Abstract
Background/purpose
Conventional treatment methods for acne vulgaris have various side effects such as the development of bacterial resistance, phototoxicity, vertigo, gastro-intestinal problems, and drug eruptions. To minimize such side effects, light and thermal methods have been alternately suggested. This study characterized a new acne vulgaris treatment device (AVTD) that combines both light and thermal methods and evaluated its clinical efficacy.
Methods
We characterized the thermal and light properties of the AVTD itself and evaluated its thermal characteristics in ex vivo porcine skin samples. The Arrhenius equation was used to calculate the skin thermal injury coefficient to confirm the skin safety of the AVTD. Finally, the clinical efficacy of the AVDT was evaluated by analyzing cross-polarization and erythema index images, which were obtained from 13 volunteers undergoing treatment with the AVTD.
Results
The temperature of the AVTD itself was maintained at 49.1 °C on the tip and 39.7 °C in the porcine skin samples. The peak intensity of the light-emitting diode (LED) light was observed at 468 nm. The skin safety of the AVTD was confirmed and 84.2% of the volunteers presented positive treatment results.
Conclusion
The treatment of acne using the AVTD resulted in a high treatment rate in a clinical study, minimizing side effects. On the basis of these results, we can be sure that the AVTD may be effectively used for the treatment of acne vulgaris.
Keywords: acne vulgaris, Propionibacterium acnes, light, heat, therapy
Acne vulgaris, which occurs in approximately 70% of adolescents, is the most common skin disorder (1). There are four main factors that contribute to acne’s formation: (1) increased sebum production due to androgenic stimulation of sebaceous glands (2); (2) obstruction of sebaceous follicle pores by abnormal keratinization, which causes solidification and pigmentation (3); (3) propagation of Propionibacterium acnes, which produce free fatty acids that cause follicular wall irritation and chemotactic factors that cause follicular wall damage; and (4) inflammation of acne vulgaris deteriorated by P. acnes. Of these, P. acnes is considered the primary cause of acne vulgaris (1, 4–8).
Typical treatments include the topical application of retinoid medicines, systemic treatments, photodynamic therapy using photosensitizers, and natural approaches (1, 5, 8). In general, these methods are successful in 29% of cases and unsuccessful in 66% (9). Additionally, many approaches tend to have unpleasant side effects (1). For example, the efficacy of antibiotic treatments is limited by the tolerance of bacterial strains and inflammatory pustules (5, 10). Bacterial resistance requires higher concentrations of medicines, which may cause phototoxicity, vertigo, gastro-intestinal problems, and drug eruptions. Hormone-related therapy is limited to a small numbers of pustules for acne vulgaris in women. The side-effects of photodynamic therapy include hyperpigmentation, exfoliation, and crusting.
To avoid these and other drawbacks associated with conventional acne treatments, light and thermal techniques have been suggested as possible alternatives (2, 5–8, 10–13). The light treatment method mainly uses ultraviolet-A (6), soret (400–410 nm), and Q-band (500–700 nm) light (7). However, a previous study demonstrated that 370, 385, 395, 405, and 470 nm are the most effective wavelengths for sterilizing P. acnes (11). The thermal treatment method is based on the thermal shock mechanism: increasing the skin temperature to 47–49 °C results in the synthesis of heat-shock proteins (HSPs), mainly groEL (HSP60) and dank (HSP70), which are the main factors of immune response for bacterial pathogenic organs (12–13).
In order to enhance the treatment efficacy of acne vulgaris and to minimize the side effects of conventional treatment methods, we developed a new acne vulgaris treatment device (AVTD) that uses the principles of both light and thermal treatments. Herein, we characterize the AVTD and evaluate its clinical efficacy.
