Abstract
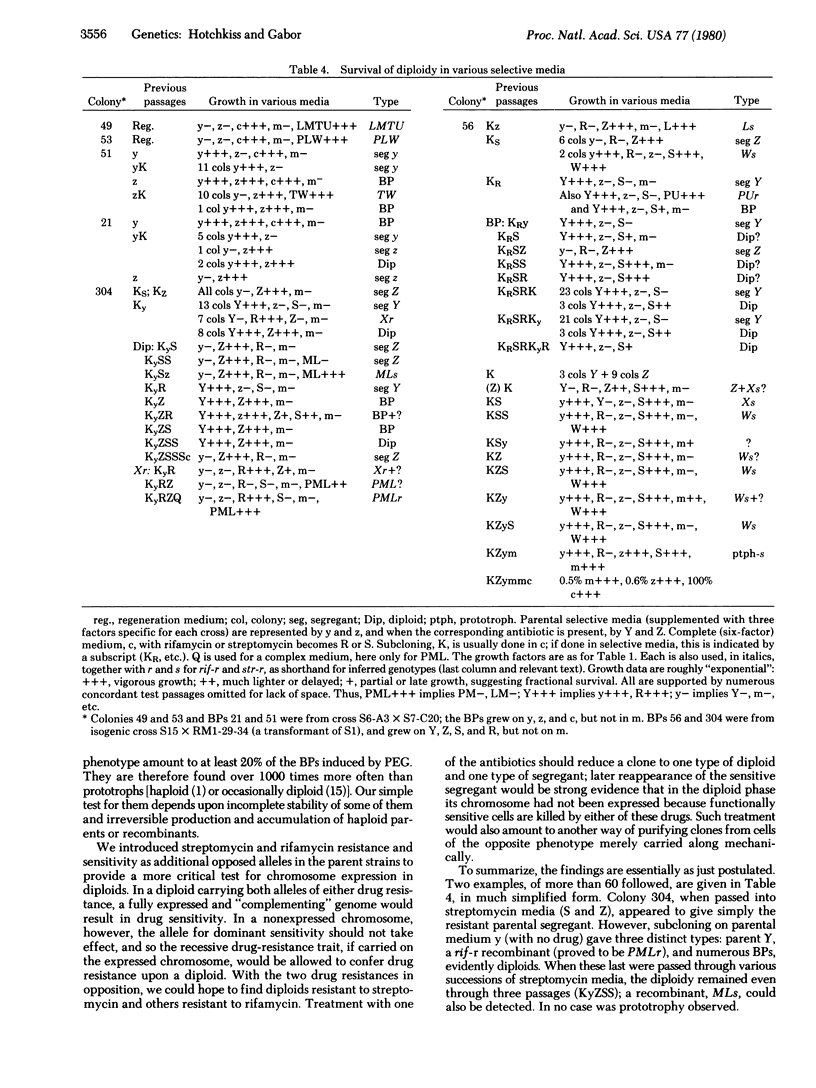
Efficiently regenerated single colonies from mixed multiply auxotrophic Bacillus subtilis protoplasts, fused with polyethylene glycol, reveal colonies carrying each of the parent types (biparentals) and recombinant colonies. The latter appear in high yields (up to 1% of certain recombinant classes); the yield of biparentals may be as large as 10%, in the range of the indicated physical fusion events. Many of the biparentals are diploids although, contrary to expectation, they are not complementing prototrophs, but show precisely the phenotype of one (either one) of the parent strains. Extensive pedigree analysis and subcloning of diploid lines show that they can propagate with varying stability on the appropriate parental selective medium to reproduce diploid progeny, parental segregants, and late-appearing recombinants, including some prototrophs. Thus, the principal product of intertype protoplast fusion is a diploid carrying two chromosomes, only one of which is expressed in each particular clone. The extinction of one parental genome is especially well demonstrated when it includes suppression of a normally dominant antibiotic sensitivity marker. In transformation experiments, DNA made from a selected diploid clone was able to transfer several of the unexpressed genes. The structural or topological character of DNA associated with the chromosome extinction remains unexplained.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCastro-Costa M. R., Landman O. E. Inhibitory protein controls the reversion of protoplasts and L forms of Bacillus subtilis to the walled state. J Bacteriol. 1977 Feb;129(2):678–689. doi: 10.1128/jb.129.2.678-689.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Fodor K., Alföldi L. Fusion of protoplasts of Bacillus megaterium. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2147–2150. doi: 10.1073/pnas.73.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., Lheritier A. M., Sanchez-Rivas C., Schaeffer P. Electron microscopic study of Bacillus subtilis protoplast fusion. J Bacteriol. 1979 Mar;137(3):1354–1361. doi: 10.1128/jb.137.3.1354-1361.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor M. H., Hotchkiss R. D. Parameters governing bacterial regeneration and genetic recombination after fusion of Bacillus subtilis protoplasts. J Bacteriol. 1979 Mar;137(3):1346–1353. doi: 10.1128/jb.137.3.1346-1353.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. Bacterial protoplast fusion: recombination in fused protoplasts of Streptomyces coelicolor. Mol Gen Genet. 1978 Jul 4;162(3):307–317. doi: 10.1007/BF00268856. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rivas C., Garro A. J. Bacterial fusion assayed by a prophage complementation test. J Bacteriol. 1979 Mar;137(3):1340–1345. doi: 10.1128/jb.137.3.1340-1345.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Cami B., Hotchkiss R. D. Fusion of bacterial protoplasts. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2151–2155. doi: 10.1073/pnas.73.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Hotchkiss R. D. Fusion of bacterial protoplasts. Methods Cell Biol. 1978;20:149–158. doi: 10.1016/s0091-679x(08)62017-8. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick P. B., Rogers H. J. Isolation and characterization of cell wall-defective variants of Bacillus subtilis and Bacillus licheniformis. J Bacteriol. 1973 Oct;116(1):456–465. doi: 10.1128/jb.116.1.456-465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



