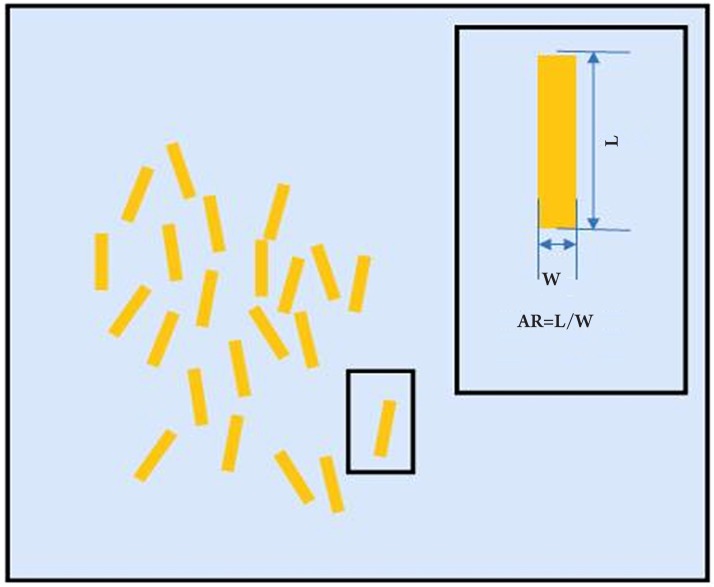
Gold-Nano-Rods (GNR) can be used to improve image contrast in THz images of cancer (66). A schematic diagram of the GNRs is given in Figure 9. The GNRs are specially made so that they can only be absorbed by cells with a high concentration of epidermal growth factor receptors. Although the cancer cells have preferential uptake of the GNRs, the THz signal of the cells with GNRs is not noticeably different from those without GNRs until after illumination by an infrared (IR) laser. The IR illumination increases the contrast because the cells containing nano-particles are heated up with surface plasma polaritons upon illumination (67),(68), and this causes an increase in the reflected THz signal amplitude. This is because biological cells have high water content and the THz properties of water such as the complex refractive index, which in turn affect reflectivity, are temperature dependent (69). In the study by Oh et al., the reflected signal of cells containing GNRs was increased by 20% upon illumination whereas the signal from the cells without GNRs showed no significant change. The difference images of the cells before and after illumination therefore showed excellent contrast and clearly showed the presence of the GNRs: the differential THz signal from cancer tissue with GNR was 30 times higher than without GNR (66). Therefore the sensitivity of the THz response is significantly enhanced using IR illumination. Additionally, since the positioning of IR light illumination can be controlled with micron resolution, the image resolution of this THz-differential image measurement technique can also be of the order of microns, which is much higher resolution than conventional THz imaging. It should also be noted that high concentrations of GNRs and intense exposure of NIR can kill the cell, so if the GNRs can target the cancer cells with high enough specificity then the technique also has potential for hyperthermia therapy of cancer (70). However, further research into the safety of proposed techniques needs to be fully investigated first.
Figure 9. Schematic diagram of gold nanorods.
The aspect ratio, AR, is defined as the length of the rod (L) divided by its width (W). The wavelength of the peak absorbance increases with increasing AR.