Materials and Methods
Device
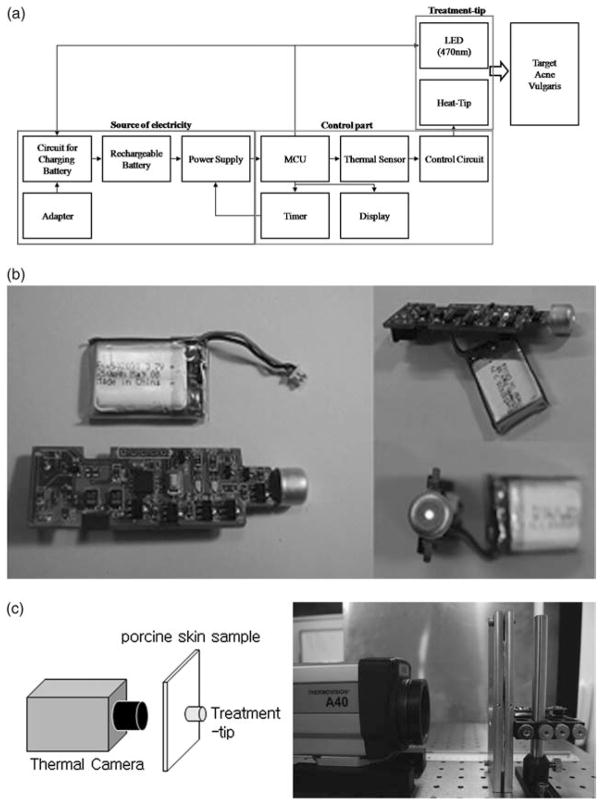
The AVDT (Fig. 1a) consists of three parts: (1) a highly conductive aluminum treatment tip (Fig. 1b) that has a 9 mm diameter to generate heat and a 1.47 mm center hole to irradiate light-emitting diode (LED) light; (2) a microprocessor control unit controller that allows the adjustment of temperature of the treatment tip and treatment duration; and (3) an electric part consisting of a display and battery (3.7 V and 250 mA).
Fig. 1.
Acne vulgaris treatment device and experimental setup. (a) Block diagram of the acne vulgaris treatment device. (b) Prototype of the developed device. (c) Experimental setup for evaluating thermal characteristics during pre-heating and treatment periods.
Characterization of AVTD
The thermal properties of the treatment tip during the pre-heating (90 s) and treatment periods (150 s) were measured at room temperature (18–20 °C) using a thermal camera (THERMOVISION™ A40, FLIR SYSTEM™, Danderyd, Sweden) placed at a distance of 11 cm from the AVTD (Fig. 1c). The thermal characteristics of the AVTD in biological tissues were evaluated using 1.95-mm-thick ex vivo porcine skin samples. Thermal profiles were measured by gently placing the AVTD on one side of each sample while the thermal camera was positioned on the opposite side (Fig. 1c).
A pulsed wave mode of LED was used to allow light to travel deeper into the skin. This occurs when the photons at the beginning of the pulse excite the chromophore molecules in the upper tissue, thereby opening the optical path (14). The wavelength and intensity of the LED were measured using an optical spectrometer (USB4000, Ocean Optics, Dunedin, FL, USA). The pulse duration and shape of the LED were measured using a photo detector (DET10A, Thorlabs, Newton, NJ, USA) connected to a digital oscilloscope (Waverunner 6050, LeCroy, Chestnut Ridge, NY, USA). The working distance for the measurements was appropriately adjusted to avoid light saturation.
In vivo evaluation of treatment efficacy and safety
Thirteen volunteers (10 males and three females; mean age = 24.5 years) with facial acne vulgaris participated in this study. All volunteers had an initial treatment by placing the AVTD tip on the acne vulgaris pustules. Cross-polarization images were acquired using a multimodal facial color imaging modality (DERMAVISION PRO, Optobiomed Co. Ltd, Wonju-si, Korea) at 6, 24, and 48 h after the initial treatment. Treatment efficacy was quantitatively evaluated by computing the erythema index (EI) images (15) from the cross-polarization images. Additionally, dermatological visual grading criteria were used to subjectively evaluate erythema and pustule formation (Table 1) on a scale of −3 to +3.
TABLE 1.
Visual grading criteria used by dermatologists to evaluate acne vulgaris subjectively
| Criterion | Grade | Description |
|---|---|---|
| Degree of erythema/size of pustule | − 3 | Huge decrement |
| − 2 | Moderate decrement | |
| − 1 | Slight decrement | |
| 0 | Before treatment (0 h) | |
| +1 | Slight increment | |
| +2 | Moderate increment | |
| +3 | Huge increment |
Skin safety was evaluated by placing the treatment tip on normal human skin and computing the skin thermal injury coefficient (w). This was accomplished using the Arrhenius equation (16) developed by Henriques to predict the probability of thermal injury to skin:
| (1) |
where T is the temperature in Kelvin as a function of depth, and t is the contact time. First-degree burns result from w = 0.53; second-degree burns result from w<0.40; and third-degree burns result from w = 1.0. Thus, w<0.53 is required for the AVTD to be safe for human use.
