Abstract
Type 1 diabetes mellitus results in impaired insulin production by pancreatic islets due to autoimmunity. Islet transplantation has recently emerged as a promising treatment for this disease. To visualize and monitor endogenous and transplanted islets, non-invasive strategies are currently being developed. These include strategies for in vivo magnetic resonance imaging of microvascular changes during diabetes development, tracking the recruitment of diabetogenic T cells to the pancreas, and imaging of endogenous and transplanted islet mass. The combination of MR imaging agents with therapy is a novel state-of-the-art theranostic approach that has a tremendous potential for type 1 diabetes management. Though still in its infancy, theranostic MR imaging has shown certain encouraging progress. Here we provide an overview of the latest accomplishments in this area as it applies to changes in islet vasculature during diabetes development, monitoring autoimmune attack mediated by T cells, and imaging of transplanted islets. Future challenges and opportunities in the area of theranostic MRI are discussed as well.
Key Words: Theranostic, magnetic resonance imaging, islet, diabetes
Introduction
Type 1 diabetes (T1D) develops as a consequence of a synergistic combination of genetic predisposition, largely unknown environmental triggers, and immunologic events (1). Microvasculature alterations (2), diabetogenic T cells infiltration (3), and autoimmune destruction of pancreatic β-cells are three characteristics among the pathological changes during T1D development.
Next to whole pancreas transplantation, islet transplantation (Tx) has become one of the most effective therapeutic procedures for T1D treatment during the past decade (4). However, a number of immunological and non-immunological factors contribute to islet graft loss after treatment (5,6). Among these factors, allogeneic immune response (7,8) and recurrence of autoimmunity (9) are the most important ones responsible for long-term graft loss. These are followed by microvasculature leakage (10,11) and ultimate islet destruction.
Although remarkable advances have been made in recent years in the understanding of T1D pathogenesis and graft loss after islet transplantation, noninvasive and real-time tools for visualization and/or monitoring the disease development in clinical application are still lacking. For more than a decade, researchers have been trying to apply in vivo magnetic resonance imaging (MRI) for the visualization of disease-relevant phenomena in T1D and monitoring transplanted islets (12,13). Various contrast MRI agents targeting key pathological changes in T1D, such as microvascular alterations and T cell infiltration as well as the damage in endogenous or transplanted islets have been developed (14). These probes include T1 contrast agents based on Gadolinium-diethylenetriaminepentaacetic acid (GdDTPA) and Manganese (Mn) and T2 contrast agents based on superparamagnetic iron oxide nanoparticles (SPIO). Contrast agents featuring fluorine-19 in the context with perfluoropolyether (PFPE) have also been reported (15).
The term “theranostic” was coined in 2002 by Funkhouser et al. (16) and is defined as an approach that combines therapy with diagnostic imaging. Thus, theranostics deliver diagnostic imaging agents and therapeutics at the same time, allowing for monitoring of their delivery. A theranostic approach combines two agents into one “package,” which has the potential to overcome undesirable differences in biodistribution and selectivity that currently exist between distinct imaging and therapeutic agents (17-20). The aim of this review is to outline the rationale, methodology, and current progressin the area of theranostic MRI as it applicable to endogenous islets and transplanted grafts.
Microvascular leakage - targeted theranostic imaging
An early biomarker of pancreatic islet damage is islet microvascular dysfunction, leading to alterations in vascular volume, flow, and vascular permeability reported in numerous models of T1D (21,22). It is essential to develop approaches for monitoring changes in pancreatic microvasculature non-invasively. This can be done by exploiting the leakiness of islet vasculature using long-circulating blood pool agents. In the absence of inflammation, these contrast agents would outline the existing vasculature. During inflammation, they would slowly leak through the gaps and accumulate in islet interstitium via the Enhanced Permeability and Retention effect (EPR) causing changes in tissue contrast.
For the noninvasive semiquantitative evaluation of vascular changes in a streptozotocin (STZ)-induced mouse model of type 1 diabetes, Medarova et al. (23) utilized a long-circulating protected graft copolymer (PGC) covalently linked to gadolinium-diethylenetriaminepentaacetic acid residues (GdDTPAs) labeled with fluorescein isothiocyanate (PGC-GdDTPA-F). PGC is based on a conjugate of a polylysine backbone to which methoxypoly (ethylene glycol) (MPEG) chains are covalently linked in a random fashion via N-ε-amino groups (24). MRI was used for monitoring diabetic animals and nondiabetic controls after intravenous injection of PGC-GdDTPA-F. The results of this study demonstrated a significantly greater accumulation of PGC-GdDTPA-F in the pancreata of diabetic animals compared to controls. Ex vivo histology confirmed considerable leakage of PGC-GdDTPA-F into the islet interstitium of diabetic animals. By contrast, in nondiabetic controls, contrast agent was largely restricted to the pancreatic vasculature at the islet periphery. This study demonstrated that high-molecular weight paramagnetic blood pool contrast agent was valuable for the in vivo definition of pancreatic microvasculature dynamics by MRI. Furthermore, this method permitted the in vivo semiquantitative assessment and direct visualization of the microvascular leakage in diabetic pancreata (Figure 1). Following these initial experiments, this agent was used for the evaluation of vascular changes in a more clinically relevant animal model. Autoimmune diabetes in BBDR rats was generated by depletion of regulatory ART2+ T cells (25,26). Animals were imaged before as well as 1 and 24 hrs after intravenous injection of PGC-GdDTPA-F. A significantly higher accumulation of the agent was observed in the pancreata of diabetic BBDR rats, as compared to non-diabetic controls at 1 hour post-injection. No differences were seen in the blood pool, kidney, or muscle, indicating that the effect was specific to diabetic pancreas. Histology studies revealed a marked increase in contrast agent availability in the pancreas of diabetic animals (27). These observations lead to the conclusion that accumulation of PGC-GdDTPA-F in the pancreas via EPR effect could be exploited for therapeutic drug delivery.
