Abstract
Numerous papers on heparin nanoparticles have been reported regarding targeting therapy and biomedical imaging. Here, we have summarized the prospects and opportunities of heparin as a carrier for cancer targeting and imaging. First, we proposed heparin-anticancer drug conjugates showing higher anticancer activity than free drug. The conjugated heparin (heparin-deoxycholate sodium) retained its ability to bind with angiogenic factors, showing a significant decrease in endothelial tubular formation. Second, targeting ligands conjugated heparin derivatives have introduced for a receptor mediated delivery of anticancer drug. Heparin-folic acid-retinoic acid (HFR) bioconjugates for treating cancer cells showed 3 fold higher efficacy than heparin-retinoic acid (HR). Besides active and passive targeting drug delivery, several papers have been reported regarding delivery of imaging agents by heparin nanoparticles. Finally, this research highlight has covered imaging agents such as gold nanoparticles and quantum dots (QDs) for noninvasive biomedical imaging. Very recently our group demonstrated that semiconductor QDs loaded heparin nanoparticles could also be administered through orally for noninvasive imaging. Due to promising features of heparin such as less toxic polysaccharide and easier modification, it was considered as a potent carrier for imaging agent and drug delivery.
Key Words: Heparin, nanoparticles, cancer, targeting, noninvasive imaging
Introduction
The current researches on drug delivery are developing multifunctional nanoparticles for cancer targeting and imaging. Biocompatible and functionalized nanoparticles have been shown to target tumors and produce optical, magnetic, and radioactive signals for enhancing sensitivity and specificity of noninvasive tumor imaging. The unique features of nanoparticles that make them suitable for receptor-targeted imaging include having a prolonged blood retention time (1-3). The physical and chemical moieties of heparin provide reactive functional groups and a large surface area for loading large numbers or multiple types of tumor-targeting ligands.
Heparin nanoparticles can be used as a remarkably strong optical imaging agent carrier and versatile surface chemistry for surface functionalization and introduction of biomolecules. Heparin itself is a safe biomaterials and widely using as a therapeutic agent (4). Heparin derivatives are also considered to deliver optical imaging agent such as iron oxide nanoparticles to detect human cancer cells (5). By the last two decades various imaging modalities have been developed regarding diagnosis of cancer cell based on photoluminescence or optical imaging strategies. Among the imaging modalities magnetic resonance imaging (MRI), optical imaging, single photon emission computed tomography (SPECT), and positron emission tomography (PET) are widely used in biomedical field (3-6). Recently Hwang et al. demonstrated the features of heparin for delivery of super paramagnetic iron oxide nanoparticles (SPIONs) as a MRI imaging agent (7). In this research highlight we have been focused on optical imaging modalities as well as cancer targeted therapy using heparin based nanoparticles.
Optical imaging modalities are proved as a safer technique compared to that of ultrasound and other medical imaging technique like X-ray (8). Beside organic dyes, QDs (9) and gold nanoparticles have been developed and reported as promising candidates for noninvasive imaging. These inorganic nanoparticles have been used as a real time imaging agent for organ, tissue, single cell and early detection of cancer cells (10). Even deep tissues like heart, kidney, lung and spleen could be noninvasively detected by near-infrared (near IR) QDs (11,12).
The selection of polymer as a carrier is very important issue to deliver those optical imaging candidate into cells or body. Poly (ethylene glycol) (PEG) (2), poly (lactic-co-glycolic acid) (PLGA) (3), poly acrylic acid (PAA) (13), polysaccharide (14) and poly (ε-caprolactone) (15) along with other biocompatible polymers are used as a carrier molecule of imaging agent. Those biopolymers play an important role in coating the nanoparticles for increased solubility in water. Recently, our group has been used heparin as a carrier to improve biocompatibility and enhance efficacy (16-18).