Results
Characterization of AVTD
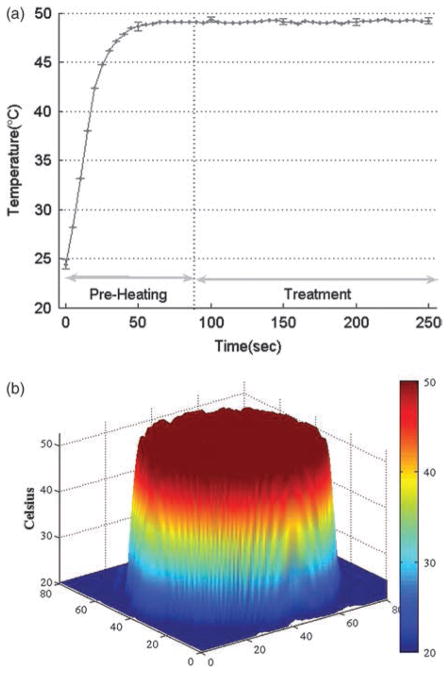
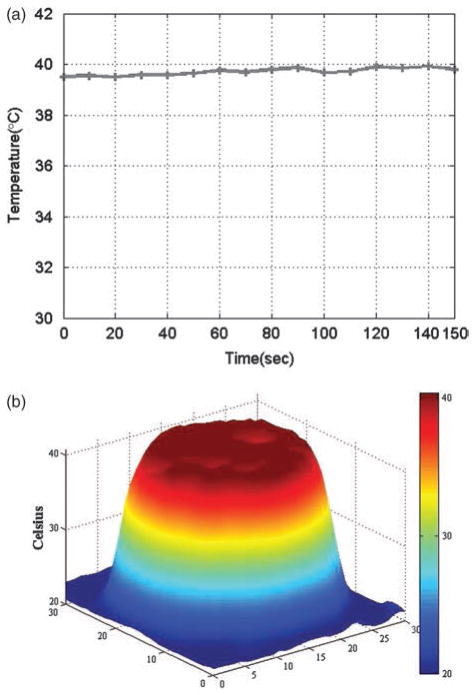
During the 90-s pre-heating period, the temperature of the treatment tip increased rapidly from 24.4 °C to 48.4 °C. The temperature then remained constant at 49.1 °C during the 150-s treatment period (Fig. 2a). Little temperature fluctuation was observed over five repetitions of this experiment. Additionally, the surface of the treatment tip was uniformly distributed at 49 °C during the treatment period (Fig. 2b). This consistency translated to skin samples during the treatment period: temperatures in the samples were maintained at 39.7 – 0.1 °C (Fig. 3a) and were uniformly distributed across the regions of interest (Fig. 3b), with even narrower thermal profiles than were observed during the initial experiments (Fig. 2b).
Fig. 2.
Thermal characteristics of the treatment tip. (a) Temperature variation of the treatment tip during pre-heating and treatment periods. (b) Temperature distribution of the treatment tip surface.
Fig. 3.
Thermal characteristics by the treatment tip used on ex vivo porcine skin samples. (a) Temperature variation during the treatment period. (b) Temperature distribution in the porcine skin sample.
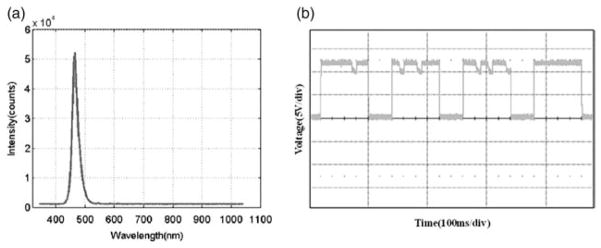
The spectral range of the LED was 426–533 nm, with a peak intensity at 468 nm (Fig. 4a). The pulse period was 119.82 ms, with a pulse duration of 66.43%; the peak-to-peak voltage was 11.9 V (Fig. 4b).
Fig. 4.
Characterization of the LED light source. (a) Optical spectrum and (b) pulse mode of LED.