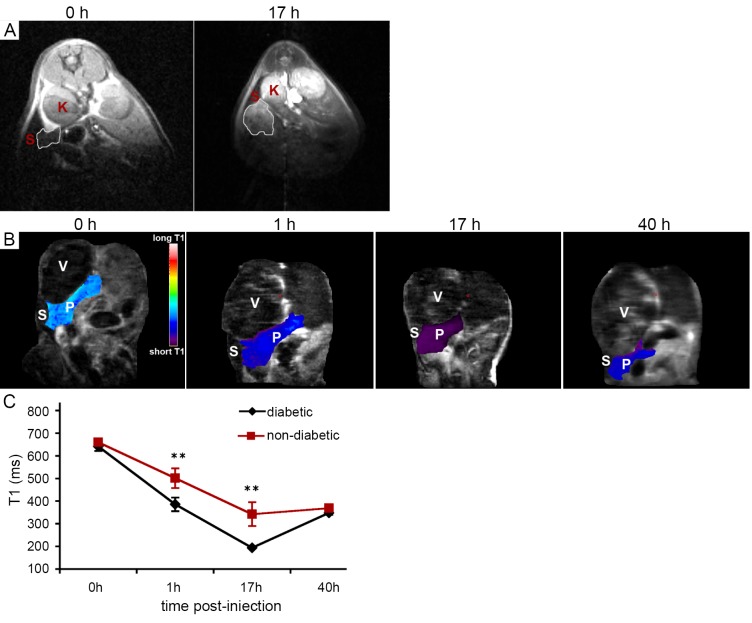
Figure 1.
T1-weighted MRI of PGC-GdDTPA-F accumulation in the diabetic pancreas. A. Transverse T1-weighted MRI of an STZ induced diabetic mouse obtained before and 17 h after injection of PGC-GdDTPA-F. The area of the pancreas is outlined. Note the increase in signal intensity after injection of the contrast agent due to local accumulation of the agent. K, kidney; S, spleen; B. Coronal T1 maps of an STZ-induced diabetic mouse obtained before and 1, 17, and 40 h after injection of PGC-GdDTPA-F. The area of the pancreas is color-coded based on its T1 relaxation time. P, pancreas; S, spleen; V, stomach; C. Quantitative analysis of T1 relaxation times of the pancreata of STZ-induced diabetic and control mice. There was a drop in the T1 of the pancreata at 1 h after injection of PGC-GdDTPA-F. This drop was most significant at 17 h after injection and was followed by a gradual increase, consistent with contrast agent washout. T1 map analysis demonstrated a significant difference in the T1 of the pancreata between diabetic and control animals at 1 and 17 h after injection of PGC-GdDTPA-F, reflecting a higher accumulation of the contrast agent in diabetic pancreata. **P<0.05. Reproduced with permission from American Diabetes Association (23)
In the first feasibility study, Castillo et al. (28) utilized fatty acid containing PGC as a stabilizing excipient for delivery of glucagon-like peptide-1 (GLP-1). GLP-1 is a multi-functional incretin hormone that enhances endocrine pancreatic function by restoring glucose sensitivity of pancreatic β-cells, inhibiting pancreatic β-cell apoptosis, and stimulating their proliferation and differentiation (29,30). This peptide and its analogues are promising drug candidates for T1D treatment (31-33). Unfortunately, native GLP-1 has a very short blood half-life due to the cleavage by dipeptidyl peptidase-4 (DPP-4) (34). In this study, PGC was used for stabilization of GLP-1, prolonging its blood half-life and ultimately delivering it to pancreatic islets. Stability studies showed that PGC-GLP-1 was significantly more stable than free GLP-1 (26% vs. ~60% degradation by DPP-4 after 24-hour digestion) (28). Detailed pharmacokinetic studies indicated that formulation of GLP-1 with PGC extended GLP-1 half-life from minutes to at least 6 hours when administered intravenously, and to at least 20 hours in both rats and mice when administered subcutaneously, which is significantly longer than that of existing products (Exenatide, Liraglutide, Byetta). In vivo data demonstrated PGC-GLP-1 efficacy in the regeneration of β-cells in diabetic male Zucker fatty (fa-/fa-) rats (28). Treatment with PGC-GLP-1 performed once a week for 7 weeks was as effective in improving HbA1c as Exendin-4, which is a GLP-1 receptor antagonist, given twice a day. Furthermore, twice a week injections of PGC-GLP-1 showed a significantly lower HbA1c at week 7 compared to Exendin-4. In addition, significant increase in β-cell division was observed in rats that received PGC-GLP-1 than in control groups. This study demonstrated the potential for a long circulating blood pool agent to serve not only as an imaging reporter of leaky vasculature, but also as a delivery vehicle for existing and developmental therapies in diabetes.
In vivo MR imaging and therapy of diabetogenic T cells
Chronic infiltration of islets by autoreactive T cells results in the destruction of insulin producing β-cells, leading to the onset of T1D (35,36). Development of theranostic imaging techniques for tracking immune cells in vivo could not only aid in understanding the mechanisms behind inflammatory and autoimmune reactions that happen prior to the onset of hyperglycemia, but would also provide potential therapeutic solutions.
Several studies have already demonstrated the potential for in vivo imaging of the autoimmune destruction of insulin-producing pancreatic β-cells by cytotoxic T lymphocytes (CTL). In one of the early studies, T lymphocytes purified from 18-20 week old, non-obese diabetic (NOD) mice were labeled with iron oxide nanoparticles conjugated with a membrane translocation Tat peptide (37). These labeled T cells were then adoptively transferred into NOD.scid (severe combined immunodeficiency) mice, which were selected as recipients because these mice do not develop diabetes due to the scid mutation. The infiltration of labeled lymphocytes in the mouse pancreas was visualized using ex vivo MRI. These first results established that labeling of diabetogenic T cells with Tat-conjugated nanoparticles resulted in highly efficient uptake of the agent that enabled detection of these cells by MRI (37).
