Abstract
Background
Exacerbations of chronic obstructive pulmonary disease (COPD) lead to significant increases in resource utilization and cost to the health care system. COPD patients with chronic bronchitis and a history of exacerbations pose an additional burden to the system. This study examined health care utilization and cost among these patients.
Methods
For this retrospective analysis, data were extracted from a large national health plan with a predominantly Medicare population. This study involved patients who were aged 40–89 years, had been enrolled continuously for 24 months or more, had at least two separate insurance claims for COPD with chronic bronchitis (International Classification of Diseases, Ninth Revision, Clinical Modification code 491.xx), and had pharmacy claims for COPD maintenance medications between January 1, 2007, and March 31, 2009. Two years of data were examined for each patient; the index date was defined as the first occurrence of COPD. Baseline characteristics were obtained from the first year of data, with health outcomes tracked in the second year. Severe exacerbation was defined by COPD-related hospitalization or death; moderate exacerbation was defined by oral or parenteral corticosteroid use. Adjusted numbers of exacerbations and COPD-related costs per patient were estimated controlling for demographic and clinical characteristics.
Results
The final study sample involved 8554 patients; mean age was 70.1 ± 8.6 years and 49.8% of the overall population had exacerbation, 13.9% had a severe exacerbation only, 29.1% had a moderate exacerbation only, and 6.8% had both a severe and moderate exacerbation. COPD-related mean annual costs were $4069 (all figures given in US dollars) for the overall population and $6381 for patients with two or more exacerbations. All-cause health care costs were $18,976 for the overall population and $23,901 for patients with history of two or more exacerbations. Severity of exacerbations, presence of cardiovascular disease, diabetes, and long-term oxygen use were associated with higher adjusted costs.
Conclusions
The results indicate that despite treatment with maintenance medications, COPD patients continue to have exacerbations resulting in higher costs. New medications and disease management interventions are warranted to reduce the severity and frequency of exacerbations and the related cost impact of the disease.
Keywords: COPD with chronic bronchitis, moderate exacerbation, severe exacerbation, Medicare patients
Introduction
Chronic obstructive pulmonary disease (COPD) is a major public health problem because of its high incidence and COPD-related morbidity and mortality.1 Chronic lower respiratory diseases are the third-leading cause of death in the United States and have both a significant health impact and a significant economic impact on society.2 In fact, a diagnosis of COPD was reported for 5.1% of adults in the United States between 2007 and 2009, and the disease was more prevalent in older age groups and in women more than men.3
Patients with COPD have a progressive condition in which airflow is consistently obstructed, making it difficult for these patients to breathe.4 These patients may experience an acute worsening of respiratory symptoms such as elevated sputum volume, sputum purulence, and dyspnea, defined as a COPD exacerbation, which is associated with poor outcomes in terms of both morbidity and mortality.5 While exacerbations are infrequent in early COPD, they can be life threatening and can occur on average three times a year in patients with moderate to severe COPD.4,6
Exacerbations are associated with disease progression by accelerating reductions in lung function measured by forced expiratory volume in one second (FEV1).5,7 Additionally, the literature suggests that nearly half of all exacerbations remain unreported, which may also have an impact on health status.8 An increase in the number of exacerbations experienced by a patient may have a deleterious effect on the patient’s health-related quality of life1 and can greatly increase the burden of health-related services and economic costs attributable to this disease.9–11
Acute episodes leading to inpatient care incur the greatest medical costs in patients with COPD.10,11 Nationally, the direct cost of COPD in the United States for 2010, as estimated by the National Heart, Lung, and Blood Institute, was $29.5 billion (all figures given in US dollars), with $13.2 billion in hospital care costs, $5.5 billion in physician services, and $5.8 billion in prescription drugs.12 As demonstrated by Dalal et al13 in a study of commercially insured patients with COPD, direct costs associated with increasing severity of exacerbations resulting in hospitalization significantly drove the cost of health care, regardless of whether or not patients were treated in hospital intensive care units.
While early treatment and recognition of exacerbation symptoms have been shown to improve health outcomes, approximately 50% of exacerbations remain unreported and consequently do not receive adequate treatment.8,14,15 Once a treatment regimen has been initiated, management of stable COPD is meant to reduce the number and intensity of exacerbations.12,16 Severely ill patients may receive oxygen therapy, but maintenance medications are generally added as the disease progresses and can include short-acting β2-agonists, long-acting β-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), inhaled glucocorticosteroids (ICSs), phosphodiesterase-4 inhibitor, or theophylline.17
Various studies have demonstrated the effectiveness of these COPD medications and their combinations for reducing exacerbations; however, the results have been mixed.18–20 Few studies have examined the influence of multiple exacerbations, differing treatment regimens, and population disparities in health care cost and utilization. The current study addresses these gaps through examination of the frequency of moderate and severe exacerbations, and examination of associated health care utilization and costs in detail, of patients with a history of exacerbations diagnosed with COPD and chronic bronchitis in a predominantly Medicare population.