Heparin derivatives for cancer targeting and therapy
Heparin shows anticancer activity through anti-angiogenesis process. A study conducted by our group reported that heparin derivatives could potentially inhibit the growth of tumor (19). These studies show that the anticancer activity is directly proportional to the number of deoxycholate (DOC) coupled with heparin. Larger antitumor effects of the DOC-heparin (8.5 mol of DOC coupled with 1.0 mol of heparin) were achieved in animal studies, compared to those of heparin alone. That report also confirmed that the conjugated heparin retained its ability to inhibit binding with angiogenic factors, showing a significant decrease in endothelial tubular formation (Figure 1A). Figure 1B shows that heparin itself is an anticancer agent and the efficacy was increased by 3 folds while conjugated with DOC. The result demonstrated that the conjugation of hydrophobic DOC with hydrophilic heparin facilitates the formation of self-assembly nanoparticles in aqueous solution, resulting in tumor uptake. It was increased through EPR effect. The heparin-DOC nanoparticles do not express any significant toxicity in mice as the body weight of each mouse was gradually increased simultaneously like control mice. Park et al. continued their work to investigate the potential of these nanoparticles as drug carriers for cancer therapy (20). Doxorubicin (DOX), a widely used chemotherapy drug, was entrapped into the amphiphilic heparin-DOC nanoparticles by hydrophobic interaction with the DOC moiety of the particles. These particles were tested for their cytotoxicity in mouse models, where no significant or unexpected side effects found, while free DOX caused a significant loss of weight in the models. DOX-loaded nanoparticles also significantly inhibited proliferation of squamous cell carcinoma and human umbilical vascular endothelial cell, with a ~60% and ~70% inhibition, respectively, compared with those of free heparin.
Figure 1.
Inhibition of tubular formation by heparin and DOC-heparin conjugates at different concentrations (A). Shrinkage in tumor volume (B) and changes in body weight (C) after treatment with saline (□), 10 mg/kg heparin (●), 5 mg/kg of DOC-heparin VI (▼), and 10 mg/kg of DOC-heparin VI (Δ), respectively. All data represent mean ± s.e. Reprinted (adapted) with permission from [Bioconjugate chem., 2008;19. Copyright (2008) with permission from American Chemical Society]
Development of heparin nanoparticles
Among biopolymers, the water-soluble natural polysaccharide heparin has attracted intense attention because it demonstrates a variety of biological activities including anticoagulant activity, inhibition of angiogenesis, tumor development, and proliferation of arterial smooth muscle cells. Chemical modifications of heparin with deoxycholate and lithocholate with reduced anticoagulant activity have demonstrated a great potential as anti-tumor drugs and drug carriers. Heparin forms self-assembled micelles upon conjugation with hydrophobic polymer or moieties like deoxycholic acid (DOCA) (17), retinoic acid (RA) (21), photodynamic therapeutic agent (PDTA) (22), poly(b-benzyl-L-aspartate (PBLA) (23), poly(L-lysine) (24) and grapheme (25) and the size of the micelles could be optimized by controlling the coupling ratio of that hydrophobic moiety. Recently, research has begun to combine the useful biological activities of heparin with the useful properties of nanomaterials. The combination of the two substances can provide synergistic improvements enhancing already existing properties and applications, and also create novel uses for these composites. This review focuses on studies on heparin based nanoparticles and the potential therapeutic applications as well as optical imaging.
Loading of QDs in heparin nanoparticles
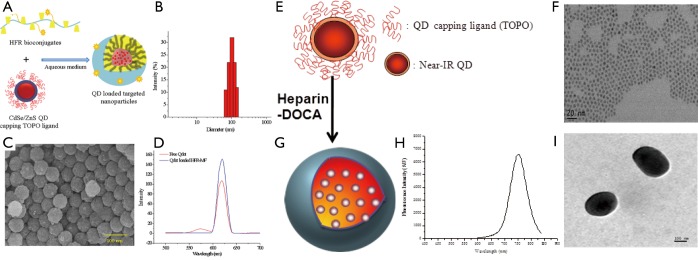
The use of QDs in biology is one of the fastest moving and most exciting interfaces of nanotechnology and nanomedicine. The unique optical properties of QDs make them appealing as in vivo and in vitro fluorophores in a variety of biological investigations, in which traditional fluorescent labels fall short of providing long-term stability and simultaneous detection of multiple signals. Various coating materials have been used to make QDs soluble in water and to target the specific cell for noninvasive optical imaging. Tran et al. demonstrated that QDs has been loaded into the heparin-folic acid-retinoic acid (HFR) bioconjugates for imaging cancer cells in vitro (26). The conjugation amount of folic acid and retinoic acid in HFR bioconjugates was increased through microfluidic approach. HFR conjugates form self-assembly micelle in aqueous solution; the particle size was less than 100nm in diameter confirmed by DLS and SEM (Figure 2A,B,C,D). They explained that the QDs-loaded HFR nanoparticles could be accumulated by cancer cell and confirmed by confocal laser scanning microscope (CLSM). Zehedina et al. (27) reported that hydrophobic QDs loaded into the heparin-DOCA micelle and formed nano-size particle ranged 120-150 nm in diameter confirmed by dynamic light scattering (DLS) and transmittance electron microscopy (TEM) (Figure 2E,F,G,H,I). The release profile of QDs from the heparin-DOCA nanoparticles showed that the release amount of QDs was very low at different buffer solution, which may prevent the possible toxicity, exert from the QDs. The QDs-loaded heparin-DOCA nanoparticles also tested for stability observation in different conditions like pH buffer and 10% FBS solution showed that the fluorescence intensity does not decreased much. The QDs-loaded heparin nanopartciles has been applied for both therapy and diagnosis of cancer cell.