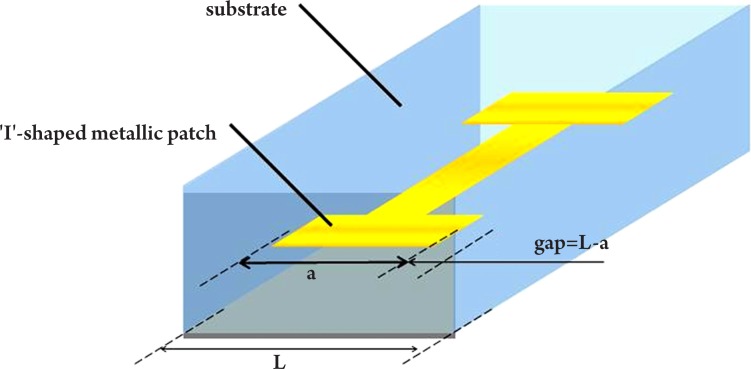
An alternative method to improve image contrast using a high refractive index meta-material has been demonstrated by Choi et al. (71) - this could also potentially be applied to cancer imaging. In their study, I-shaped metallic patches (Figure 10) were fabricated symmetrically in a substrate to achieve an extremely high refractive index. This was done by maximizing the effective permittivity ε, while suppressing the diamagnetic effect. The metal used in their study was gold (on chromium or aluminum) and the substrate was made of flexible polyimide with the real part of the refractive index n equal to 1.8. A single layer meta-material was first measured using both the ‘effective parameter retrieving’ method and THz-TDS. The predicted result of the effective parameter retrieving method was a peak index of n=27.25 at 0.516 THz and the corresponding THz-TDS measurement showed a peak index of n=24.34 at 0.522 THz. The two results correlate well considering the uncertainties in the material parameters and the errors in gap-width measurement. To investigate the 3D properties of the meta-material, samples containing up to five layers were fabricated and tested. The highest refractive index of 33.22 was observed at 0.851 THz. The new artificial material with extremely high refractive index at THz frequencies has great potential in the development of sub-wavelength-scale functional devices including small-footprint cloaking devices and wide-angle meta-material lenses. Moreover, if this or similar meta-material nano-particles can be preferentially absorbed by cancer cells, they could potentially be combined with THz imaging and used as a biomarker for cancer diagnosis.
Figure 10. The unit cell of the I-shaped high refractive index meta-material.
Comparison with other technologies
Numerous groups have investigated direct transmission or reflection THz imaging as a means of distinguishing tissue types (30),(41),(72) and recognizing diseases including tumors penetrating below the surface layers of skin or into organs (42),(44),(50). Although progress is being made, the competition from other more developed imaging modalities is fierce. Optical coherence tomography, ultrasound, near-IR, and Raman spectroscopy, MRI, positron emission tomography, in situ confocal microscopy, and X-ray techniques have all received much more attention and currently offer enhanced resolution, greater penetration, higher acquisition speeds, and specifically targeted contrast mechanisms. This does not preclude THz imaging from finding a niche in this barrage of already favorable modalities. There is still no technique that can readily distinguish benign from malignant lesions macroscopically at the surface or sub-dermally. The sensitivity of THz signals to skin moisture, which is often a key indictor, is very high, and competing techniques such as high-resolution MRI are less convenient and more costly.
The resolution of ordinary THz imaging is diffraction limited however its high sensitivity to water content and great surface imaging capability provides motivation for further development and sub-wavelength resolution has been achieved in near-field studies (73). Indeed shallow subsurface images can be the very revealing and the first few hundred micrometers are hard to image with other modalities. The high sensitivity of THz radiation to fluid composition and the variable conductivity in tissue (74) is likely to lead to statistically significant differences between nominally identical samples taken at different locations in the body at different times or from different subjects. This may ultimately prove advantageous; however in the short term, it will tend to mask sought for differences that are indicative of diseases.
Conclusion
THz imaging is still in the early stages of development - it is only recently that we have seen the first demonstration of in vivo breast cancer detection and this was with a mouse model. However, THz imaging has great potential to be a valuable imaging technique in the future, particularly for cancer diagnosis. Its inherent sensitivity to water can be exploited as a natural contrast agent as well as by employing nano-particles and IR illumination to enhance cancer cell contrast. The latter approach is particularly promising because by combining THz imaging with IR illumination it will be possible to achieve micron resolution and this will enable further applications to be investigated.
Acknowledgments
The authors gratefully acknowledge partial financial support for this work from the Research Grants Council of the Hong Kong Government (project 419609) and the Shun Hing Institute of Advanced Engineering (project BME -p4/09), Hong Kong.
Footnotes
No potential conflict of interest.
References
- 1.Shen Y, Lo T, Taday P, et al. Detection and identification of explosives using terahertz pulsed spectroscopic imaging. Appl Phys Lett. 2005;86:241116. [Google Scholar]
- 2.Duling I, Zimdars D. Terahertz imaging: Revealing hidden defects. Nature Photonics. 2009;3:630–2. [Google Scholar]
- 3.Fitzgerald AJ, Wallace VP, Pye R, et al. Terahertz imaging of breast cancer, a feasibility study. In Infrared and Millimeter Waves, 2004 and 12th International Conference on Terahertz Electronics, 2004. Conference Digest of the 2004 Joint 29th International Conference on. 2004:823–4. [Google Scholar]
- 4.American Cancer Society Cancer facts & figures 2011. 2011. American Cancer Society, Atlanta. Available Online: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures-2011.