Methods
Data source
De-identified health care claims data from a large national health care company were used for this analysis. Data included administrative claims for more than 3 million members from a fully insured commercial health plan and Medicare Advantage Prescription Drug Plan members. The data included medical claims, pharmacy claims, and eligibility files from the health plan database. Patient privacy was maintained by masking identification in accordance with the Health Insurance Portability and Accountability Act of 1996.
Patient population
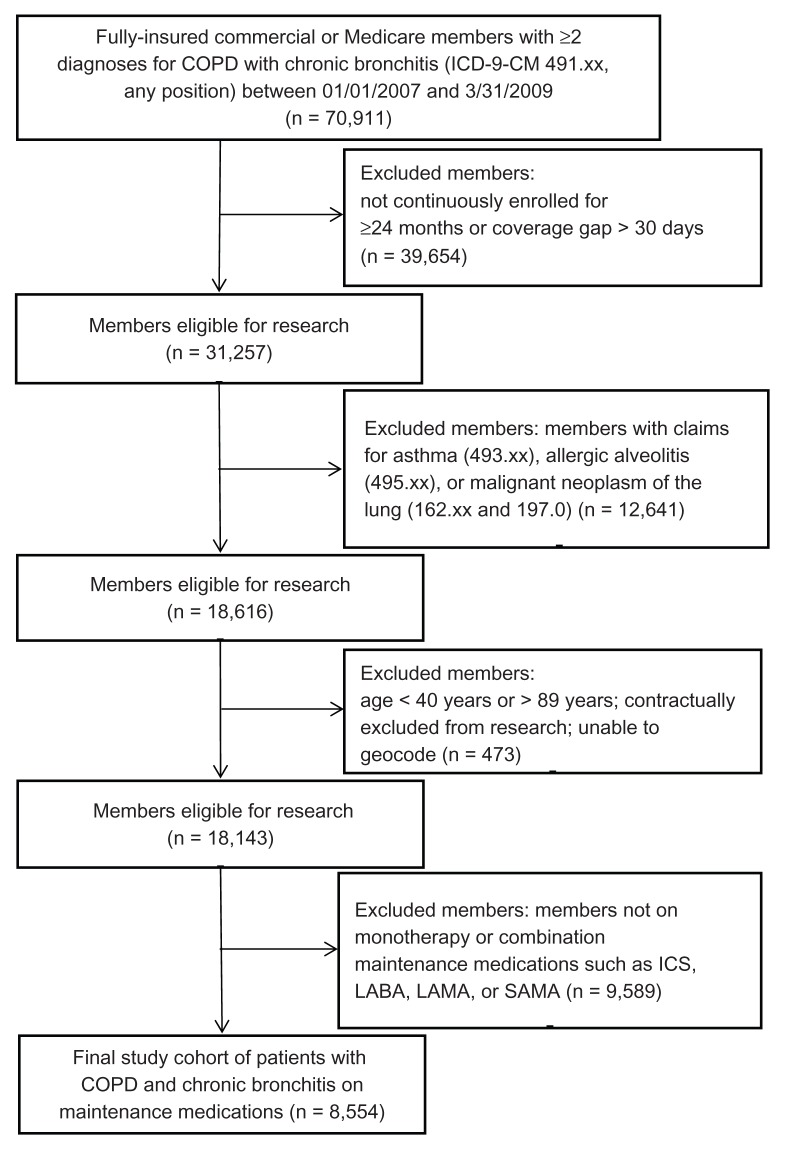
Fully insured patients aged between 40 and 89 years at the index date (defined as the first occurrence of COPD) who were enrolled continuously for 24 months or more, who had at least two separate inpatient or outpatient insurance claims for COPD with chronic bronchitis – as identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code of 491.xx in any diagnosis position – from January 1, 2007, to March 31, 2009, and who had pharmacy claims for COPD maintenance medications were included in the study ( Figure 1). Maintenance medications included LABAs, LAMAs, ICSs, and SAMAs and are listed in Table 1. Patients were excluded if they had medical claims for any malignant neoplasm of the lung (ICD-9-CM codes 162.xx and 197.0), asthma (ICD-9-CM code 493.xx) or allergic alveolitis (ICD-9-CM code 495.xx) during the study time period.
Figure 1.
Selection of study participants: claims data from a large national health plan were used to identify patients diagnosed with chronic obstructive pulmonary disease (COPD) and chronic bronchitis who experienced two or more exacerbations within the study time period.