Figure 2.
Schematic figures (A), hydrodynamic size (B), SEM image (C) and fluorescence spectrum (D) of QDs-loaded heparin-rolic acid-retinoic acid conjugates. Reproduce by permission of The Royal Society of Chemistry. Schematic representation of QDs (E) TEM images of QDs (F) QDs-loaded heparin-DOCA nanoparticle (G) fluorescence intensity (h) and TEM images
Oral delivery of optical imaging agent
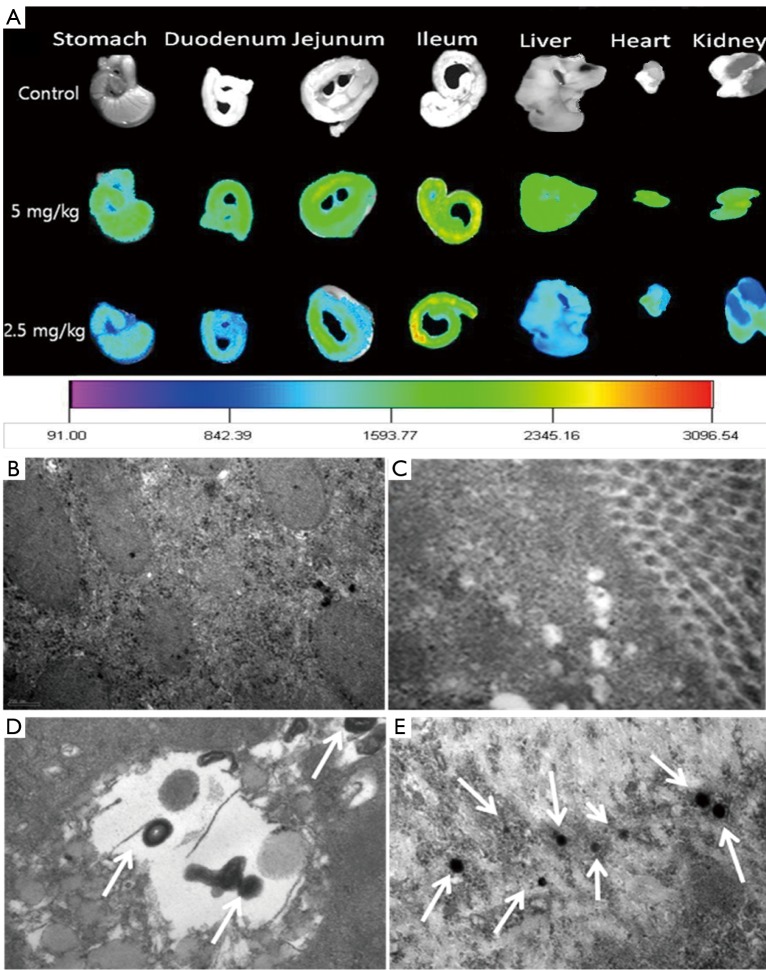
The oral delivery becomes a very interesting alternative to the intravenous injection therapy. Our research group has developed heparin-bile acids conjugates for oral devliery of heparin. From the result, we confirmed that heparin derivatives are absorbed through bile acid transporter in the small intestine. In addition, we used a organic dye to find absorption site of heparin-bile acids conjugates in small intestine. However, it was hard to show a precise evidence for absorption mechanism due to degradation or loosing fluorescent intensity of organic dye in vivo. Nowdays the use of dyes for diagnosis is replacing by semiconductor quantum dots due to existing its high fluorescence and stability for long time in appropriate condition. Embedding biocompatible QDs into heparin nanoparticles is an effective method of enhancing the functions of materials. From the results of real time imaging, we found that QDs in heparin nanoparticles were distributed in the liver, lung and spleen of normal mice after oral administration (Figure 3A). QDs loaded heparin nanoparticles absorbed mainly through duodenum, jejunum and ileum of small intestine. The transmittance electron microscope (TEM) images of the stomach, duodenum, jejunum and ileum confirmed that the maximum amount of QDs loaded heparin nanoparticles absorbed through the ileum part of small intestine as the quantity of bile acid transporters are higher at that portion (Figure 3B). A competitive oral absorption profile of heparin was also observed by QD-loaded heparin nanoparticles (28). The study demonstrated the optical imaging agent such as QDs may be used to observe the drug absorption, bio-distribution and elimination study. The QD loaded heparin-bile acid nanoparticles introduce a novel strategy for oral delivery of optical imaging agent for a long-term observation.
