Abstract
During ripening of grape (Vitis labruscana L. cv Concord) berries, abundance of several proteins increased, coordinately with hexoses, to the extent that these became the predominant proteins in the ovary. These proteins have been identified by N-terminal amino acid-sequence analysis and/or function to be a thaumatin-like protein (grape osmotin), a lipid-transfer protein, and a basic and an acidic chitinase. The basic chitinase and grape osmotin exhibited activities against the principal grape fungal pathogens Guignardia bidwellii and Botrytis cinerea based on in vitro growth assays. The growth-inhibiting activity of the antifungal proteins was substantial at levels comparable to those that accumulate in the ripening fruit, and these activities were enhanced by as much as 70% in the presence of 1 m glucose, a physiological hexose concentration in berries. The simultaneous accumulation of the antifungal proteins and sugars during berry ripening was correlated with the characteristic development of pathogen resistance that occurs in fruits during ripening. Taken together, accumulation of these proteins, in combination with sugars, appears to constitute a novel, developmentally regulated defense mechanism against phytopathogens in the maturing fruit.
Plants have evolved a number of strategies to resist fungal infection. One strategy involves the accumulation of defense proteins that have direct inhibitory activity against the hyphae and/or germinating spores of the pathogen. Among these are PR proteins including chitinases (PR-3 family), thaumatin-like proteins (PR-5 family), and nsLTPs. Typically, these antifungal proteins are expressed constitutively at low levels in cells and accumulate in response to fungal attack or in response to other inducers of acquired resistance (Uknes et al., 1992). Reproductive organs are apparently an exception to induced acquired resistance. Presumably, the importance of flowers and ovaries to the maintenance of the species has mandated that reproductive organs acquire pathogen resistance during development. Developmentally regulated expression of PR proteins has been observed in floral organs (Lotan et al., 1989; Neal et al., 1990), including numerous examples of defensive gene mRNA accumulation in fruits. However, comparatively few data are available that interrelate developmental accumulation of antifungal proteins and the acquisition of resistance against fruit pathogens (Fils-Lycaon et al., 1996; Meyer et al., 1996).
Another physiological adaptation of plants that affects fungal pathogenesis, but one that has received considerably less attention, is the accumulation of sugars. Results from studies of several host/pathogen systems have implicated accumulation or depletion of sugars in resistance to fungal infection (VanderPlank, 1984). Increased susceptibility to fungi in sugar-depleted vegetative tissues, a phenomenon termed “sink-induced loss of resistance,” has been documented in tomato (Horsfall, 1975), cotton (Eaton and Rigler, 1946), and maize (Holbert et al., 1935). Conversely, moderate levels of sugars can enhance colonization rates of some fungal pathogens, presumably because these are important sources of C for the microbes (Mains, 1917). However, higher levels of sugars can reverse this effect, leading to decreased susceptibility, a phenomenon termed “high-sugar resistance” (Horsfall and Dimond, 1957). This reversal of susceptibility has been suggested to occur as a result of high sugar levels furnishing an osmotic challenge to the fungi and suppressing their colonization of the plant (VanderPlank, 1984).
We report here that accumulation of hexoses in grape (Vitis labruscana L. cv Concord) berries is accompanied by a developmental-stage-specific increase in a suite of proteins that are homologous to proteins known to be antifungal determinants. These proteins have been identified as a thaumatin-like protein (Salzman et al., 1994) (here we have named it GO), chitinases (both CBC and AC forms), and a nsLTP. Physiological levels of GO or CBC assayed in vitro alone or in combination with physiological levels of Glc exhibited individual and/or combinatorial activities against the important grape pathogens Guignardia bidwellii, the causal agent of black rot, and Botrytis cinerea. The interaction of the antifungal proteins and hexoses appears to constitute a developmentally regulated defense mechanism to restrict fungal pathogen infection as seeds are maturing in ripening berries.
MATERIALS AND METHODS
Unless otherwise stated, all plant material was obtained from field-grown vines of grape (Vitis labruscana L. cv Concord).
Protein Purification
For antifungal protein purification, grape berries were destemmed, crushed, and squeezed in a horizontal press (Vaslin Bucher, Chalonnes-sur-Loire, France) to obtain juice. The juice was clarified by centrifugation at 10,000g for 15 min at 4°C. The supernatant was adjusted to pH 3.7 with malic acid, and passed through a column of Amberlite CG 50 cation-exchange resin (Rohm and Haas, Philadelphia, PA) that was preequilibrated with 10 mm KH2PO4 buffer, pH 3.7. The column was washed with buffer until A280 was undetectable. The proteins were eluted with 1.5% NH4OH (adjusted to pH 9.5 with malic acid). The eluate from the ion-exchange column was dialyzed against 100 volumes of distilled water, with one change, for 48 h. The dialyzed ion-exchange fraction was adjusted to 20 mm KH2PO4, pH 6.0, and applied to an S-300 cation-exchange HPLC column (Synchropak, Lafayette, IN). The flow-through fraction was collected for subsequent chromatographic separation. The column was then eluted using NaCl and pH-gradient fractionation (0–0.5 m NaCl in 20 mm KH2PO4, pH 6.0–8.0, for a period of 60 min) to isolate the CBC and nsLTP. After elution, the column was equilibrated with 10 mm KH2PO4, pH 3.5. The flow-through fraction was adjusted to pH 3.5 with malic acid and then applied to the column. GO and AC were eluted using a gradient of 0 to 0.5 m NaCl in 10 mm KH2PO4, pH 3.5, during a period of 60 min. The fractions were dialyzed as described above, and lyophilized to dryness. The proteins were resolubilized to a concentration of 2 mg mL−1 in distilled water. Chitin-binding capacity was assessed using a colloidal chitin column (Pierpoint, 1983) and elution with 10 mm NaOH.
