Port wine stain (PWS), also called nevus flammeus, is a congenital, cutaneous vascular malformation involving post-capillary venules which produce a light pink to dark-red-violet discoloration of the skin. The most likely hypothesis for the development of PWS is the deficiency or absence of surrounding neurons regulating blood flow through the ectatic post-capillary venules. As a result, the blood vessels are unable to constrict normally and remain permanently dilated.
The standard treatment for PWS is the pulsed dye laser (PDL), which induces photocoagulation of the subsurface-targeted blood vessels without inducing thermal damage in the normal overlying epidermis. Through selective photo-thermolysis, light emitted by the PDL is preferentially absorbed by hemoglobin, a major chromophore in blood, in the PWS blood vessels where, after being converted to heat, it causes thermal damage and thrombosis [1]. Thereafter, remnants of the blood and vascular wall components are assimilated within the skin and replaced by dermal collagen.
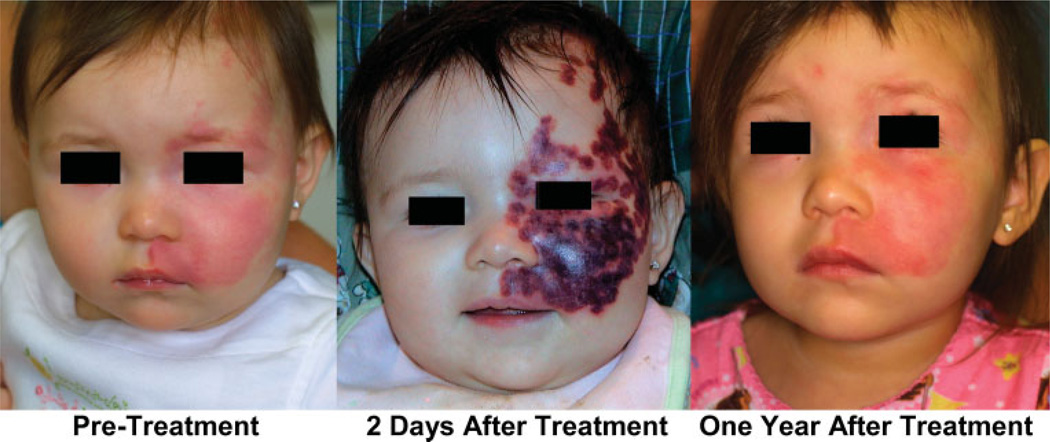
PDL treatment of PWS induces an acute purpuric response, which is associated with inflammation, edema, and hypoxia in the upper layers of the skin. Although clinically resulting in intense purpura (Fig. 1), with histological documentation of vascular wall necrosis in vessels located 2–3 mm below the skin surface, the laser-induced wound healing response to PDL treatment often results in reformation of PWS blood vessels within 1 month after laser exposure. In simple terms, the laser does what it is supposed to do, namely, cause blood vessel wall necrosis. Regrettably, the body also does what it is supposed to do, namely, repair the laser-induced damage.
Fig. 1.
PWS before PDL treatment and 2 days and 1 year after PDL. Despite intense purpuric response induced by PDL treatment, the PWS blood vessels have reformed.