- 5.Pickwell-MacPherson E, Wallace VP. Terahertz pulsed imaging--a potential medical imaging modality? Photodiagnosis Photodyn Ther. 2009;6:128–34. doi: 10.1016/j.pdpdt.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Mittleman D, Gupta M, Neelamani R, et al. Recent advances in terahertz imaging. Appl Phy B. 1999;68:1085–94. [Google Scholar]
- 7.Zhang XC. Terahertz wave imaging: horizons and hurdles. Phys Med Biol. 2002;47:3667–77. doi: 10.1088/0031-9155/47/21/301. [DOI] [PubMed] [Google Scholar]
- 8.Siegel PH. Terahertz technology in biology and medicine. IEEE Transactions on Microwave Theory and Techniques. 2004;52:2438–47. [Google Scholar]
- 9.Wallace VP, Taday PF, Fitzgerald AJ, et al. Terahertz pulsed imaging and spectroscopy for biomedical and pharmaceutical applications. Faraday Discuss. 2004;126:255–63; discussion 303-11. doi: 10.1039/b309357n. [DOI] [PubMed] [Google Scholar]
- 10.Withayachumnankul W, Png GM, Yin X, et al. T-ray sensing and imaging. Proceedings of the IEEE. 2007;95:1528–58. [Google Scholar]
- 11.Brown E, McIntosh K, Nichols K, et al. Photomixing up to 3.8 THz in low-temperature-grown GaAs. Appl Phys Lett. 1995;66:285–7. [Google Scholar]
- 12.Jeong YU, Lee BC, Kim SK, et al. First lasing of the KAERI compact far-infrared free-electron laser driven by a magnetron-based microtron. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2001;475:47–50. [Google Scholar]
- 13.Smet JH, Fonstad CG, Hu Q. Intrawell and interwell intersubband transitions in multiple quantum wells for far-infrared sources. J Appl Phys. 1996;79:9305–20. [Google Scholar]
- 14.Baker C, Gregory IS, Tribe WR, et al. Continuous-wave terahertz photomixing in low-temperature InGaAs. In Infrared and Millimeter Waves, 2004 and 12th International Conference on Terahertz Electronics, 2004. Conference Digest of the 2004 Joint 29th International Conference on. 2004:367–8. [Google Scholar]
- 15.Auston D. Picosecond optoelectronic switching and gating in silicon. Appl Phys lett. 1975;26:101–3. [Google Scholar]
- 16.Zhang XC, Ma X, Jin Y, et al. THz optical rectification from a nonlinear organic crystal. Appl Phys Lett. 1992;61:3080–2. [Google Scholar]
- 17.Huber R, Tauser F, Brodschelm A, Bichler M, et al. How many-particle interactions develop after ultrafast excitation of an electron-hole plasma. Nature. 2001;414:286–9. doi: 10.1038/35104522. [DOI] [PubMed] [Google Scholar]
- 18.Roskos HG, Nuss MC, Shah J, et al. Coherent submillimeter-wave emission from charge oscillations in a double-well potential. Phys Rev Lett. 1992;68:2216–2219. doi: 10.1103/PhysRevLett.68.2216. [DOI] [PubMed] [Google Scholar]
- 19.McClatchey K, Reiten M, Cheville R. Time resolved synthetic aperture terahertz impulse imaging. Appl Phys Lett. 2001;79:4485. [Google Scholar]
- 20.Woolard DL, Globus TR, Gelmont BL, et al. Submillimeter-wave phonon modes in DNA macromolecules. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;65:051903. doi: 10.1103/PhysRevE.65.051903. [DOI] [PubMed] [Google Scholar]
- 21.Globus T, Bykhovskaia M, Woolard D, et al. Sub-millimetre wave absorption spectra of artificial RNA molecules. J Phys D. 2003;36:1314–22. [Google Scholar]