Electrophoresis and Detection Methods and Antibody Production
For determination of protein levels in fruit, fresh berries were deseeded and ground in a mortar and pestle with 5× sample buffer (Laemmli, 1970), 250 μL g−1 fresh weight of fruit. The homogenate was boiled for 3 min and centrifuged at 13,000g for 10 min, and the supernatant was applied to SDS-PAGE 15% gels, 25 μL of extract per lane. For immunodetection, proteins were electroblotted onto nitrocellulose according to the method of Zhu et al. (1996) and detected by reaction with IgY polyclonal antibodies (Salzman et al., 1996) raised against tobacco (Nicotiana tabacum L.) osmotin, tobacco chitinase T2 (Yun et al., 1996), or grape LTP. After reaction with the primary antibody, blots were incubated with alkaline phosphatase-linked rabbit anti-chicken IgY secondary antibodies (Jackson Immunoresearch, West Grove, PA), and the color product was developed according to standard protocols (Bio-Rad).
Amino Acid Sequence Determination
Purified proteins were subjected to SDS-PAGE and then electroblotted to a PVDF (Bio-Rad) membrane (Zhu et al., 1996). Protein bands were visualized with Coomassie blue R and excised from the membrane. The N-terminal amino acid sequence determination was performed by the Purdue Laboratory for Molecular Structure (West Lafayette, IN) on a sequencer (model 470A, Applied Biosystems) using the Edman degradation method.
Measurement of Total Sugars
Total soluble sugars in grape berries were determined from measurements of the refractive index, which accurately reflects total soluble solids. In grape berries Glc and Fru typically represent 95 to 99% of soluble solids (Zoeklein et al., 1996). Molarity (as Glc equivalents) of total sugars in freshly expressed juice was calculated from refractive index measurements using a Glc standard curve.
Chitinase and Antifungal Assays
Chitinase activity was determined colorimetrically as described previously (Yun et al., 1996). Hyphal growth measurements were determined on one-half-strength potato dextrose-broth (Sigma) medium (Abad et al., 1996). Autoclaved potato dextrose-broth medium was cooled to 40°C, followed by inclusion of purified antifungal proteins, individually or in combination, and without or with Glc or Xyl. The medium was distributed into 2.5-mm (width) by 6-mm (depth) slots in assay plates and allowed to solidify. The medium in each slot was then inoculated at one end with a small block of potato dextrose-broth medium containing fungal hyphae excised from the edge of an actively growing culture (Abad et al., 1996). Hyphal elongation was quantified with the aid of a stereomicroscope equipped with a micrometer (Olympus Optical Co., Ltd., Tokyo, Japan).
Each treatment contained six replicates per experiment, and each experiment was conducted at least twice. Dose-response data from one representative experiment are presented for each fungus/protein/solute combination.
RESULTS
Identification of Major Proteins Accumulating in Grape Berries
Polypeptides with molecular masses of 32, 29, 27, and 9 kD were the predominant proteins recovered from the juice of grape berries at the fully ripe stage (Fig. 1). These proteins were purified to apparent homogeneity based on Coomassie blue staining of SDS-PAGE gels (Fig. 2). The 27-kD protein (GO) has an N-terminal amino acid sequence that is 72% identical to VVTL1 from Vitis vinifera (Tattersall et al., 1997), and is 68% identical to TPM-1, an osmotin-like protein from tomato (Fig. 3). GO has a sequence divergent from that of VVTL1, possibly because the proteins were obtained from different genotypes. GO reacted strongly with tobacco anti-osmotin. The 9-kD protein was identified as nsLTP based on the N-terminal amino acid sequence identity (79%) with NLT4 from V. vinifera × Vitis berlanchen (Coutos-Thevenot et al., 1993) and conservation of motifs prevalent in other LTPs (Fig. 3). The molecular mass determined by SDS-PAGE and heat stability are also characteristics of plant LTPs. Amino acid sequence information was not obtained for the 29- and 32-kD proteins because these were N-terminally blocked. However, both of these proteins had potent endochitinase activity but not exochitinase activity (Table I). These proteins reacted strongly with tobacco anti-chitinase (Yun et al., 1996) and comigrated with the tobacco homologs on SDS-PAGE gels (data not shown). Increased accumulation of these two proteins was also correlated with increases in chitinase activity levels shown to occur in ripening grapes (Robinson et al., 1997). The 29-kD chitinase (AC) was more acidic than the 32-kD form based on elution from a cation-exchange column, and lacked chitin-binding activity, analogous to class II chitinases. The chitin-binding 32-kD protein (CBC) was more basic and presumably contained a chitin-binding domain (Fig. 4), both characteristics of class I chitinases.