In the next step, Billotey et al. (38) demonstrated the possibility of in vivo imaging of T cells from NOD mice labeled with anionic magnetic nanoparticles (AMNPs). Labeled T cells were then adoptively transferred to irradiated NOD mice. MR images of the pancreas were obtained in these NOD mice at 11 and 20 days after adoptive transfer of AMNP-labeled T cells. Homing of T cells in pancreatic lymph nodes was revealed as a focal dark spot with T2* effect in the caudal area of the pancreas 11 days after the transfer. After 20 days, adoptively transferred animals developed diabetes, which was seen as a more diffuse negative enhancement of the whole pancreas than the control (38). This study demonstrated that AMNP loaded T cells could be detected using in vivo MRI.
Proof-of-concept studies listed above applied non-specific contrast agent for labeling and tracking of T cells infiltrating pancreatic islets in vivo. Meanwhile, the pool of diabetogenic lymphocytes consists of multiple specificities that target autoantigens present on the surface of β-cells in the context with major histocompatibility complex (MHC) Class I molecules during an autoimmune attack (39). To selectively label T cells involved in autoantigen recognition, our group subsequently designed antigen-specific iron oxide nanoparticles conjugated through avidin-biotin linkage to MHC-1-NRP-V7 peptide [a high-avidity mimotope of islet specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) (40,41)], which could be recognized by H-2Kd restricted CD8+ T cells (42) (Figure 2). The CD8+ T cells from NOD mice and 8.3/NOD mice (transgenic mice strain expressing NRP-V7 specific T cell receptor on NOD background) were incubated with the nanoparticles and analyzed by flow cytometry. The probe labeled about 92% of CD8+ T cells from 8.3-NOD-mice whereas only 1.83% CD8+ T cells from NOD mice, which proved that the probe was specific to NRP-V7 reactive CD8+ T cells. For the in vivo studies, CD8+ T cells from 8.3/NOD mice were labeled with NRP-V7-specific nanoparticles and adoptively transferred into 5-week-old NOD.scid mice. The decrease in signal intensity of pancreatic tissues on T2-weighted images was already noticeable 2 days after T cell transfers and continued until the 16th day. Histological studies confirmed the presence of labeled CD8+ T cell infiltration in the pancreas tissues. This study served as the first demonstration of imaging adoptively transferred autoimmune reaction by MRI in live animals (42).
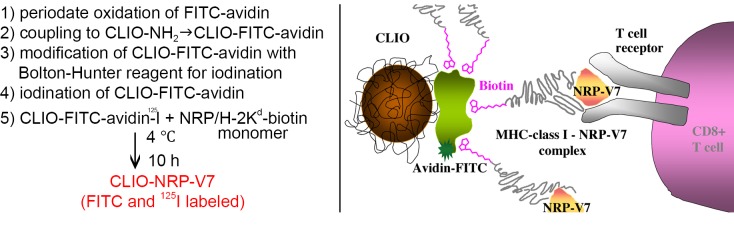
Figure 2.
Left. Step-by-step synthesis of CLIO-NRP-V7 probe; Right. Schematic representation of CLIO-NRP-V7 probe. Biotinylated NRP-V7/H-2Kd complex was coupled to CLIO particles modified with avidin. CLIO-NRP-V7 probe is recognized by the TCR on NRP-V7-reactive CD8+ T-cells. Note that there are four binding sites for biotin on avidin and, hence, for biotinylated peptide/MHC complex. Reproduced with permission from American Diabetes Association (42)
For the future clinical application of these approaches, it would be necessary to deliver the nanoparticle probe to endogenous CD8+ T cells that already relocated to the diabetic pancreas. Towards this step, magnetic nanoparticles conjugated to MHC-1-NRP-V7 complex were injected intravenously in 5, 8, 15 and 24-week old NOD mice followed by in vivo MRI. Accumulation of the nanoparticles (as determined by MRI analysis of pancreas-associated T2 relaxation time) was age-dependent, correlated well with the progression of insulitis, and provided quantitative information on the infiltration of CD8+ T cells carrying TCR specific for NRP-V7 peptides. Ex vivo analysis showed that the percentage of islet-associated specific IGRP-reactive CD8+ T cells from mice of different age groups correlated well with the change in T2 signal intensity on MR images (43) (Figure 3).
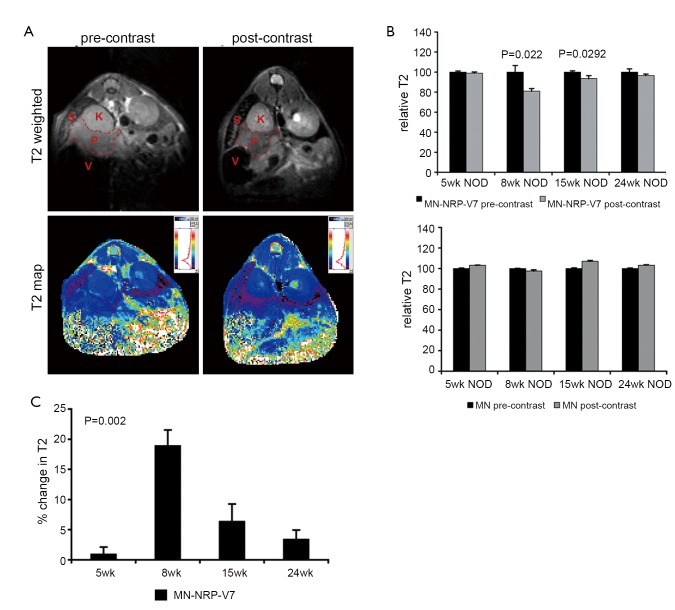
Figure 3.