Note: Exclusion criteria were applied to remove patients with a claims history that did not meet the study requirements.
Abbreviations: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICSs, inhaled glucocorticosteroids; LABAs, long-acting β2-agonists; LAMAs, long-acting muscarinic antagonists; SAMAs, short-acting muscarinic antagonists.
Table 1.
Demographic and clinical characteristics in sample (n = 8554)
| Characteristic | Results |
|---|---|
| Age (years) [mean (SD)] | 70.1 (8.6) |
| Aged 65 years or older [n (%)] | 6759 (79.0) |
| Gender (% female) | 50.2 |
| Race/ethnicity [n (%)] | |
| White | 5806 (68.7) |
| Black | 659 (7.8) |
| Hispanic | 466 (5.5) |
| Other | 1515 (17.9) |
| United States geographic region [n (%)] | |
| Midwest | 2076 (24.3) |
| Northeast | 147 (1.7) |
| South | 5670 (66.5) |
| West | 639 (7.5) |
| Income level [n (%)]a | |
| Low (≤$33,541) | 2317 (27.1) |
| Medium (>$33,541 to <$52,480) | 4195 (49.0) |
| High (≥$52,480) | 2042 (23.9) |
| DCI score [mean (SD)]b | 1.9 (1.8) |
| Top five comorbidities [n (%)] | |
| Related COPD diagnosesc | 1968 (23.0) |
| Diabetes without complication | 1900 (22.2) |
| Congestive heart failure | 1568 (18.3) |
| Peripheral vascular disease | 972 (11.4) |
| Renal disease | 822 (9.6) |
| Maintenance medications (total) [n (%)]d | |
| LABA alone | 360 (4.2) |
| LAMA alone | 3708 (43.4) |
| Any ICS | 8554 (100) |
| ICS + LABA combination | 4472 (52.3) |
| ICS + LABA + LAMA combination | 2130 (24.9) |
| Other respiratory medications (total) [n (%)] | |
| SAMA and combinations | 4611 (53.9) |
| Methylxanthines | 520 (6.1) |
| Oral/IV corticosteroids | 5289 (61.8) |
| Antibiotics | 7203 (84.2) |
| Long-term oxygen use | 3525 (41.2) |
| Mean number of total drugs taken for the year [n (SD)] | 14.65 (6.9) |
Notes:
All figures given in US dollars;
the range for the DCI score was 0–16;
related COPD diagnoses (chronic pulmonary heart disease, COPD and allied conditions other than ICD-9-CM code 491.x, and respiratory conditions due to external agents) are ICD-9-CM codes 416.8, 416.9, 490, 492, 493, 494, 495, 496, 500, 501, 502, 503, 504, 505, 506.4, 508.1, and 508.8;
categories of maintenance medication prescriptions are not mutually exclusive.
Abbreviations: DCI, Deyo-Charlson Comorbidity Index; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICS, inhaled glucocorticosteroid; IV, intravenous; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; SAMA, short-acting muscarinic antagonist; SD, standard deviation.
Study design
Patients were identified by the first occurrence of COPD with chronic bronchitis (ICD-9-CM code 491.xx), which, as mentioned earlier, was defined as the index date. Claims activity for each patient was followed for 2 years after the index date. Year 1 was defined as the 12 months following the index date and was used to gather baseline information and define specific subgroups (patients with one or more exacerbations, those with two or more exacerbations, and those aged 65 years or older); Year 2 (the follow-up year) was defined as the 13–24 months post index and was used to measure outcomes and costs.
The occurrence of severe and moderate exacerbations was determined through claims data and this was used for tracking health care costs and utilization. The definition of a moderate or severe exacerbation was adapted and slightly modified from the method previously described by Yu et al.21 A severe exacerbation was defined by COPD-related hospitalization or death (identified by discharge status of “died” or by ICD-9 -CM code 798) within 7 days of a COPD diagnosis (defined by the following ICD-9-CM codes: 490.xx, 491.xx, 492.xx, 494.1, or 496.xx). A moderate exacerbation was defined by oral or parenteral corticosteroid use on the same day or within 7 days of a COPD-related diagnosis claim (as identified by the same ICD-9-CM codes: 490.xx, 491.xx, 492.xx, 494.1, or 496.xx). Allergic alveolitis (495.xx) was excluded from this study’s definition of COPD exacerbation.
The Deyo-Charlson adaptation of the Charlson Comorbidity Index (DCI) was used to determine the disease burden for each patient.22 The Deyo adaptation includes 17 diseases that were selected and weighted on the basis of the strength of their association with mortality.