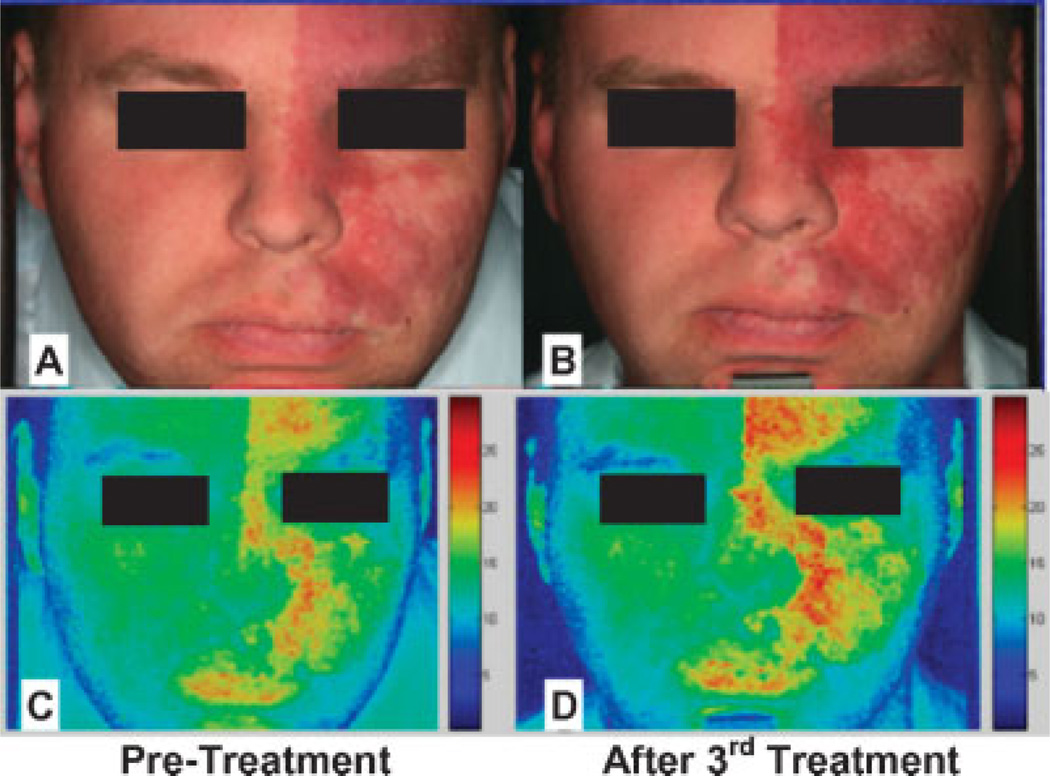
Based on more than 20 years of clinical experience, it may be concluded that the PDL produces reasonably good results in terms of PWS lesion blanching in most patients. However, if the ultimate standard required is complete blanching of the lesion, the average success rate is below 20% [2–4]. The degree of PWS blanching seen following PDL treatment remains variable and unpredictable, and consequently it is often very difficult to estimate how many treatments will be required to achieve the desired level of improvement. Moreover, due to the reformation of PWS blood vessels, there have been reports of PWS becoming darker and redder after PDL treatment (Fig. 2).
Fig. 2.
PWS before (A,C) and after (B,D) PDL treatment using conventional photography (A,B) and cross-polarized imaging (C,D).
To date, most researchers seeking to improve PWS laser therapeutic outcome have approached the problem from an engineering and tissue optics perspective. Although the delivery and photothermal interaction of light with subsurface blood vessels has been reasonably well characterized, the wound healing response of human skin to laser induced vascular photothermolysis remains incompletely understood. The perplexing clinical results achieved after PWS laser therapy raise the following question: can the wound healing response of human skin after laser therapy be modulated? More intriguing, can the effects of laser exposure be extended beyond the immediate light-target interaction?
Rapamycin (RPM) is a specific inhibitor of mammalian target of rapamycin (mTOR) and has a long history of human use as an immunosuppressive agent. Recent investigations have explored the use of RPM as an anticancer drug through its ability to inhibit mTOR-mediated functions, such as protein synthesis, cell proliferation and tumor angiogenesis [5,6]. The anti-angiogenic function of RPM is thought to involve downregulation of hypoxia-inducible factor (HIF-1α) synthesis, which in turn acts as a transcriptional factor that regulates vascular endothelial growth factor (VEGF) expression [7,8].