MRI of NRP-V7-specific CD8+ T lymphocyte infiltration of the pancreas. A. A representative T2-weighted image (top) and its corresponding multiecho T2 map (bottom) before (left) and 24 h after (right) intravenous injection of MN-NRP-V7. The pancreas is outlined in red. V = stomach, S = spleen, K = kidney, P = pancreas; B. Quantitative representation of the change in T2 relaxation times (ΔT2, ms) as a function of age in 5- (n=7), 8- (n=4), 15- (n=4), and 24-week-old (n=10) NOD mice injected with MN-NRP-V7 (left) or unmodified MN nanoparticles (right). In animals injected with MN-NRP-V7, there was a significant change in T2 relaxation times after injection at 8 (P=0.022) and 15 (P=0.0292) weeks of age. By contrast, in animals injected with unmodified MN nanoparticles, there was no significant change in T2 values. Data (mean ± SEM) were analyzed by two-tailed Student’s t test; C. The change in T2 in animals injected with MN-NRP-V7 followed a trend of age dependence, with a peak at 8 weeks of age. Data (mean ± SEM) were analyzed by one-way ANOVA with Tukey’s post-hoc pairwise comparison test. **P=0.002. Reproduced with permission from John Wiley and Sons Publishing Group (43)
Similar to long circulating agent PGC (23,27), the initial accumulation of iron oxide nanoparticles in pancreatic islets occurs through leaky islet vasculature, followed by extravasation into islet interstitium from where it could be potentially taken up by specific pools of diabetogenic T cells. Based on the fact that inhibition of antigen-specific T cells could lead to T1D prevention and treatment (44), the next study investigated the utility of iron nanoparticles conjugated with disease-relevant peptide complexes for therapeutic purposes. Studies in NOD mice showed that accumulation of nanoparticles coated with disease-relevant peptide-MHC (pMHC) complexes led to protection from and the reversal of diabetes in these animals by expanding (in an epitope-specific manner) a subset of antigen-experienced autoreactive CD8+ cells (Tregs) that suppressed the activation and recruitment of noncognate specificities to islets (45). These autoregulatory T cells suppressed local presentation of autoantigens in an interferon-gamma-, indoleamine 2,3-dioxygenase-, and perforin-dependent manner. Interestingly, nanoparticles coated with human diabetes-relevant pMHC complexes restored normoglycemia in a humanized model of diabetes. The authors conclude that nanoparticles coated with disease-relevant pMHC complexes have the potential to become powerful vaccines capable of blunting and resolving polyclonal autoimmune responses in a disease-specific manner (45).
MRI contrast agents described thus far are used with 1H MRI (termed proton MRI). Delivered contrast agents affect relaxation properties of water protons in the sense that MR scanner detects the contrast agents indirectly. A more direct approach is heteronuclear MRI. In principle, any nucleus with a nonzero magnetic spin and a large enough magnetic sensitivity could be used for imaging. 19-Fluorine (19F) is a popular choice because it exhibits favorable MR imaging characteristics with a magnetic sensitivity close to that of protons, and is 100% naturally abundant. Srinivas et al. (46) described an in vivo imaging method for visualizing and quantifying diabetogenic T cells in T1D animal model using 19F MRI. In these experiments, cells were labeled ex vivo with perfluoropolyether nanoparticles and then monitored in vivo using 19F MRI following cell transfer. 19F MRI selectively visualized only the labeled cells with no background, showing early homing of diabetogenic T cells to the pancreas. According to their data, approximately 2% of the transferred cells homed to the pancreas after 48 hours (46). The utility of this agent for the imaging of endogenous immune cells or for delivery of therapeutic agent is yet to be determined.
Theranostic MR imaging of transplanted islets
MRI offers the best spatial resolution and unlimited depth penetration for the anatomical imaging of islet transplantation (47,48). Islets have been labeled with SPIOs (49,50) or paramagnetic gadolinium based contrast agent (51) for in vivo monitoring by MRI. Low toxicity and good sensitivity make SPIOs by far the most widely used contrast agent for MR cellular imaging. The basic structure of SPIOs includes an iron oxide core (3-5 nm) coated with dextran (52) or siloxanes (53). Magnetic nanoparticles could be made multimodal by conjugation to additional imaging, targeting, or therapeutic moieties (54). Our group labeled human pancreatic islets with iron oxide magnetic nanoparticles (MNs) that were modified with a near-infrared fluorescent dye (MN-NIRF) and then transplanted under the kidney capsule of immunocompromised mice. Islets were detected for up to 188 days by MRI post-transplantation (49,55). Furthermore, we investigated the applicability of in vivo MRI for detection of intra-hepatically transplanted islets. Human islets labeled with iron oxide agent ferumoxides and transplanted into the liver appeared as distinct hypo-intense foci, representing single islets and/or islet clusters. This approach was also used to monitor immune rejection of ferumoxides-labeled human islets in an immunocompetent mouse model (56). A follow-up study by Tai et al. showed application of clinical imaging systems for imaging of Feridex-labeled transplanted pancreatic islets. Using a steady-state acquisition imaging sequence and 1.5 T scanner, the authors detected as few as 200 SPIO-labeled islets transplanted under a rat kidney capsule (50). Potential translation of these findings to the clinical scenario requires validation of this methodology in a non-human primate model, which was carried out in baboons (Papio hamadryas) (57). The Food and Drug Administration (FDA) approved SPIOs contrast agent Feridex was utilized for islet labeling in these studies. T2*-weighted MR images generated using a 1.5 T clinical scanner revealed the presence of labeled grafts transplanted under the kidney capsule and in the liver (57).