Figure 3.
Biodistribution of QDs-loaded heparin nanoparticles. TEM images show maximum amount of QDs-loaded nanoparticle absorbed through ileum (A,E) compared to that of stomach (B), duodenum (C) and jejunum (D). Reprinted from Carbohydrate polymers, 10.1016/j.carbpol.2012.07.016, Khatun Z. et al., Imaging of the GI Tract by QDs Loaded Heparin-Deoxycholic Acid (DOCA) Nanoparticles. Copyright (2012) with permission from Elsevier
Gold loaded heparin nanoparticles for optical imaging
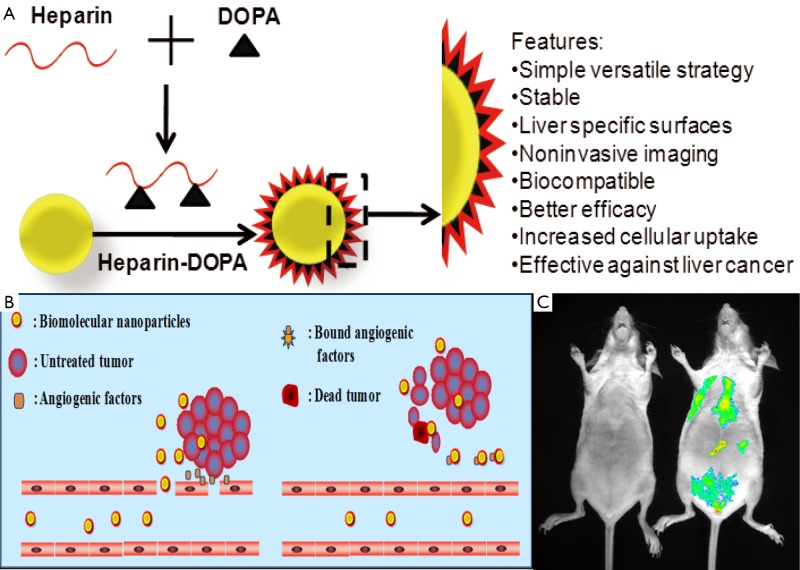
Gold nanoparticles (AuNPs) is the another promising optical imaging candidate to detect the cancer cell as well as tumor tissue in both in vitro and in vivo conditions. Non-toxicity of AuNPs is the main feature as an imaging candidate along with other advantages like biocompatibility and easy surface modification. Gua et al. demonstrated that the AuNPs showed negative charges in the aqueous solution and the size and shape of the AuNPs could be controlled by changing the concentration of the heparin. Moreover, the gold nanoparticles obtained with relatively high concentration of heparin were very stable and had relative narrow size distribution (29). Richard M. Pino has reported that heparin coated gold nanoparticles could be used to mark the diaphragm-fenestrated endothelium of the rat choriocapillaris (30). Sun et al., has demonstrated that AuNPs has been loaded into the heparin-3, 4-dihydroxyphenylalanine (DOPA) conjugated nanoparticle and showed very promising outcome for noninvasive optical imaging of liver (31). The authors explained that heparin enhanced the biocompatibility of AuNPs as well as career molecule of the drugs (Figure 4). The heparin-coated AuNPs showed enhanced liver-specific CT images in vivo. The outstanding properties of this platform system highlight its potential as a novel liver-specific CT imaging agent and a molecular imaging probe for assessment of the therapeutic effect of anticancer drugs against liver cancer by a noninvasive method.