One variable indicating use of long-term oxygen therapy was created for “severity” of disease, based on baseline period treatments consistent with the Global Initiative for Chronic Obstructive Lung Disease guidelines17 for severe and very severe patients. Very severe patients are recommended long-term oxygen therapy. This variable was used to control for severity in regression analyses.
Race data were available for Medicare patients in the study sample; however, race assignment for commercial health plan members required the use of geocoding at the census tract level to impute race/ethnicity values.
Statistical analysis
All analyses were conducted using SAS (v 9.3; SAS Institute Inc, Cary, NC) and Enterprise Guide (v 4.3; SAS Institute Inc). P-values ≤ 0.05 were considered to indicate statistically significant differences. A generalized linear model (GLM), with log link and gamma distribution for the error term, was used to estimate adjusted health care costs, controlling for demographic and clinical characteristics. A GLM is an extension of linear regression models that can handle non- normally distributed data with nonconstant variances. 23 A fitted GLM was used to produce an adjusted mean cost estimate for patients with each type of exacerbation. Independent variables in the models included age, gender, race/ethnicity, geographic region, income level, and comorbidities recorded at baseline. Covariates included whether or not a patient experienced a severe and/or a moderate exacerbation (reference group: no exacerbations), and various types of medication treatment.
Results
The final study sample involved 8554 patients. The demographic and clinical characteristics of the COPD population taking maintenance medications as measured in the identification period (Year 1) are reported in Table 1. The mean age of the population was 70.1, with 79% of patients aged 65 years or older. The population was mainly made up of Medicare health plan members (96.5%), and was split evenly between the genders. The sample consisted predominantly of white members residing in the southern United States, with nearly half (49%) of the sample reporting a medium income level. The medium income level was made up of incomes between $33,541 and $52,480 after the sample income was divided into tertiles.
For all patients, the mean DCI score was 1.9 (standard deviation, 1.8; range, 0–16). Among the comorbidities used to calculate the DCI score, the top comorbidity was related COPD diagnoses (23.0%, including chronic pulmonary heart disease, COPD and allied conditions other than 491.x, and respiratory conditions due to external agents). This was closely followed by diabetes without complication and congestive heart failure (22.2% and 18.3%, respectively). Peripheral vascular disease and renal disease were also included in the top five comorbidities (11.4% and 9.6%, respectively).
Among the maintenance medications taken by the patients in this study, 100% of the population had filled one or more prescriptions for ICS, 52.3% for ICS and LABA combination drugs, 43.4% for LAMAs, and 24.9% for triple ICS, LABA, and LAMA combination therapy. These categories were not mutually exclusive – that is, a patient may have been included in more than one category. In addition to COPD maintenance medications, the majority of patients (84.2%) filled one or more prescriptions for antibiotics during the course of the year, 61.8% were treated with oral or parenteral corticosteroids, and 41.2% were receiving long-term oxygen therapy.
The proportion of patients with each type of exacerbation, as well as number of exacerbations during the follow-up year, is provided in Table 2 and is categorized as patients with one or more exacerbations, those with two or more exacerbations, and those aged 65 years or older. Of the overall population on maintenance medications, 49.8% had any type of exacerbation. Within this group, 13.9% had severe exacerbations only, 29.1% had moderate exacerbations only, and 6.8% had both a severe and a moderate exacerbation. The overall population was also stratified into three patient categories: (1) patients with one or more exacerbations during the baseline period, (2) patients with two or more exacerbations during the baseline period, and (3) patients aged 65 years or older. As with maintenance medications, these subgroups were not mutually exclusive. As expected, patients with two or more exacerbations during the baseline period had the highest rate of exacerbations per patient in the follow-up year among the three subgroups, followed by patients with one or more exacerbations (Table 2). The rates of exacerbations per patient per year during the follow-up year for all subgroups were as follows: 1.26 for the subgroup with one or more exacerbations at baseline, 1.77 for the subgroup with two or more exacerbations at baseline, and 0.99 for patients aged 65 years or older. Rates of exacerbations for subgroups with a history of exacerbations at baseline were significantly different from the overall population (P < 0.05, Table 2).
Table 5.