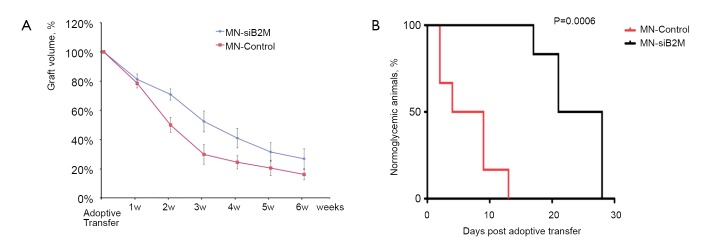
Since dextran-coated iron oxide nanoparticles can be linked and functionalized with therapeutic moieties, the next step was to explore their utility for islet protection prior to transplantation. Taking advantage of the propensity of pancreatic islets to avidly take up dextran-coated SPIOs, our group designed a probe that consisted of a dextran-coated iron oxide core, conjugated to small interfering RNA (siRNA). siRNAs represent a powerful therapeutic tool by selectively silencing the expression of any gene of choice with single nucleotide specificity (58). As proof of concept, our studies showed that siRNA to green fluorescent protein (GFP) used as a model target tagged to iron nanoparticles, could accumulate in pancreatic islets in quantities sufficient for detection by MRI in vitro and for silencing GFP (59). Two more recent studies by our group have used a dual-purpose therapy/imaging nanoparticle probes to target the apoptotic-related gene caspase-3 (MN-siCaspase-3) and MHC class 1 molecule beta-2 microglobulin (MN-siB2M) in pancreatic islets prior to transplantation (Figures 4,5,6). We suggested that silencing caspase-3 would prevent islet cells from undergoing apoptosis, whereas silencing of B2M would protect islet cells from immune rejection. In both cases, islets were incubated with corresponding probes prior to transplantation. The results showed that these “two-in-one” probes could reduce the expression of the key apoptotic-related gene and MHC class 1 molecule B2M, significantly protecting grafts from damage post transplantation. Simultaneously, iron oxide nanoparticles allowed for monitoring the fate of the grafts noninvasively (60,61) (Figures 4,5). Islets labeled with nanoparticles conjugated to siRNA to caspase-3 showed reduced caspase-3 expression and, as a consequence, allowed for better graft survival (60) (Figure 4). Islets immunoprotected by MN-siB2M nanoparticles showed a significantly delayed onset of hyperglycemia caused by T cell challenge compared to control non-protected islets. As evident from Kaplan-Meier analysis, the mean time for developing diabetes in the control group was 6.5±4.5 days whereas in the experimental group it was delayed up to 23.8±4.8 days (61) (Figure 6). These studies have laid the groundwork for future applications in which genes responsible for islet damage (immunological or non-immunological) can be targeted, either by delivering a cocktail of nanoparticles or nanoparticles decorated with various sets of siRNAs for improving graft outcome. Furthermore, the inherent imaging capabilities of this approach permit the noninvasive tracking of the contrast agent conjugated therapeutic moiety and its relationship to graft fate (62).
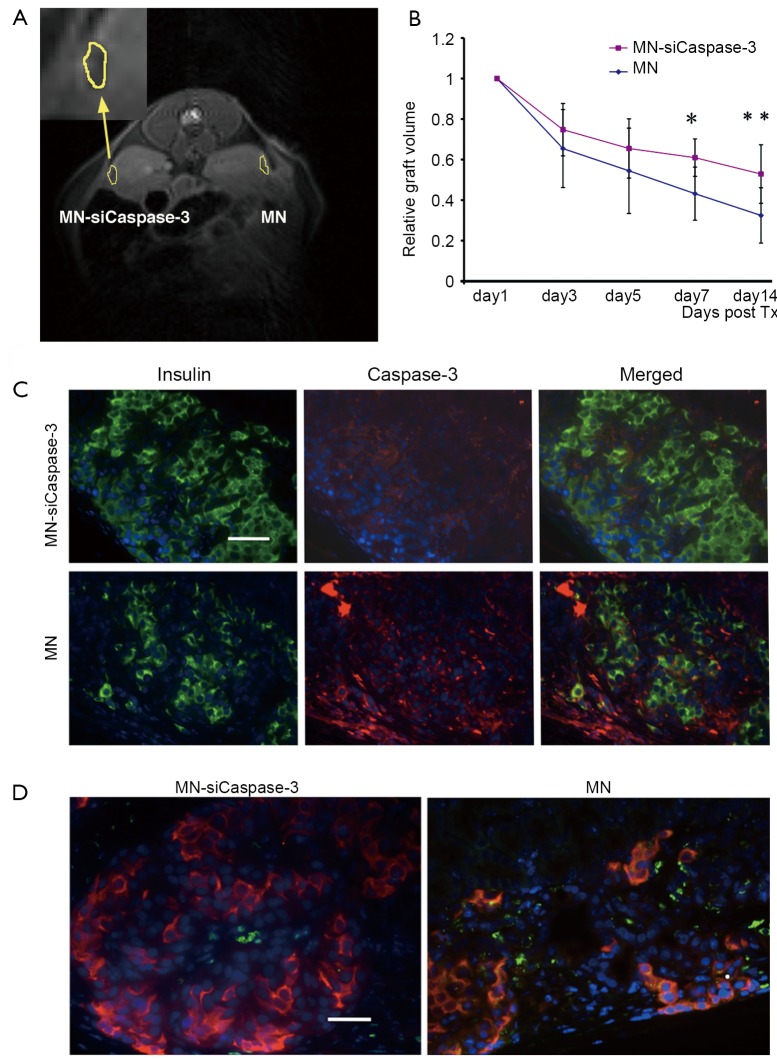
Figure 4.
A. Representative in vivo MRI of islet transplantation showing MN-siCaspase-3-treated islets implanted under the left kidney (inset) and parental MN-treated islets implanted under the right kidney. The dark area outlined under both kidney capsules represents the labeled grafts (day 3 shown); B. Semiquantitative assessment of the relative changes in graft volumes revealed protective effect in MNsiCaspase-3-labeled grafts (*day 7, P<0.05; **day 14, P<0.05); C. Fluorescence microscopy revealed higher expression of insulin and lower expression of caspase-3 inMN-siCaspase-3-treated grafts compared with MN-labeled islet grafts on the 14th day after-transplantation (Tx) (green, insulin; red, cleaved caspase-3; blue, DAPI nuclear stain) (magnification bar =50 mm); D. TUNEL assay on insulin-stained sections confirmed lower apoptotic rate and higher insulin expression in islets treated with MN-siCaspase-3 compared with islets treated with MN on the 14th day post-Tx (red, insulin; green, TUNEL; blue, DAPI nuclear stain) (magnification bar =50 µm). Reproduced with permission from American Diabetes Association (60)
Figure 5.