In vivo evaluation of treatment efficacy and skin safety
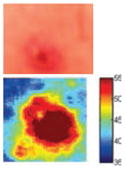
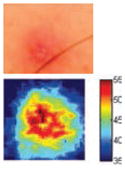
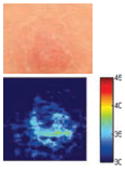
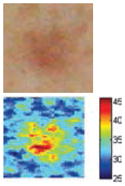
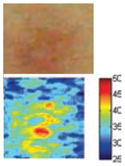
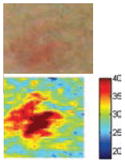
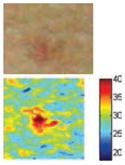
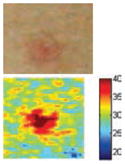
The cross-polarization and EI images quantitatively show the degree of erythema before and after treatments (Table 2). Eleven of 13 volunteers showed in positive treatment outcomes in which an obvious reduction of EI was observed 48 h after treatment and the EI images indicated reductions in the size of the area of the acne outbreaks with a white or a pale yellow pustule. Two of the 13 volunteers showed negative treatment outcomes, which resulted in the increment of EI 48 h after the treatment.
TABLE 2.
Representative examples of cross-polarization (upper) and erythema index (lower) images used to evaluate the treatment efficacy of acne vulgaris as a function of time
| Before treatment | 6 h after treatment | 24 h after treatment | 48 h after treatment | |
|---|---|---|---|---|
| Positive result |
|
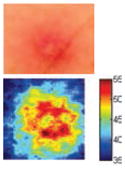
|
|
|
| Acne without pustule (Volunteer B) | ||||
| Cross-polarization image | ||||
| Erythema index image | ||||
| Acne without pustule (Volunteer K) |
|
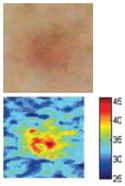
|
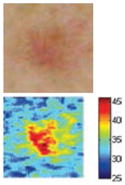
|
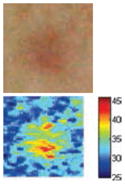
|
| Cross-polarization image | ||||
| Erythema index image | ||||
| Acne with pustule (Volunteer G) |
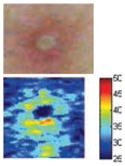
|
|
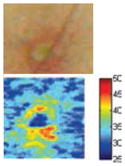
|
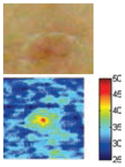
|
| Cross-polarization image | ||||
| Erythema index image | ||||
| Negative result |
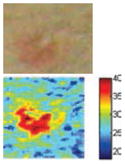
|
|
|
|
| Acne without pustule (Volunteer M) | ||||
| Cross-polarization image | ||||
| Erythema index image |
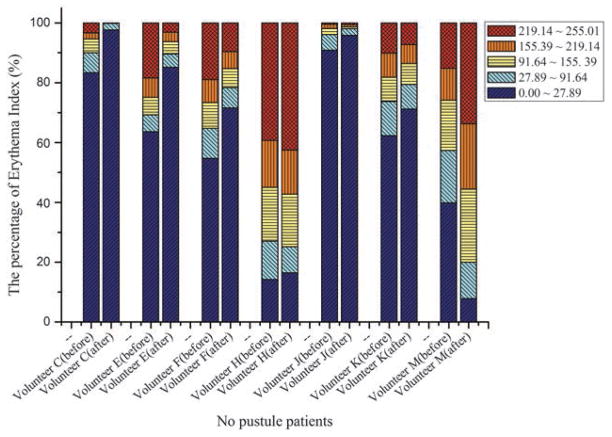
The efficacy of the AVTD treatment was determined objectively by evaluating the degree and size of erythema and inflammation using EI images of ten non-pustule acne volunteers. Eight volunteers (A, B, C, D, E, F, J, and K) showed remarkable decrements in the 219.14–255.01 EI range, and increments in the 0–27.89 EI range 48 h after treatment (Fig. 5). However, two volunteers (H and M) showed negative treatment outcomes with the opposite pattern.
Fig. 5.
Percentage variation in the erythema index in 10 no-pustule acne volunteers.
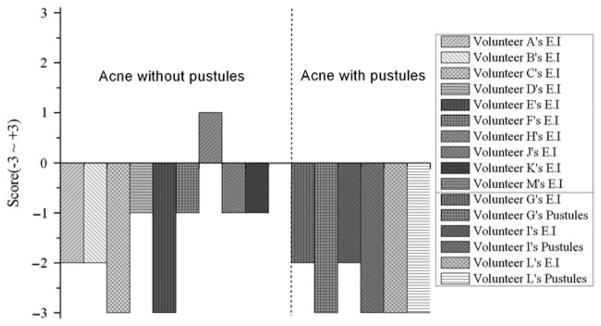
Subjective evaluations of acne vulgaris with and without pustules support these patterns (Fig. 6). Across all cases of acne vulgaris with pustules, the pustule size was scored as − 3 and the EI was relatively lower (average score = − 2.3 in three volunteers) than in cases of acne vulgaris without pustules (average score = − 1.3 in 10 volunteers). The pustules disappeared and erythema decreased remarkably 48 h after treatment. Both objective and subjective evaluation outcomes show that 84.6% of the volunteers experienced positive treatment outcomes by 48 h after the treatment.