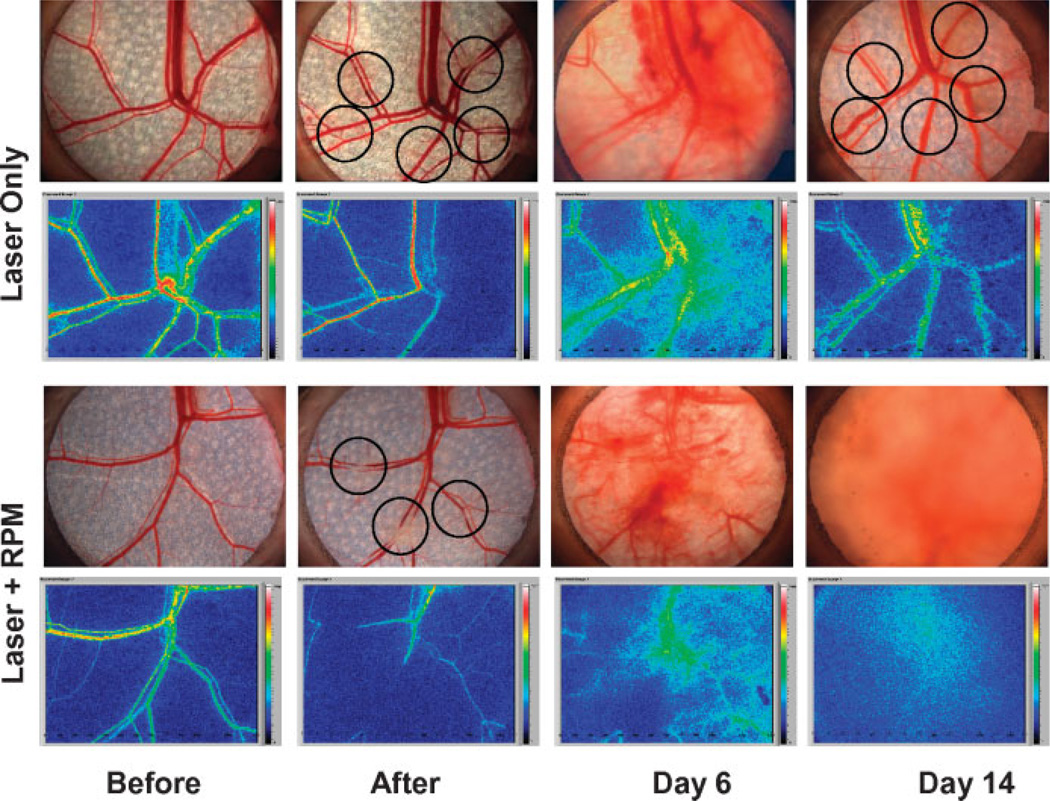
We investigated the effects of RPM as an off label topical agent to modulate the skin wound healing response by inhibiting the reformation of blood vessels after light-induced photothermolysis using the in vivo rodent window chamber model (RWCM). Photomicrographs of the RWCM skin microvasculature and laser speckle images of blood flow in response to either laser alone (with topical control) or laser plus daily topical application of RPM are shown in Figure 3. When the RWCM skin was exposed to laser alone, acute vessel injury with a reduction in blood flow immediately soon after exposure. However, reformation and reperfusion of the blood vessels were completed by Day 14, a process that is normally observed within 10 days post-laser irradiation. In contrast, when RWCM skin was exposed to laser and topical RPM for 14 days, there was no reformation and reperfusion of blood vessels after light-induced photothermolysis. Even after RPM treatment was discontinued for 21 days, only 20% revascularization was observed in the skin microvasculature during this period.
Fig. 3.
Digital photographs of the subdermal side of the RWCM before and immediately after laser exposure, and 6 and 14 days later with or without topical RPM treatment. Circles in “After” panel indicate sites of laser irradiation. Images on Day 6 “Laser Only” panel indicate early signs of blood vessel revascularization. Images on Day 14 “Laser Only” panel indicate complete blood vessel revascularization. Laser speckle blood flow images show changes consistent with complete reperfusion of these vessels. Images on Day 14 “Laser + RPM” panel show no revascularization and reperfusion 14 days after laser irradiation and topical RPM treatment.