- 22.Pal SK, Peon J, Zewail AH. Biological water at the protein surface: dynamical solvation probed directly with femtosecond resolution. Proc Natl Acad Sci U S A. 2002;99:1763–8. doi: 10.1073/pnas.042697899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourne N, Clothier RH, D'Arienzo M, et al. The effects of terahertz radiation on human keratinocyte primary cultures and neural cell cultures. Altern Lab Anim. 2008;36:667–84. doi: 10.1177/026119290803600610. [DOI] [PubMed] [Google Scholar]
- 24.Berry E, Walker GC, Fitzgerald AJ, et al. Do in vivo terahertz imaging systems comply with safety guidelines? J Laser Appl. 2003;15:192. [Google Scholar]
- 25.Zeni O, Gallerano GP, Perrotta A, et al. Cytogenetic observations in human peripheral blood leukocytes following in vitro exposure to THz radiation: a pilot study. Health Phys. 2007;92:349–57. doi: 10.1097/01.HP.0000251248.23991.35. [DOI] [PubMed] [Google Scholar]
- 26.Wilmink GJ, Rivest BD, Ibey BL, et al. Quantitative investigation of the bioeffects associated with terahertz radiation. Proceedings of SPIE. 2010;7562:75620L. [Google Scholar]
- 27.Wilmink GJ, Grundt JE. Invited review article: current state of research on biological effects of terahertz radiation. Journal of Infrared, Millimeter and Terahertz Waves. 2011:1–49. [Google Scholar]
- 28.Kawase K, Ogawa Y, Watanabe Y, et al. Non-destructive terahertz imaging of illicit drugs using spectral fingerprints. Opt Express. 2003;11:2549–54. doi: 10.1364/oe.11.002549. [DOI] [PubMed] [Google Scholar]
- 29.Ning L, Shen J, Jinhai S, et al. Study on the THz spectrum of methamphetamine. Opt Express. 2005;13:6750–5. doi: 10.1364/opex.13.006750. [DOI] [PubMed] [Google Scholar]
- 30.Hu BB, Nuss MC. Imaging with terahertz waves. Opt Lett. 1995;20:1716. doi: 10.1364/ol.20.001716. [DOI] [PubMed] [Google Scholar]
- 31.Mittleman DM, Hunsche S, Boivin L, et al. T-ray tomography. Opt Lett. 1997;22:904–6. doi: 10.1364/ol.22.000904. [DOI] [PubMed] [Google Scholar]
- 32.Pearce J, Choi H, Mittleman D, et al. T-ray reflection computed tomography. CLEO. 2005 [Google Scholar]
- 33.Pearce J, Choi H, Mittleman DM, et al. Terahertz wide aperture reflection tomography. Opt Lett. 2005;30:1653–5. doi: 10.1364/ol.30.001653. [DOI] [PubMed] [Google Scholar]
- 34.Wallace VP, Macpherson E, Zeitler JA, et al. Three-dimensional imaging of optically opaque materials using nonionizing terahertz radiation. J Opt Soc Am A Opt Image Sci Vis. 2008;25:3120–33. doi: 10.1364/josaa.25.003120. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson B, Zhang XC. Materials for terahertz science and technology. Nat Mater. 2002;1:26–33. doi: 10.1038/nmat708. [DOI] [PubMed] [Google Scholar]
- 36.Beard MC, Turner GM, Schmuttenmaer CA. Subpicosecond carrier dynamics in low-temperature grown GaAs as measured by time-resolved terahertz spectroscopy. J Appl Phys. 2001;90:5915–23. [Google Scholar]
- 37.Hartwick TS, Hodges DT, Barker DH, et al. Far infrared imagery. Appl Opt. 1976;15:1919–22. doi: 10.1364/AO.15.001919. [DOI] [PubMed] [Google Scholar]
- 38.Siegel P, Dengler R, Mueller E, et al. Scanned pixel heterodyne terahertz imaging. British Royal Soc, Terahertz Gap Meeting. 2003:2003. [Google Scholar]
- 39.Siebert KJ, Löffler T, Quast H, et al. All-optoelectronic continuous wave THz imaging for biomedical applications. Phys Med Biol. 2002;47:3743–8. doi: 10.1088/0031-9155/47/21/310. [DOI] [PubMed] [Google Scholar]