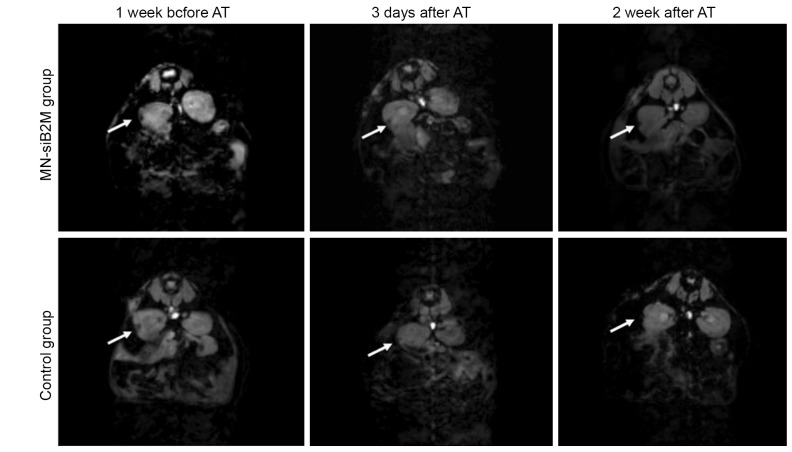
Representative T2* weighted MR images of grafts labeled with MN-siB2M or control probes under the left kidney capsule of NOD (B2M null)/scid mice 1 week before and 3 days and 2 weeks after adoptive transfer (AT) of T cells (white arrows indicate the islet grafts areas). Reproduced with permission from American Diabetes Association (61)
Figure 6.
A. Semiquantitative assessment of relative changes in graft volumes revealed the protective effect of MN-siB2M probe (n=6; P<0.05 starting 2 weeks after adoptive transfer compared with control group); B. Kaplan-Meier curve shows animals transplanted with control (red) and MN-siB2M-treated (black) islets. Development of diabetes was defined as the occurrence of hyperglycemia (blood glucose level >250 mg/dL on two consecutive measurements, n=6; P=0.0006). Reproduced with permission from American Diabetes Association (61)
A different theranostic imaging strategy for transplanted islets utilized islet encapsulation for imaging and concurrent immunoprotection (63,64). Barnett et al. reported the use of Feridex-labeled alginate magnetocapsules for islet monitoring, combined with immunoprotection. Magnetocapsules were functional in vivo because mouse β-cells restored normal glycemia in STZ-induced diabetic mice and human islets induced sustained C-peptide levels in swine (63). Further, MRI provided the ability to monitor distribution and localization of infused pancreatic islets over time in yet another large animal model. This study demonstrated theranostic capabilities of magnetocapsules paving a way to potential clinical application, taking into account that magnetocapsules comprise clinically applicable materials. Later, Barnett et al. developed a technique to include perfluorocarbon emulsions in alginate islet capsules (65). Perfluorocarbon emulsions, developed in the 1980s as oxygen-rich blood substitutes, can be detected by 19F MR imaging and ultrasound. In addition, when a bromine-containing perfluorocarbon is used, such as perfluorooctyl bromide, these emulsions are also visible at CT. The authors termed the perfluorocarbon emulsion-alginate islet capsules fluorocapsules and found that the perfluorocarbons did not alter the permeability of the capsules or affect islet function (66). Arifin et al. (67) introduced a biohybrid theranostic agent, which was used for islets encapsulation. A porous matrix mediated the therapeutic efficacy and inorganic nanoparticles served as trimodal diagnostic markers for islet monitoring. The encapsulated β-cells were transplanted intra-abdominally into mice with STZ-induced diabetes. Noninvasive localization of the grafts was successfully achieved with all three imaging modalities: CT, ultrasonic imaging, and T1-weighted MR imaging. In these diabetic mice, within 1 week after the transplantation, glucose levels returned to normal and remained stable for at least 6 weeks without the need of immunosuppressive drugs. However, long-term effects of co-encapsulated imaging markers on β-cells and on the surrounding host tissue have yet to be evaluated (64).
Challenges and conclusions
Here, we have summarized the evolution of the theranostic MR imaging in T1D during the last decade. Though still in its infancy, it has shown certain encouraging progress. A few fundamental challenges have to be resolved that currently hinder the translation of theranostic MRI imaging of T1D into clinic. One of the concerns is circulation time and the proper dosage of the multifunctional MRI probes that target islet microvasculature. A significant portion of systemically injected probes is rapidly taken up and shuttled out of circulation to the liver and spleen by the reticuloendothelial system (RES) (18). Proper design of these probes is needed to shield recognition and degradation by RES. In addition, therapeutic strategy theoretically requires the higher probe accumulation at the target site for drug release than the amount needed for diagnostic application (18,68). With regard to theranostic imaging of infiltrated autoimmue T cells and endogenous β-cells, the lack of specific two-in-one delivery tools is a significant obstacle in this field. Ideally, such probe should target a specific molecule that is abundant on the surface of targeted cells or, alternatively, be incorporated by them (69). The search for candidates based on the screening of genomic, epigenetic, proteomic and metabolomic libraries should be performed to produce such eligible molecules (70). We believe that close collaboration among biologists, chemists, radiologists, pharmacologists, and physicists would undoubtedly generate dramatic advances in this multidisciplinary field in the coming decade. With the maturation of theranostic imaging, the diagnoses and treatment of T1D will definitely be taken to a new level in the future.
Acknowledgements
This paper describes the work supported in part by NIH Grants R24DK080264, 5RO1DK072137, 5RO1DK078615, and 1RO1DK080784 to A. M.