Factors associated with annual cost of exacerbation by type
| Variable | Coefficient estimate | P-value (95% CI) |
|---|---|---|
| Age | −0.0072 | <0.0001 (−0.0098 to −0.0046) |
| Race/ethnicity (versus white) | ||
| Other | 0.0772 | 0.0114 (0.0174–0.1371) |
| Hispanic | 0.0930 | 0.0763 (−0.0098–0.1959) |
| Black | 0.2250 | <0.0001 (0.1372–0.3128) |
| Income (high versus not high)a | 0.0719 | 0.0086 (0.0183–0.1256) |
| Comorbidity | ||
| CVD (versus no CVD) | 0.5265 | <0.0001 (0.4714–0.5816) |
| Diabetes (versus no diabetes) | 0.3765 | <0.0001 (0.3208–0.4323) |
| Maintenance medications | ||
| ICS + LABA | 0.1031 | 0.0005 (0.0452–0.1611) |
| LAMA | 0.0939 | 0.0005 (0.0413–0.1465) |
| LABA | 0.1361 | 0.0085 (0.0347–0.2376) |
| Long-term oxygen use | 0.2164 | <0.0001 (0.1691–0.2636) |
| Severe exacerbation | 0.9925 | <0.0001 (0.9285–1.0566) |
| Moderate exacerbation | 0.1567 | <0.0001 (0.0995–0.2139) |
Note:
High income was defined by income ≥$52,480 (in US dollars) (see Table 1).
Abbreviations: CI, confidence interval of coefficient estimate; CVD, cardiovascular disease; ICS, inhaled glucocorticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
Table 2.
Proportion of patients with exacerbations and number of exacerbations in Year 2 (follow-up period)
| Variable | COPD patients with bronchitis receiving maintenance medications (n = 8554) | One or more exacerbations at baseline (n = 6298) | Two or more exacerbations at baseline (n = 3242) | Aged 65 years or older (n = 6759) |
|---|---|---|---|---|
| Sample with any exacerbation [n (% of column)] | 4259 (49.8) | 3620 (57.5) | 2284 (70.5) | 3277 (48.5) |
| Sample with severe exacerbations only [n (% of column)] | 1188 (13.9) | 993 (15.8) | 602 (18.6) | 946 (14.0) |
| Sample with moderate exacerbations only [n (% of column)] | 2491 (29.1) | 2091 (33.2) | 1275 (39.3) | 1903 (28.2) |
| Sample with both severe and moderate exacerbations [n (% of column)] | 580 (6.8) | 536 (8.5) | 407 (12.6) | 438 (6.5) |
| Exacerbation rate per patient per year [mean (SD)] | ||||
| Rate of any exacerbation | 1.04 (1.51) | 1.26 (1.64)* | 1.77 (1.90)* | 0.99 (1.46) |
| Rate of severe exacerbations | 0.33 (0.84) | 0.40 (0.93)* | 0.56 (1.12)* | 0.32 (0.81) |
| Rate of moderate exacerbations | 0.70 (1.31) | 0.86 (1.44)* | 1.21 (1.74)* | 0.67 (1.26) |
Note:
Significantly different from overall COPD patients with bronchitis on maintenance medications, using the Wilcoxon rank-sum test (P < 0.05).
Abbreviations: COPD, chronic obstructive pulmonary disease; SD, standard deviation.
In line with the observed numbers of filled prescriptions reported in Table 1, annual COPD-related utilization in the overall population reported in Table 3 was dominated by pharmacy claims (91.6%), which were closely followed by physician office visits (75.8%). Approximately one-fifth of the cohort (20.6%) had COPD-related inpatient hospitalizations, and 13.9% had emergency room visits during the study time period. As expected, patients with two or more exacerbations dominated consumption in all utilization categories.
Table 3.
Annual chronic obstructive pulmonary disease (COPD)–related utilization and costs for COPD patients on maintenance medications
| Variable | Overall COPD patients on maintenance medications (n = 8554) | One or more exacerbations at baseline (n = 6298) | Two or more exacerbations at baseline (n = 3242) | Aged 65 years or older (n = 6759) |
|---|---|---|---|---|
| Utilization per patient [mean (SD; % of patients)] | ||||
| Inpatient hospitalizations | 1.2 (4.0; 20.6) | 1.5 (4.4; 24.2) | 2.2 (5.5; 31.0) | 1.1 (3.6; 20.3) |
| ER visits | 0.2 (0.7; 13.9) | 0.3 (0.8; 16.7) | 0.4 (1.0; 22.0) | 0.2 (0.7; 13.2) |
| Physician office visits | 7.9 (10.1; 75.8) | 9.4 (10.8; 80.7) | 12.4 (11.8; 89.0) | 7.6 (9.9; 75.7) |
| Pharmacy claims | 9.7 (7.4; 91.6) | 10.5 (7.7; 93.0) | 12.2 (8.3; 96.4) | 9.6 (7.3; 91.4) |
| Costs per patient [mean (SD)]a | ||||
| Inpatient hospitalizations | $1576 ($7578) | $1903 ($8330) | $2835 ($10,954) | $1396 ($5407) |
| ER visits | $87 ($367) | $108 ($414) | $158 ($524) | $78 ($324) |
| Physician office visits | $1121 ($1865) | $1334 ($2011) | $1785 ($2316) | $1070 ($1816) |
| Pharmacy claims | $1401 ($1465) | $1479 ($1475) | $1663 ($1564) | $1393 ($1438) |
| Total COPD-related costs | $4069 ($8635) | $4720 ($9430) | $6381 ($12,092) | $3817 ($6478) |
Note:
All figures given in US dollars.