Fig. 6.
Qualitative evaluation of acne vulgaris treatment efficacy using cross-polarization and EI images. Left and right sides indicate volunteers with acne vulgaris both without and with pustules, respectively.
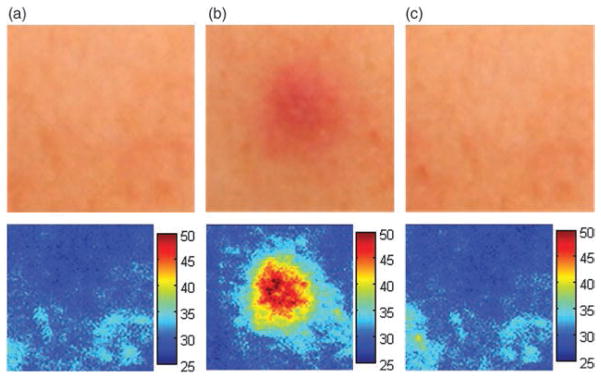
The thermal injury coefficient, w, was measured as 6.3189 × 10−5, which is much smaller than the value required to cause first-degree burns. Figure 7 shows the temperature changes on normal human skin surfaces during the treatment.
Fig. 7.
Skin safety evaluation. Cross-polarization (upper) and erythema index (lower) images (a) before contact, (b) during treatment period, and (c) after 1 min of treatment.
Discussion
The AVTD uses both light and heat to kill P. acnes. A previous study demonstrated that blue light illuminated to P. acnes colonies induces photoexcitation of bacterial porphyrins and stimulates the production of single oxygen and, finally, results in the endogenous photodynamic destruction of P. acnes (4, 6–7). The peak intensity of the blue LED (468 nm) was very close to the wavelength (470 nm) previously shown to be effective in acne vulgaris phototherapy (11). Therefore, it is expected that the blue LED used in this study might contribute to a similar treatment outcome as the previous study (11). Thermal treatment of skin causes changes in nutrient concentrations and oxygen tension by a heat-shock effect contributing to the synthesis of HSPs. During treatment with the AVTD, skin samples maintained a temperature of approximately 40 °C. This temperature is the most effective in promoting HSP synthesis as it is sufficiently warm to instigate HSP synthesis but not so warm to restrict the activity of the HSP promoter σ32 (13).
Clinical tests yielded positive treatment outcomes in eleven volunteers, and negative treatment outcomes in two volunteers. Among the positive treatment outcomes, cross-polarization images clearly showed that three volunteers with pustules (G, I, and L) were completely improved 48 h after treatment. Treatment efficacy for three out of ten volunteers without pustules could not be easily confirmed with cross-polarization images alone but could be discriminated with EI images. Although it was difficult to evaluate some of the cross-polarization images (e.g., volunteers F, J, and K), the percentage of EI above 91.64 was clearly reduced (Fig. 5). In other words, the size of the acne vulgaris was reduced, indicating a positive treatment outcome.
In the case of the two negative treatment outcomes, the cross-polarization images did not show any noticeable changes, but EI above 91.64 was increased at 48 h after treatment. These results may have been caused by various factors, such as skin thickness and water and fat content ratio, which might potentially influence the depth of light penetration and thermal diffusion.
The thermal safety experiments showed that erythema increased during the treatment period but recovered to normal conditions 1 min after the treatment was terminated. In fact, no skin burn side effects were observed in all 13 volunteers during the treatment, thus indicating that the skin safety of the AVTD is promising.
The cross-polarization images enhance the color image contrast of skin lesions and EI images can be effectively utilized to quantitatively evaluate erythema information. Therefore, cross-evaluation using both imaging methods resulted in the enhancement of the evaluation accuracy of acne vulgaris.
Conclusion
The AVTD may have much higher success rate than conventional methods used to treat acne vulgaris. Additionally, this treatment does not have any significant side effects. On the basis of these results, we recommend this treatment modality for patients with acne vulgaris both with and without pustules.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0076112).