Authors thank Alana Ross, B.S. (Molecular Imaging Laboratory, MGH/MIT/HMS Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital) for proofreading the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- 1.Lebastchi J, Herold KC. Immunologic and Metabolic Biomarkers of β-Cell Destruction in the Diagnosis of Type 1 Diabetes. Cold Spring Harb Perspect Med 2012;2:a007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaparro RJ, Dilorenzo TP. An update on the use of NOD mice to study autoimmune (Type 1) diabetes. Expert Rev Clin Immunol 2010;6:939-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppieters K, Amirian N, von Herrath M.Intravital imaging of CTLs killing islet cells in diabetic mice. J Clin Invest 2012;122:119-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCall M, James Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med 2012;2:a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol 2005;77:587-97 [DOI] [PubMed] [Google Scholar]
- 6.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol 2004;4:259-68 [DOI] [PubMed] [Google Scholar]
- 7.Rickels MR, Kamoun M, Kearns J, et al. Evidence for allograft rejection in an islet transplant recipient and effect on beta-cell secretory capacity. J Clin Endocrinol Metab 2007;92:2410-4 [DOI] [PubMed] [Google Scholar]
- 8.Campbell PM, Salam A, Ryan EA, et al. Pretransplant HLA antibodies are associated with reduced graft survival after clinical islet transplantation. Am J Transplant 2007;7:1242-8 [DOI] [PubMed] [Google Scholar]
- 9.Worcester Human Islet Transplantation Group Autoimmunity after islet-cell allotransplantation. N Engl J Med 2006;355:1397-9 [DOI] [PubMed] [Google Scholar]
- 10.Menger MD, Wolf B, Höbel R, et al. Microvascular phenomena during pancreatic islet graft rejection. Langenbecks Arch Chir 1991;376:214-21 [DOI] [PubMed] [Google Scholar]
- 11.Gudemez E, Turegun M, Zins J, et al. Microvascular permeability following composite tissue transplantation. Ann Plast Surg 1998;41:519-29 [DOI] [PubMed] [Google Scholar]
- 12.Medarova Z, Moore A.MRI in diabetes: first results. AJR Am J Roentgenol 2009;193:295-303 [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Medarova Z, Moore A. Molecular imaging: a promising tool to monitor islet transplantation. J Transplant 2011;2011:202915. [DOI] [PMC free article] [PubMed]
- 14.Leoni L, Roman BB. MR imaging of pancreatic islets: tracking isolation, transplantation and function. Curr Pharm Des 2010;16:1582-94 [DOI] [PubMed] [Google Scholar]
- 15.Borot S, Crowe LA, Toso C, et al. Noninvasive imaging techniques in islet transplantation. Curr Diab Rep 2011;11:375-83 [DOI] [PubMed] [Google Scholar]
- 16.Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug Chem 2011;22:1879-903 [DOI] [PubMed] [Google Scholar]
- 17.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev 2010;62:1052-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jokerst JV, Gambhir SS. Molecular imaging with theranostic nanoparticles. Acc Chem Res 2011;44:1050-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Zhang N.Gadolinium loaded nanoparticles in theranostic magnetic resonance imaging. Biomaterials 2012;33:5363-75 [DOI] [PubMed] [Google Scholar]
- 20.Ma X, Zhao Y, Liang XJ. Theranostic nanoparticles engineered for clinic and pharmaceutics. Acc Chem Res 2011;44:1114-22 [DOI] [PubMed] [Google Scholar]
- 21.Carlsson PO, Flodström M, Sandler S. Islet blood flow in multiple low dose streptozotocin-treated wild-type and inducible nitric oxide synthase-deficient mice. Endocrinology 2000;141:2752-7 [DOI] [PubMed] [Google Scholar]
- 22.Papaccio G.Insulitis and islet microvasculature in type 1 diabetes. Histol Histopathol 1993;8:751-9 [PubMed] [Google Scholar]
- 23.Medarova Z, Castillo G, Dai G, et al. Noninvasive magnetic resonance imaging of microvascular changes in type 1 diabetes. Diabetes 2007;56:2677-82 [DOI] [PubMed] [Google Scholar]
- 24.Bogdanov AA, Jr, Mazzanti M, Castillo G, et al. Protected Graft Copolymer (PGC) in Imaging and Therapy: A Platform for the Delivery of Covalently and Non-Covalently Bound Drugs. Theranostics 2012;2:553-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moedes J, Serreze D, Greiner D, et al. Animal modles of autoimmune diabetes mellitus. In: LeRoith D, Taylor S, Olefsky J. eds. Diabetes Mellitus: A Fundamental and Clinical Text. Philadelphia: Lippincott Williams & Wilkins, 2004:591-610. [Google Scholar]
- 26.Moedes JP, Poussier P, Rossini A, et al. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. In: Shafrir E. eds. Animal models of Diabetes: Frontiers in Research. Boca Raton: CRC Press Taylor & Francis Group, 2007:1-39. [Google Scholar]
- 27.Medarova Z, Greiner DL, Ifediba M, et al. Imaging the pancreatic vasculature in diabetes models. Diabetes Metab Res Rev 2011;27:767-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castillo GM, Reichstetter S, Bolotin EM. Extending residence time and stability of peptides by protected graft copolymer (PGC) excipient: GLP-1 example. Pharm Res 2012;29:306-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev 2005;21:91-117 [DOI] [PubMed] [Google Scholar]
- 30.Vilsbøll T.The effects of glucagon-like peptide-1 on the beta cell. Diabetes Obes Metab 2009;11:11-8 [DOI] [PubMed] [Google Scholar]
- 31.Kielgast U, Holst JJ, Madsbad S. Treatment of type 1 diabetic patients with glucagon-like peptide-1 (GLP-1) and GLP-1R agonists. Curr Diabetes Rev 2009;5:266-75 [DOI] [PubMed] [Google Scholar]
- 32.Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual β-cell function. Diabetes 2011;60:1599-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Issa CM, Azar ST. Possible Role of GLP-1 and Its Agonists in the Treatment of Type 1 Diabetes Mellitus. Curr Diab Rep 2012;12:560-7 [DOI] [PubMed] [Google Scholar]
- 34.Ahrén B, Schmitz O.GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res 2004;36:867-76 [DOI] [PubMed] [Google Scholar]