- 40.Fitzgerald A, Berry E, Zinov'ev N, et al. Catalogue of human tissue optical properties at terahertz frequencies. J Biol Phys. 2003;29:123–128. doi: 10.1023/A:1024428406218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Löffler T, Siebert K, Czasch S, et al. Visualization and classification in biomedical terahertz pulsed imaging. Phys Med Biol. 2002;47:3847–52. doi: 10.1088/0031-9155/47/21/324. [DOI] [PubMed] [Google Scholar]
- 42.Löffler T, Bauer T, Siebert K, et al. Terahertz dark-field imaging of biomedical tissue. Opt Express. 2001;9:616–21. doi: 10.1364/oe.9.000616. [DOI] [PubMed] [Google Scholar]
- 43.Kan WC, Lee WS, Cheung WH, et al. Terahertz pulsed imaging of knee cartilage. Biomed Opt Express. 2010;1:967–974. doi: 10.1364/BOE.1.000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knobloch P, Schmalstieg K, Koch M, et al. THz imaging of histo-pathological samples. Proceedings of SPIE. 2001;4434:239–45. [Google Scholar]
- 45.Woodward RM, Wallace VP, Pye RJ, et al. Terahertz pulse imaging of ex vivo basal cell carcinoma. J Invest Dermatol. 2003;120:72–8. doi: 10.1046/j.1523-1747.2003.12013.x. [DOI] [PubMed] [Google Scholar]
- 46.Woodward RM, Cole BE, Wallace VP, et al. Terahertz pulse imaging in reflection geometry of human skin cancer and skin tissue. Phys Med Biol. 2002;47:3853–63. doi: 10.1088/0031-9155/47/21/325. [DOI] [PubMed] [Google Scholar]
- 47.Pickwell E, Cole BE, Fitzgerald AJ, et al. In vivo study of human skin using pulsed terahertz radiation. Phys Med Biol. 2004;49:1595–607. doi: 10.1088/0031-9155/49/9/001. [DOI] [PubMed] [Google Scholar]
- 48.Cole BE, Woodward RM, Crawley DA, et al. Terahertz imaging and spectroscopy of human skin in vivo. Proceedings of SPIE. 2001;4276:1. [Google Scholar]
- 49.Huang SY, Wang YX, Yeung DK, et al. Tissue characterization using terahertz pulsed imaging in reflection geometry. Phys Med Biol. 2009;54:149–60. doi: 10.1088/0031-9155/54/1/010. [DOI] [PubMed] [Google Scholar]
- 50.Woodward RM, Wallace VP, Cole BE, et al. Terahertz pulse imaging in reflection geometry of skin tissue using time-domain analysis techniques. Proceedings of SPIE. 2002;4625:160. [Google Scholar]
- 51.Strachan CJ, Taday PF, Newnham DA, et al. Using terahertz pulsed spectroscopy to quantify pharmaceutical polymorphism and crystallinity. J Pharm Sci. 2005;94:837–46. doi: 10.1002/jps.20281. [DOI] [PubMed] [Google Scholar]
- 52.Rubin AI, Chen EH, Ratner D. Basal-Cell Carcinoma. N Engl J Med. 2005;353:2262–9. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 53.Chen JH, Avram HE, Crooks LE, et al. In vivo relaxation times and hydrogen density at 0.063-4.85 T in rats with implanted mammary adenocarcinomas. Radiology. 1992;184:427–34. doi: 10.1148/radiology.184.2.1620841. [DOI] [PubMed] [Google Scholar]
- 54.Ross KF, Gordon RE. Water in malignant tissue, measured by cell refractometry and nuclear magnetic resonance. J Microsc. 1982;128:7–21. doi: 10.1111/j.1365-2818.1982.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 55.Wallace VP, Fitzgerald AJ, Pickwell E, et al. Terahertz pulsed spectroscopy of human Basal cell carcinoma. Appl Spectrosc. 2006;60:1127–33. doi: 10.1366/000370206778664635. [DOI] [PubMed] [Google Scholar]
- 56.Fitzgerald AJ, Wallace VP, Jimenez-Linan M, et al. Terahertz pulsed imaging of human breast tumors. Radiology. 2006;239:533–40. doi: 10.1148/radiol.2392041315. [DOI] [PubMed] [Google Scholar]