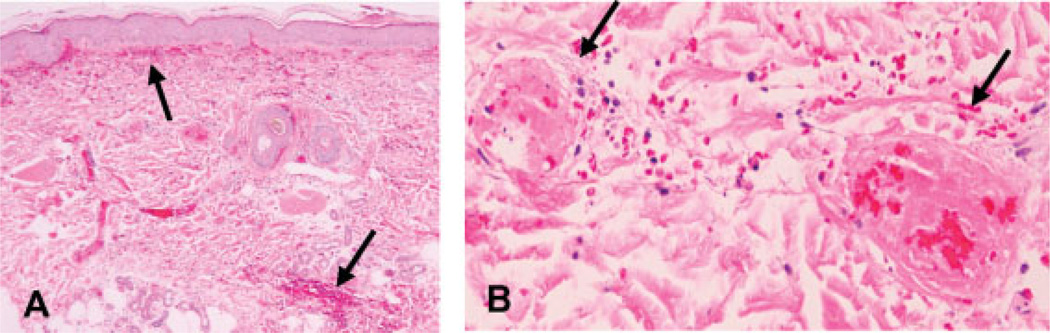
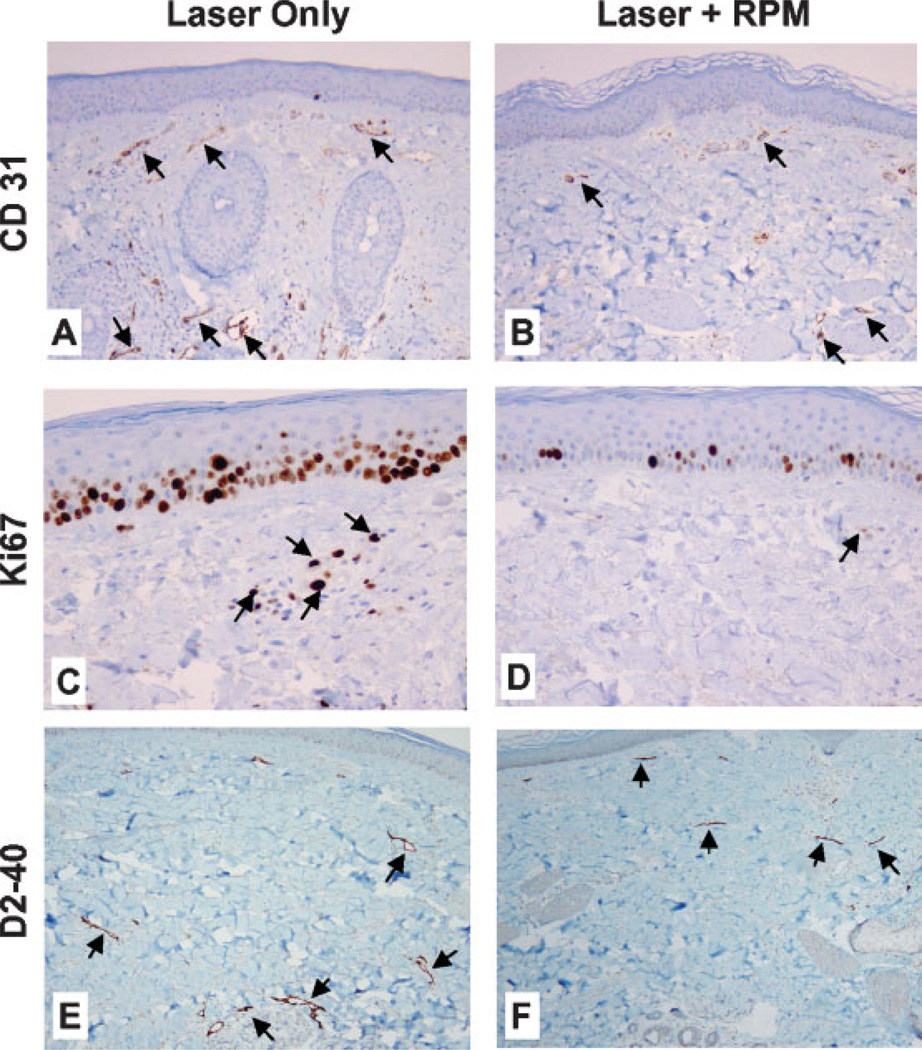
Preliminary studies on normal human skin in situ have compared the effects of laser alone or in combination with a single daily topical application of RPM (compounded as a cream placed on the skin surface under occlusion) for 14 days. As expected, there was marked vascular destruction of the vascular plexuses in the skin 4 days after laser irradiation. There was necrosis of small and large blood vessels walls, some with evidence of luminal obliteration and intravascular thrombi 3–5 mm below the skin surface (Figs. 4A,B). With laser treatment alone, there was regrowth of upper and deep dermal blood vessels 14 days after treatment as highlighted in immunostains to CD31, an endothelial cell marker (Fig. 5A). In contrast, combined laser and topical RPM treatment significantly inhibited the regrowth of new blood vessels in the skin (Fig. 5B). The decrease in new blood vessels with RPM treatment correlates with an overall decrease in the proliferative activity of cells in the epidermis and dermis as seen in Ki67 immunostains (Figs. 5C,D). Assessment of the skin lymphatics with D2-40 immunostain showed that the vast majority of lymphatics were entirely collapsed in RPM treated skin relative to the more ectatic lymphatics in skin treated with laser alone (Figs. 5E,F), suggesting inhibition of vascular leak and dermal edema with RPM.
Fig. 4.
H&E stains of tissue sections of human skin 4 days after laser exposure showing hemorrhage (arrows in A, magnification 40×) and vascular destruction with intravascular thrombi (arrows in B, magnification 400×).
Fig. 5.