- 35.Phillips JM, Parish NM, Raine T, et al. Type 1 diabetes development requires both CD4+ and CD8+ T cells and can be reversed by non-depleting antibodies targeting both T cell populations. Rev Diabet Stud 2009;6:97-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai S, Shameli A, Santamaria P.CD8+ T cells in type 1 diabetes. Adv Immunol 2008;100:79-124 [DOI] [PubMed] [Google Scholar]
- 37.Moore A, Sun PZ, Cory D, et al. MRI of insulitis in autoimmune diabetes. Magn Reson Med 2002;47:751-8 [DOI] [PubMed] [Google Scholar]
- 38.Billotey C, Aspord C, Beuf O, et al. T-cell homing to the pancreas in autoimmune mouse models of diabetes: in vivo MR imaging. Radiology 2005;236:579-87 [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Santamaria P.Lessons on autoimmune diabetes from animal models. Clin Sci (Lond) 2006;110:627-39 [DOI] [PubMed] [Google Scholar]
- 40.Amrani A, Verdaguer J, Serra P, et al. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 2000;406:739-42 [DOI] [PubMed] [Google Scholar]
- 41.Amrani A, Serra P, Yamanouchi J, et al. Expansion of the antigenic repertoire of a single T cell receptor upon T cell activation. J Immunol 2001;167:655-66 [DOI] [PubMed] [Google Scholar]
- 42.Moore A, Grimm J, Han B, et al. Tracking the recruitment of diabetogenic CD8+ T-cells to the pancreas in real time. Diabetes 2004;53:1459-66 [DOI] [PubMed] [Google Scholar]
- 43.Medarova Z, Tsai S, Evgenov N, et al. In vivo imaging of a diabetogenic CD8+ T cell response during type 1 diabetes progression. Magn Reson Med 2008;59:712-20 [DOI] [PubMed] [Google Scholar]
- 44.Lee M, Liu S, Lee W, et al. In Vivo Monitoring of Inflammation and Regulation in Type 1 Diabetes. In: Vagner D. eds. Type 1 Diabetes - Pathogenesis, Genetics and Immunotherapy. InTech, 2011: 253-68. [Google Scholar]
- 45.Tsai S, Shameli A, Yamanouchi J, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 2010;32:568-80 [DOI] [PubMed] [Google Scholar]
- 46.Srinivas M, Morel PA, Ernst LA, et al. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med 2007;58:725-34 [DOI] [PubMed] [Google Scholar]
- 47.Medarova Z, Moore A.MRI as a tool to monitor islet transplantation. Nat Rev Endocrinol 2009;5:444-52 [DOI] [PubMed] [Google Scholar]
- 48.Arifin DR, Bulte JW. Imaging of pancreatic islet cells. Diabetes Metab Res Rev 2011;27:761-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evgenov NV, Medarova Z, Dai G, et al. In vivo imaging of islet transplantation. Nat Med 2006;12:144-8 [DOI] [PubMed] [Google Scholar]
- 50.Tai JH, Foster P, Rosales A, et al. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes 2006;55:2931-8 [DOI] [PubMed] [Google Scholar]
- 51.Biancone L, Crich SG, Cantaluppi V, et al. Magnetic resonance imaging of gadolinium-labeled pancreatic islets for experimental transplantation. NMR Biomed 2007;20:40-8 [DOI] [PubMed] [Google Scholar]
- 52.Shen T, Weissleder R, Papisov M, et al. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn Reson Med 1993;29:599-604 [DOI] [PubMed] [Google Scholar]
- 53.Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging 1995;13:661-74 [DOI] [PubMed] [Google Scholar]
- 54.Doane TL, Burda C. The unique role of nanoparticles in nanomedicine: imaging, drug delivery and therapy. Chem Soc Rev 2012;41:2885-911 [DOI] [PubMed] [Google Scholar]
- 55.Medarova Z, Evgenov NV, Dai G, et al. In vivo multimodal imaging of transplanted pancreatic islets. Nat Protoc 2006;1:429-35 [DOI] [PubMed] [Google Scholar]
- 56.Evgenov NV, Medarova Z, Pratt J, et al. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes 2006;55:2419-28 [DOI] [PubMed] [Google Scholar]
- 57.Medarova Z, Vallabhajosyula P, Tena A, et al. In vivo imaging of autologous islet grafts in the liver and under the kidney capsule in non-human primates. Transplantation 2009;87:1659-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 2007;59:75-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medarova Z, Kumar M, Ng SW, et al. Multifunctional magnetic nanocarriers for image-tagged SiRNA delivery to intact pancreatic islets. Transplantation 2008;86:1170-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang P, Yigit MV, Medarova Z, et al. Combined small interfering RNA therapy and in vivo magnetic resonance imaging in islet transplantation. Diabetes 2011;60:565-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang P, Yigit MV, Ran C, et al. A theranostic siRNA nanoprobe protects pancreatic islet grafts from adoptively transferred immune rejection. Diabetes. 2012 doi: 10.2337/db12-0441. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gotthardt M.A therapeutic insight in beta-cell imaging? Diabetes 2011;60:381-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnett BP, Arepally A, Karmarkar PV, et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med 2007;13:986-91 [DOI] [PubMed] [Google Scholar]
- 64.Kiessling FM. Science to practice: are theranostic agents with encapsulated cells the key for diabetes therapy? Radiology 2011;260:613-5 [DOI] [PubMed] [Google Scholar]
- 65.Barnett BP, Ruiz-Cabello J, Hota P, et al. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology 2011;258:182-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cormode DP, Mulder WJ, Fayad ZA. Science to practice: versatile method to track transplanted encapsulated islet cells with multiple imaging modalities. Radiology 2011;258:1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arifin DR, Long CM, Gilad AA, et al. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology 2011;260:790-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caldorera-Moore ME, Liechty WB, Peppas NA. Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Acc Chem Res 2011;44:1061-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andralojc K, Srinivas M, Brom M, et al. Obstacles on the way to the clinical visualisation of beta cells: looking for the Aeneas of molecular imaging to navigate between Scylla and Charybdis. Diabetologia 2012;55:1247-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bouckenooghe T, Flamez D, Ortis F, et al. Identification of new pancreatic beta cell targets for in vivo imaging by a systems biology approach. Curr Pharm Des 2010;16:1609-18 [DOI] [PubMed] [Google Scholar]