Figure 4.
Heparin-based gold nanoparticles for biomedical imaging. Schematic presentations show the formation of nanoparticles (A) and the mechanism of tumor uptake through porous and leaky blood vessels (B). The in vivo imaging represents gold nanoparticles in heparin nanoparticles (C)
Conclusions
We have developed several kinds of highly fluorescent, extremely photostable, biodegradable QD-encapsulated heparin nanoparticles. The proposed QD-heparin nanoparticles can be promising candidates as a carrier molecule for anticancer drug and optical imaging agent by chemical conjugation or physical loading for in vivo imaging. It also would provide a novel way for the diagnosis and curative effect observation of cancer cells.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021427).
Disclosure: The authors declare no conflict of interest.
References
- 1.Blanco E, Hsiao A, Mann AP, et al. Nanomedicine in cancer therapy: innovative trends and prospects. Cancer Sci 2011;102:1247-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nurunnabi M, Cho KJ, Choi JS, et al. Targeted near-IR QDs-loaded micelles for cancer therapy and imaging. Biomaterials 2010;31:5436-44 [DOI] [PubMed] [Google Scholar]
- 3.Kim JS, Cho KJ, Tran TH, et al. In vivo NIR imaging with CdTe/CdSe quantum dots entrapped in PLGA nanospheres. J Colloid Interface Sci 2011;353:363-71 [DOI] [PubMed] [Google Scholar]
- 4.Zhao F, Ma ML, Xu B. Molecular hydrogels of therapeutic agents. Chem Soc Rev 2009;38:883-91 [DOI] [PubMed] [Google Scholar]
- 5.Min KA, Yu F, Yang VC, et al. Transcellular transport of heparin-coated magnetic iron oxide nanoparticles (Hep-MION) under the influence of an applied magnetic field. Pharmaceutics 2010;2:119-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwenzer NF, Stegger L, Bisdas S, et al. Simultaneous PET/MR imaging in a human brain PET/MR system in 50 patients-Current state of image quality. Eur J Radiol. 2012 doi: 10.1016/j.ejrad.2011.12.027. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Hwang YH, Lee DY. Magnetic resonance imaging using heparin-coated superparamagnetic iron oxide nanoparticles for cell tracking in vivo. Quant Imaging Med Surg 2012;2:118-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YK, Hong SM, Kim JS, et al. Encapsulation of CdSe/ZnS quantum dots in poly(ethylene glycol)-poly(D,L-lactide) micelle for biomedical imaging and detection. Macromol Res 2007;15:330-36 [Google Scholar]
- 9.Quek CH, Leong KW. Near-infrared fluorescent nanoprobes for in vivo optical imaging. Nanomaterials 2012;2:92-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cha EJ, Sun IC, Lee SC, et al. Development of a pH sensitive nanocarrier using calcium phosphate coated gold nanoparticles as a platform for a potential theranostic material. Macromol Res 2012;20:319-26 [Google Scholar]
- 11.Li JM, Wang YY, Zhao MX, et al. Multifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time tracking. Biomaterials 2012;33:2780-90 [DOI] [PubMed] [Google Scholar]
- 12.Peng CW, Liu XL, Chen C, et al. Patterns of cancer invasion revealed by QDs-based quantitative multiplexed imaging of tumor microenvironment. Biomaterials 2011;32:2907-17 [DOI] [PubMed] [Google Scholar]
- 13.Lee DK, Lee YK. Preparation of near-infrared quantum dots-herceptin conjugates for cancer imaging. Macromol Res 2010;18:641-47 [Google Scholar]