CD31, Ki67, and D2-40 immunohistochemical staining of human skin 14 days after laser exposure. CD-31: Relative to skin treated by laser only (A), Laser + RPM (B) leads to a reduced vessel number in both the superficial and deep vascular plexuses (arrows in A,B, magnification 100×). Ki67: Relative to the skin treated with laser only (C), Laser + RPM (D) results in a lower number of epidermal and dermal Ki67 stained cells (arrows in C,D, magnification 400×). D2-40: Relative to the skin treated with laser only (E), Laser + RPM (F) produces less ectatic lymphatics (arrows in E,F, magnification 100×).
Based on the results of these preliminary animal and human skin investigations, the wound healing response and revascularization of human skin can indeed be modulated and the effects of laser exposure extended using anti-angiogenic agents such as RPM. These initial data demonstrate that following laser irradiation, application of a topical angiogenesis inhibitor can suppress the reformation and reperfusion of blood vessels previously disrupted by photothermolysis. These results may be attributed in part to an inhibition of endothelial cell proliferation, leading to a decrease in the overall blood vessel density. Moreover, the combined procedure appears safe for human use. There was no skin irritation or necrosis observed on the sites that received topical RPM after laser exposure.
The use of anti-angiogenic modality to inhibit revascularization following laser photothermolysis of blood vessels maybe extended beyond RPM to include other angiogenesis inhibitors such as VEGF inhibitors and inhibitors of the VEGF receptor signaling pathway. Angiogenesis inhibitors have been shown to block tumor angiogenesis, lead to “normalization” of tumor blood vessels to resemble normal vasculature, and decrease vascular permeability [9–11]. These biological outcomes may be desirable in inhibiting blood vessel revascularization following laser irradiation.