Abbreviations: ER, emergency room; SD, standard deviation.
Patients with two or more exacerbations also led COPD-related unadjusted annual costs (Table 3). Specifically, inpatient costs for the subgroup were almost double that for the overall population ($2835 versus $1576). Total COPD-related annual costs were $6381 for the subgroup with a history of two or more exacerbations, versus $4069 for the overall population.
Patients who experienced both severe and moderate exacerbations led in adjusted COPD-related costs and in total health care cost per year (Table 4). After adjustment, total COPD-related costs were similar to unadjusted total costs for each of the one or more exacerbation and two or more exacerbation subgroups and for the group aged 65 years or older. COPD-related health care costs were substantially higher for all three groups than for the no exacerbation group ($4875, $6550, $4050, versus $1425, respectively), with a similar trend observed for total health care costs ($20,418, $23,346, $18,038 versus $11,747, respectively).
Table 4.
Adjusted annual chronic obstructive pulmonary disease (COPD)–related and total health care costsa in follow-up year
| Variable | Adjusted mean COPD-related annual cost (95% CI) | Total health care cost per year (95% CI) |
|---|---|---|
| Patients with severe exacerbations only | $12,765 ($12,483–$13,047) | $43,042 ($42,404–$43,681) |
| Patients with moderate exacerbations only | $3356 ($3293–$3419) | $14,188 ($14,025–$14,351) |
| Patients with both severe and moderate exacerbations | $14,721 ($14,164–$15,279) | $43,385 ($42,103–$44,668) |
| Patients with any exacerbationsb | $7022 ($6926–$7119) | $25,894 ($25,625–$26,163) |
| Patients with no exacerbationsc | $1425 ($1404–$1447) | $11,747 ($11,639–$11,854) |
| Patients with a history of one or more exacerbationsd | $4875 ($4803–$4946) | $20,418 ($20,225–$20,611) |
| Patients with a history of two or more exacerbationse | $6550 ($6451–$6650) | $23,346 ($23,069–$23,622) |
| Patients aged 65 years or older | $4050 ($3986–$4114) | $18,038 ($17,866–$18,211) |
Notes:
All figures given in US dollars;
patients with any exacerbations were patients with one or more severe and/or moderate exacerbations during the follow-up year;
patients with no exacerbations were patients who had no severe or moderate exacerbations during the follow-up year;
patients with a history of one or more exacerbations were patients who had one or more severe and/or moderate exacerbations during the baseline year – the reported mean costs shown were COPD-related annual costs during the follow-up year for this subgroup of patients;
patients with a history of two or more exacerbations were patients who had two or more severe and/or moderate exacerbations during the baseline year – the reported mean costs shown were COPD-related annual costs during the follow-up year for this subgroup of patients (note, this group is a subset of patients with a history of one or more exacerbations).
Abbreviation: CI, confidence interval.
Factors associated with the annual cost of exacerbation are provided in Table 5. Notably, experiencing a moderate or severe exacerbation was associated with incrementally higher cost than the cohort with no exacerbation. Exponentiation of the coefficients from the GLM suggested that, all else being equal, the cost ratio for a patient with a severe exacerbation relative to a patient with no exacerbations was 2.7, and for a patient with a moderate exacerbation versus a patient with no exacerbations the cost ratio was 1.17. Long-term oxygen use, a proxy for severe disease, was associated with a cost ratio of 1.24. Each of the maintenance medications was also associated with higher health care costs, but coefficients were smaller than for long-term oxygen use. Cardiovascular disease was associated with a cost ratio of 1.69, and diabetes with a cost ratio of 1.46. The parameter estimate for age was close to zero (but negative), and statistically significant. This was an unexpected result, but it may reflect the fact that costs for commercial health plan members are higher than for Medicare members.
Discussion
The impact of the severity and frequency of exacerbations in patients with COPD can affect a patient’s quality of life and can have long-term health consequences.1,5,24 The intensity and repetition of these episodes also affects health care costs for the patient, as well as for society in general because of the distribution of a number of health-related costs.25,26 Currently, there is a dearth of information available about COPD patients with chronic bronchitis. The majority of published studies examine COPD in general but do not address subgroups or the use of maintenance medications as the authors have done in the current study. In this study, the authors examined the health care utilization and costs associated with COPD patients with chronic bronchitis who experienced a range of moderate to severe exacerbations over a 12-month period.