- 57.Wu F, Wang ZB, Cao YD, et al. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer. 2003;89:2227–33. doi: 10.1038/sj.bjc.6601411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bolan PJ, Meisamy S, Baker EH, et al. In vivo quantification of choline compounds in the breast with 1H MR spectroscopy. Magn Reson Med. 2003;50:1134–43. doi: 10.1002/mrm.10654. [DOI] [PubMed] [Google Scholar]
- 59.Chen H, Chen TH, Tseng TF, et al. High-sensitivity in vivo THz transmission imaging of early human breast cancer in a subcutaneous xenograft mouse model. Opt Express. 2011;19:21552–62. doi: 10.1364/OE.19.021552. [DOI] [PubMed] [Google Scholar]
- 60.Wang K, Mittleman DM. Metal wires for terahertz wave guiding. Nature. 2004;432:376–9. doi: 10.1038/nature03040. [DOI] [PubMed] [Google Scholar]
- 61.Ji YB, Lee ES, Kim SH, et al. A miniaturized fiber-coupled terahertz endoscope system. Opt Express. 2009;17:17082–7. doi: 10.1364/OE.17.017082. [DOI] [PubMed] [Google Scholar]
- 62.Jung EA, Lim MH, Moon KW, et al. Terahertz Pulse Imaging of Micro-metastatic Lymph Nodes in Early-stage Cervical Cancer Patients. J Opt Soc Korea. 2011;15:155–60. [Google Scholar]
- 63.Reese G, Reid C, Goldin R, et al. Using terahertz pulsed imaging (TPI) to identify colonic pathology. In Infrared, Millimeter and Terahertz Waves, 2008. IRMMW-THz 2008. 33rd International Conference on. 2008:1–1. [Google Scholar]
- 64.Wahaia F, Valusis G, Bernardo LM, et al. Detection of colon cancer by terahertz techniques. Proceedings of SPIE. 2011;8001:800130. [Google Scholar]
- 65.Sy S, Huang S, Wang YX, et al. Terahertz spectroscopy of liver cirrhosis: investigating the origin of contrast. Phys Med Biol. 2010;55:7587–96. doi: 10.1088/0031-9155/55/24/013. [DOI] [PubMed] [Google Scholar]
- 66.Oh SJ, Kang J, Maeng I, et al. Nanoparticle-enabled terahertz imaging for cancer diagnosis. Opt Express. 2009;17:3469–75. doi: 10.1364/oe.17.003469. [DOI] [PubMed] [Google Scholar]
- 67.Lee J, Yang J, Ko H, et al. Multifunctional magnetic gold nanocomposites: human epithelial cancer detection via magnetic resonance imaging and localized synchronous therapy. Advanced Functional Materials. 2008;18:258–64. [Google Scholar]
- 68.Huang X, El-Sayed IH, Qian W, et al. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–20. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 69.Rønne C, Thrane L, Åstrand PO, et al. Investigation of the temperature dependence of dielectric relaxation in liquid water by THz reflection spectroscopy and molecular dynamics simulation. J Phys Chem. 1997;107:5319–31. [Google Scholar]
- 70.Oh SJ, Huh YM, Suh JS, et al. Cancer Diagnosis by Terahertz Molecular Imaging Technique. Journal of Infrared, Millimeter and Terahertz Waves. 2011:1–8. [Google Scholar]
- 71.Choi M, Lee SH, Kim Y, et al. A terahertz metamaterial with unnaturally high refractive index. Nature. 2011;470:369–73. doi: 10.1038/nature09776. [DOI] [PubMed] [Google Scholar]
- 72.Ferguson B, Wang S, Gray D, et al. Towards functional 3D T-ray imaging. Phys Med Biol. 2002;47:3735–42. doi: 10.1088/0031-9155/47/21/309. [DOI] [PubMed] [Google Scholar]
- 73.Chen HT, Kersting R, Cho GC. Terahertz imaging with nanometer resolution. Appl Phys Lett. 2003;83:3009–11. [Google Scholar]
- 74.Ulgen Y, Sezdi M. Electrical parameters of human blood. In Engineering in Medicine and Biology Society, 1998. Proceedings of the 20th Annual International Conference of the IEEE. 1998;6:2983–6. [Google Scholar]