References
- 1.Strauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56:651–663. doi: 10.1016/j.jaad.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 2.Tuchin VV, Genina EA, Bashkatov AN, Simonenko GV, Odoevskaya OD, Altshuler GB. A pilot study of ICG laser therapy of acne vulgaris: photodynamic and photothermolysis treatment. Lasers Surg Med. 2003;33:296–310. doi: 10.1002/lsm.10211. [DOI] [PubMed] [Google Scholar]
- 3.Boreli C, Merk K, Schaller M, Jacob K, Vogeser M, Weindl G, Berger U, Plewig G. In vivo porphyrin production by P. acnes in untreated acne patients and its modulation by acne treatment. Acta Derm Venereol. 2006;86:316–319. doi: 10.2340/00015555-0088. [DOI] [PubMed] [Google Scholar]
- 4.Lee WS, Shalita AR, Poh-Fitzpatrick MB. Comparative studies of porphyrin production in Propionibacterium acnes and Propionibacterium granulosum. J Bacteriol. 1977;133:811–815. doi: 10.1128/jb.133.2.811-815.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SY, You CE, Park MY. Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg Med. 2007;39:180–188. doi: 10.1002/lsm.20412. [DOI] [PubMed] [Google Scholar]
- 6.Kawada A, Aragane Y, Kameyama H, Sangen Y, Tezuka T. Acne phototherapy with a high-intensity, enhanced, narrow-band, blue light source: an open study and in vitro investigation. J Dermatol Sci. 2002;30:129–135. doi: 10.1016/s0923-1811(02)00068-3. [DOI] [PubMed] [Google Scholar]
- 7.Bhardwaj SS, Rohrer TE, Arndt K. Lasers and light therapy for acne vulgaris. Sem Cutan Med Surg. 2005;24:107–112. doi: 10.1016/j.sder.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Prieto VG, Zhang PS, Sadick NS. Evaluation of pulsed light and radiofrequency combined for the treatment of acne vulgaris with histologic analysis of facial skin biopsies. J Cosmet Laser Ther. 2005;7:63–68. doi: 10.1080/14764170500231848. [DOI] [PubMed] [Google Scholar]
- 9.Morton CA, Scholefield RD, Whitehurst C, Birch J. An open study to determine the efficacy of blue light in the treatment of mild to moderate acne. J Dermatol Treat. 2005;16:219–223. doi: 10.1080/09546630500283664. [DOI] [PubMed] [Google Scholar]
- 10.Suh DH, Shin JW, Min SU, Lee DH, Yoon MY, Kim NI, Kye YC, Lee ES, Ro YS, Kim KJ. Treatment-seeking behaviors and related epidemiological features in Korean acne patients. J Korean Med Sci. 2008;23:969–974. doi: 10.3346/jkms.2008.23.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho YJ, Suh DH. Study of the photoinactivation effect on Propionibacterium acnes after light irradiation with variable wavelengths. Korean J Dermatol. 2006;44:1332–1338. [Google Scholar]
- 12.Farrar MD, Ingham E, Holland KT. Heat shock proteins and inflammatory acne vulgaris: molecular cloning, over expression and purification of a Propionibacterium acnes GroEL and DnaK homologue. FEMS Micro Lett. 2000;191:183–186. doi: 10.1111/j.1574-6968.2000.tb09337.x. [DOI] [PubMed] [Google Scholar]
- 13.Kusukawa N, Yura T. Heat shock protein GroE of Escherichia coli: key protective roles against thermal stress. Genes Dev. 1988;2:874–882. doi: 10.1101/gad.2.7.874. [DOI] [PubMed] [Google Scholar]
- 14.Barolet D. Light-emitting diodes (LEDs) in dermatology. Sem Cutan Med Surg. 2008;27:227–238. doi: 10.1016/j.sder.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Kang H, Jung BJ, Nelson JS. Polarization color imaging system for online quantitative evaluation of facial skin lesions. Dermatol Surg. 2007;33:1350–1356. doi: 10.1111/j.1524-4725.2007.33288.x. [DOI] [PubMed] [Google Scholar]
- 16.Henriques FC. Study of thermal injury: the predictability and the significance of thermally induced rate processes leading to irreversible epidermal injury. Arch Pathol. 1947;43:489–502. [PubMed] [Google Scholar]