In resource use and cost, relative to other subgroups, patients with two or more exacerbations led in every category, including rate of COPD exacerbations, physician office visits, and COPD-related and total health care costs. These results suggest that the more severe the disease, the greater the utilization and costs incurred by the patient. In fact, reduction of two or more exacerbations to none, based on the dollar amount reported here, could save $5125 per patient per year in COPD-related health care costs and $11,599 per patient per year in total health care costs (Table 4). Furthermore, preventing any exacerbations in patients with both severe and moderate exacerbations would save $13,296 per patient per year, and simply reducing the severity of exacerbations from severe to moderate could save $9409 per patient per year (Table 4). Factors associated with higher total health care costs extended beyond COPD to comorbidities such as cardiovascular disease and/or diabetes as well (Table 5). The exponentiated coefficients (1.69 for cardiovascular disease and 1.46 for diabetes) imply that these comorbidities add to health care costs by less than having a severe COPD exacerbation but by more than having a moderate COPD exacerbation, and are significant. The financial impact of these results is particularly significant in this vulnerable population largely made up of elderly patients enrolled in Medicare.
These results are similar to those reported by previous studies. For example, Yu et al21 observed that health care costs increased in parallel with the severity of exacerbations experienced by COPD patients. Similarly, as seen in the current study, Yu and colleagues21 demonstrated that all-cause costs were double for patients with any exacerbation compared with those without any exacerbation. However, although similar trends in data were observed in both the study by Yu et al21 and the current study, the cost and utilization rates reported by Yu et al21 were lower than those observed in the current study. This may be because Yu et al21 reported the data quarterly as opposed to annually, or it may be because Yu et al21 focused on the general COPD patient rather than restricting the study to COPD patients with chronic bronchitis. Also, the difference in data sources and/or geographic location of the members observed in both Yu et al’s21 study and the current study may be other contributing factors to cost differences.
Notably, as with the study by Yu et al,21 the research reported in the current study was based on data from a claims database. One of the limitations inherent in using claims data is that clinical measures, such as FEV1, were not available to identify baseline severity of patients or to measure outcomes during follow-up. In addition, as with all claims data analyses, there is the possibility for error due to missing information that was not coded in the database. However, the use of a claims database does provide a range and depth of data not necessarily available from other resources. Importantly, studies examining the cost of COPD exacerbations in other countries (eg, France [this study included FEV1 measures],27 Canada,28 and Poland29) indicated that costs increased markedly with the severity of exacerbations, consistent with the current study.
A further limitation of the current study is that multivariate regression modeling was used to reduce selection bias and strengthen the causal inference. This type of modeling can only reduce bias caused by measured covariates; it cannot reduce bias caused by unmeasured covariates. Consequently, no causal inference can be ascertained from the current study.
Conclusion
Despite treatment with maintenance medications, COPD patients continue to have exacerbations resulting in high health care costs. Reduction of the severity and frequency of exacerbations can not only provide relief and clinical benefit to patients but also reduce the cost impact of the disease. 30 This has been demonstrated in research studies where reduction of exacerbations relates directly to the reduction of cost of care for the patient.11 In fact, the largest potential for cost savings has been suggested to be in patients who have the highest rate of exacerbations that lead to expensive hospitalizations. 11 This reflects the observation reported in the current study that patients with more exacerbations incur significantly higher cost. The development of new medications and disease management interventions are clearly needed to further prevent or reduce COPD exacerbations and to reduce the cost and suffering caused by this disease.
Acknowledgment
The authors would like to thank Mary E Costantino, PhD, for her contributions to developing the manuscript.
Footnotes
Disclosure
Margaret Pasquale, Heather Hartnett, and Stephen Stemkowski are employees of Competitive Health Analytics. Frank Song was an employee of Competitive Health Analytics at the time of this study. Shawn Sun is an employee of Forest Research Institute. Competitive Health Analytics received payment from Forest Research Institute to conduct the study and draft this manuscript.