- 14.Mattoussi H, Palui G, Na HB. Luminescent quantum dots as platforms for probing in vitro and in vivo biological processes. Adv Drug Deliv Rev 2012;64:138-66 [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Nurunnabi M, Oh YJ, et al. Herceptin conjugated PCL-PEG-PCL triblock copolymer for cancer targeting and imaging. Macromol Res 2012;20:875-82 [Google Scholar]
- 16.Park IK, Tran TH, Oh IH, et al. Ternary biomolecular nanoparticles for targeting of cancer cells and anti-angiogenesis. Eur J Pharm Sci 2010;41:148-55 [DOI] [PubMed] [Google Scholar]
- 17.Park IK, Kim YJ, Tran TH, et al. Water-soluble heparin–PTX conjugates for cancer targeting. Polymer 2010;51:3387-93 [Google Scholar]
- 18.Kim SK, Lee DY, Lee E, et al. Absorption study of deoxycholic acid-heparin conjugate as a new form of oral anti-coagulant. J Control Release 2007;120:4-10 [DOI] [PubMed] [Google Scholar]
- 19.Cho KJ, Moon HT, Park GE, et al. Preparation of sodium deoxycholate (DOC) conjugated heparin derivatives for inhibition of angiogenesis and cancer cell growth. Bioconjug Chem 2008;19:1346-51 [DOI] [PubMed] [Google Scholar]
- 20.Park K, Lee GY, Kim YS, et al. Heparin-deoxycholic acid chemical conjugate as an anticancer drug carrier and its antitumor activity. J Control Release 2006;114:300-6 [DOI] [PubMed] [Google Scholar]
- 21.Oh Ih, Cho KJ, Tran TH, et al. Biofuntional nanoparticle formation and folate-targeted antitumor effect of heparin-retinoic acid conjugates. Macromol Res 2012;20:520-27. [Google Scholar]
- 22.Li L, Moon HT, Park JG, et al. Heparin-based self-assembled nanoparticles for photodynamic therapy. Macromol Res 2011;19:487-94 [Google Scholar]
- 23.Li L, Huh KM, Lee YK, et al. Biofunctional self-assembled nanoparticles of folate-PEG-heparin/PBLA copolymers for targeted delivery of doxorubicin. J Mater Chem 2011;21:15288-97 [Google Scholar]
- 24.Na K, Kim S, Park K, et al. Heparin/poly(l-lysine) nanoparticle-coated polymeric microspheres for stem-cell therapy. J Am Chem Soc 2007;129:5788-9 [DOI] [PubMed] [Google Scholar]
- 25.Lee da Y, Khatun Z, Lee JH, et al. Blood compatible graphene/heparin conjugate through noncovalent chemistry. Biomacromolecules 2011;12:336-41 [DOI] [PubMed] [Google Scholar]
- 26.Tran TH, Nguyen CT, Kim DP, et al. Microfluidic approach for highly efficient synthesis of heparin-based bioconjugates for drug delivery. Lab Chip 2012;12:589-94 [DOI] [PubMed] [Google Scholar]
- 27.Khatun Z, Nurunnabi M, Cho KJ, et al. Imaging of the GI tract by QDs loaded heparin-deoxycholic acid (DOCA) nanoparticles. Carbohydr Polym 2012;90:1461-8 [DOI] [PubMed] [Google Scholar]
- 28.Khatun Z, Nurunnabi M, Cho KJ, et al. Oral delivery of near-infrared quantum dot loaded micelles for noninvasive biomedical imaging. ACS Appl Mater Interfaces 2012;4:3880-7 [DOI] [PubMed] [Google Scholar]
- 29.Guo Y, Yan H.Preparation and characterization of heparin - stabilized gold nanoparticles. J Carbohydr Chem 2008;5:309-19 [Google Scholar]
- 30.Pino RM. Binding and endocytosis of heparin-gold conjugates by the fenestrated endothelium of the rat choriocapillaris. Cell Tissue Res 1987;250:257-66 [DOI] [PubMed] [Google Scholar]
- 31.Sun IC, Eun DK, Na JH, et al. Heparin-coated gold nanoparticles for liver-specific CT imaging. Chemistry 2009;15:13341-7 [DOI] [PubMed] [Google Scholar]