What might be the implications of the combined laser and angiogenesis inhibitor approach for the clinical management of patients with vascular skin lesions such as PWS? A topically applied inhibitor that directly inhibits vascular regrowth after laser irradiation might lead to improved therapeutic outcome after fewer treatments. RPM effectively inhibits revascularization of both the superficial and deep vascular plexuses. Due to light attenuation, the deeper vascular plexus is exposed to less incident photon energy than the superficial vascular plexus leading to incomplete photocoagulation of deeper vessels. However, because RPM blocks the proliferation of endothelial cells, the skin is unable to repair such damage. The observed blood vessel wall necrosis 3–5 mm below the skin surface when RPM was used is consistent with this hypothesis (blood vessel wall necrosis 2–3 mm below the skin surface is typically noted when the PDL is used alone). When the combined approach is used, might lower laser light dosages be more successful thereby making PWS therapy not only more effective, but also safer? It is also hoped that the increased efficacy of PDL when used in conjunction with angiogenesis inhibitors will reduce the occurrence of PWS darkening that can appear many years after PDL treatment [12–14]. Revascularization in the deeper parts of the PWS is likely responsible for such observed lesion recurrence. The opportunity to induce more vascular destruction deeper in the skin should allow for a better and more complete result in terms of PWS blanching. It is our belief that if more PWS blanching can be obtained in response to laser therapy, the less likely it is that the lesion will recur.
Although the combined laser and angiogenesis inhibitor approach is intriguing, clinical validation in PWS is urgently needed. Prospective, comparative and controlled clinical studies conducted by experienced investigators in a multicenter trial against accepted treatment regimens are necessary to better define the role of angiogenesis inhibitors in conjunction with PWS laser therapy. The possible unintended potential for systemic effects after topical application of an angiogenesis inhibitor also requires further study.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (AR47551, GM62177 and AR48458) to JSN and research grants from the American Society for Laser Medicine and Surgery to TLP and WJ. The authors greatly appreciate the assistance of Dr. Bernard Choi, Nadia Tran, and Justin Lotfi with the rodent window chamber model.
Footnotes
This work was presented in part at the 27th Annual Conference of the American Society for Laser Medicine and Surgery in Grapcviae, TX, on April 14, 2007.
REFERENCES
- 1.Anderson RR, Parrish JA. Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 2.van der Horst CMAM, Koster PHL, deBorgie CAJM, Bossuyt PMM, van Gemert MJC. Effect of timing of treatment of port-wine stains with the flash-lamp-pumped pulsed dye laser. N Engl J Med. 1998;338:1028–1033. doi: 10.1056/NEJM199804093381504. [DOI] [PubMed] [Google Scholar]
- 3.Morelli JG, Weston WL, Huff JC, Yohn JJ. Initial lesion size as a predictive factor in determining the response of port-wine stains in children treated with the pulsed dye laser. Arch Pediatr Adolesc Med. 1995;149:1142–1144. doi: 10.1001/archpedi.1995.02170230096014. [DOI] [PubMed] [Google Scholar]
- 4.Lanigan SW. Port-wine stains unresponsive to pulsed dye laser: Explanations and solutions. Br J Dermatol. 1998;139:173–177. doi: 10.1046/j.1365-2133.1998.02351.x. [DOI] [PubMed] [Google Scholar]
- 5.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 7.Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 8.Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100:3767–3775. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 11.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 12.Orten SS, Waner M, Flock S. Port wine stains: An assessment of 5 years of treatment. Arch Otolaryngol Head Neck Surgery. 1996;122:1174–1179. doi: 10.1001/archotol.1996.01890230022005. [DOI] [PubMed] [Google Scholar]
- 13.Mork NJ, Austad J, Helsing P. Do port wine stains recur after successful treatment with pulsed dye laser? J Eur Acad Dermatol Venereol. 1998;11:S142–S143. [Google Scholar]
- 14.Huikeshoven M, Koster PHL, de Borgie CAJM, Beek J, van Gemert MJC, van der Horst CMAM. Redarkening of port-wine stains 10 years after pulsed-dye-laser treatment. N Engl J Med. 2007;356:1235–1240. doi: 10.1056/NEJMoa064329. [DOI] [PubMed] [Google Scholar]