References
- 1.Doll H, Miravitlles M. Health-related QOL in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literature. Pharmacoeconomics. 2005;23(4):345–363. doi: 10.2165/00019053-200523040-00005. [DOI] [PubMed] [Google Scholar]
- 2.Kochanek KD, Xu JQ, Murphy SL, Minino AM, Kung HC. Deaths: Preliminary Data for 2009. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 3.Akinbami LJ, Liu X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009. NCHS Data Brief. 2011;63:1–8. [PubMed] [Google Scholar]
- 4.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaldson GC, Seemungal TA, Patel IS, Lloyd-Owen SJ, Wilkinson TM, Wedzicha JA. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J. 2003;22(6):931–936. doi: 10.1183/09031936.03.00038303. [DOI] [PubMed] [Google Scholar]
- 7.Kanner RE, Anthonisen NR, Connett JE for Lung Health Study Research Group. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the Lung Health Study. Am J Respir Crit Care Med. 2001;164(3):358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 8.Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177(4):396–401. doi: 10.1164/rccm.200708-1290OC. [DOI] [PubMed] [Google Scholar]
- 9.Mapel DW, Hurley JS, Frost FJ, Petersen HV, Picchi MA, Coultas DB. Health care utilization in chronic obstructive pulmonary disease: a case-control study in a health maintenance organization. Arch Intern Med. 2000;160(17):2653–2658. doi: 10.1001/archinte.160.17.2653. [DOI] [PubMed] [Google Scholar]
- 10.Dalal AA, Shah M, D’Souza AO, Crater GD. Rehospitalization risks and outcomes in COPD patients receiving maintenance pharmacotherapy. Respir Med. 2012;106(6):829–837. doi: 10.1016/j.rmed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Mapel DW, Schum M, Lydick E, Marton JP. A new method for examining the cost savings of reducing COPD exacerbations. Pharmacoeconomics. 2010;28(9):733–749. doi: 10.2165/11535600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) Morbidity and Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: NHLBI; 2009. [Accessed 8 August 2012]. http://www.nhlbi.nih.gov/resources/docs/2009_ChartBook_508.pdf. [Google Scholar]
- 13.Dalal AA, Christensen L, Liu F, Riedel AA. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341–349. doi: 10.2147/COPD.S13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Collet JP, Shapiro S, et al. Negative impacts of unreported COPD exacerbations on health-related quality of life at 1 year. Eur Respir J. 2010;35(5):1022–1030. doi: 10.1183/09031936.00079409. [DOI] [PubMed] [Google Scholar]
- 16.Celli BR, MacNee W for ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 17.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Bethesda, MD: GOLD; 2011. [Accessed August 8, 2012]. Available from: http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdf? [Google Scholar]
- 18.Cazzola M, Hanania NA. The role of combination therapy with corticosteroids and long-acting beta2-agonists in the prevention of exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(4):345–354. doi: 10.2147/copd.2006.1.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suissa S, McGhan R, Niewoehner D, Make B. Inhaled corticosteroids in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(7):535–542. doi: 10.1513/pats.200701-024FM. [DOI] [PubMed] [Google Scholar]
- 20.Sciurba F. Current therapies and new approaches to the treatment of COPD. In: Wise RA, editor. John Hopkins Hospital Advanced Studies in Medicine. Vol. 8. Baltimore, MD: 2008. pp. 445–450. [Google Scholar]
- 21.Yu AP, Yang H, Wu EQ, Setyawan J, Mocarski M, Blum S. Incremental third-party costs associated with COPD exacerbations: a retrospective claims analysis. J Med Econ. 2011;14(3):315–323. doi: 10.3111/13696998.2011.576295. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Blough DK, Ramsey SD. Using generalized linear models to assess medical care costs. Health Serv Outcome Res Meth. 2000;1(2):185–202. [Google Scholar]
- 24.Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918–923. doi: 10.1164/rccm.201105-0939PP. [DOI] [PubMed] [Google Scholar]
- 25.Strassels SA, Smith DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States. Chest. 2001;119(2):344–352. doi: 10.1378/chest.119.2.344. [DOI] [PubMed] [Google Scholar]
- 26.Stewart AL, Greenfield S, Hays RD, et al. Functional status and wellbeing of patients with chronic conditions: results from the Medical Outcomes Study. JAMA. 1989;262(7):907–913. [PubMed] [Google Scholar]
- 27.Detournay B, Pribil C, Fournier M, et al. for SCOPE Group. The SCOPE study: health-care consumption related to patients with chronic obstructive pulmonary disease in France. Value Health. 2004;7(2):168–174. doi: 10.1111/j.1524-4733.2004.72329.x. [DOI] [PubMed] [Google Scholar]
- 28.Mittmann N, Kuramoto L, Seung SJ, Haddon JM, Bradley-Kennedy C, Fitzgerald JM. The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med. 2008;102(3):413–421. doi: 10.1016/j.rmed.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Jahnz-Rózyk K, Targowski T, From S. Costs of exacerbations of chronic obstructive pulmonary disease in primary and secondary care in 2007: results of multicenter Polish study. Pol Merkur Lekarski. 2009;26(153):208–214. Polish. [PubMed] [Google Scholar]
- 30.Anzueto A, Sethi S, Martinez FJ. Exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(7):554–564. doi: 10.1513/pats.200701-003FM. [DOI] [PubMed] [Google Scholar]