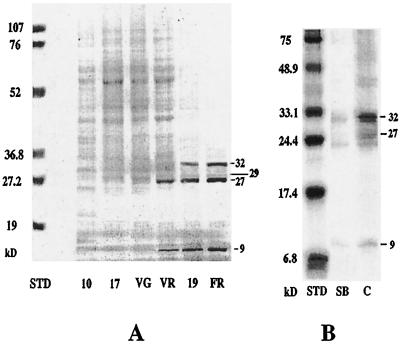
Figure 1.
Pattern of protein accumulation in grape berries during ripening. A, Total protein accumulation profile in cv Concord. Total protein from fresh berries was extracted, separated by SDS-PAGE (15% gel), and visualized after staining with Coomassie blue R. Each lane was loaded with 25 μL of total protein extract. Proteins with molecular masses of 32, 29, 27, and 9 kD were detected at the onset of ripening. Molecular mass standards (STD) are indicated on the left. 10, Green berry, 10 mm in diameter; 17, green berry, 17 mm in diameter; VG, preveraison berry (green); VR, postveraison berry (red); 19, mid-ripe-stage berry, 19 mm in diameter; and FR, fully ripe berry. B, Comparison of total protein profiles of B. cinerea-resistant versus -sensitive grape genotypes. Total protein extracts were prepared from equal fresh weights of berries of the hybrid cv SV 5276, Seyval Blanc (sensitive), and of cv Concord (resistant), respectively, at the same ripening stage (fully ripe, equivalent to 1.8 m Glc). Equal volumes of the two extracts were separated by SDS-PAGE and stained as described above. SB, cv Seyval Blanc; C, cv Concord.
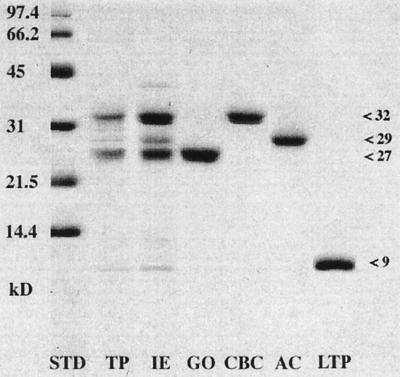
Figure 2.
Purification of proteins that accumulate in grape berries during ripening. Proteins extracted from berries at the fully ripe stage were fractionated and separated by SDS-PAGE. Molecular mass standards (STD) are indicated on the left. TP, Total fruit protein extract from clarified juice; and IE, high-pH eluate from CG 50 cation-exchange chromatography of total juice proteins. Remaining proteins were purified from IE by cation-exchange HPLC on S-300 columns: GO, purified GO; CBC, purified CBC; AC, purified AC; and LTP, purified LTP.
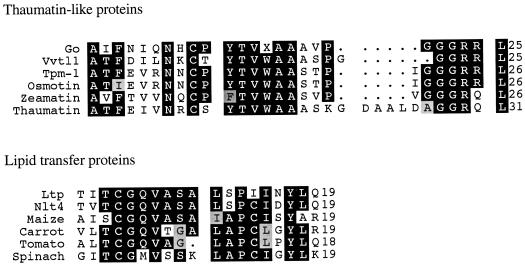
Figure 3.
N-terminal amino acid sequence comparison identifies GO and LTP. GO had high sequence homology with thaumatin-like proteins: V. vinifera (Vvtl1) (GenBank [G] accession no. AF003007), tomato (Tpm-1) (SwissProt [SP] accession no. Q01591), osmotin (SP accession no. P14170), zeamatin (SP accession no. P33679), and thaumatin (SP accession no. P02883). The grape LTP had high sequence homology with LTPs from: Vitis sp. NLT4 (SP accession no. P80274), maize (SP accession no. P19656), carrot (SP accession no. P27631), tomato (SP accession no. P27056), and spinach (SP accession no. P10976). X represents unidentified amino acid residues and dots indicate gaps in the sequence comparisons. Shaded areas are consensus regions among proteins in each respective group; identical amino acids are darkly shaded and similar amino acids are lightly shaded.
Table I.
Grape chitinases CBC and AC have high endochitinase activity
Purified CBC and AC were evaluated for chitinase activity (Yun et al., 1996). Chitinase from Streptomyces griseus (Sigma) served as a positive control for enzyme activity.
Exochitinase activity was not detected.
One kat is defined as the enzyme activity catalyzing the formation of 1 mol of GlcNAc per second.
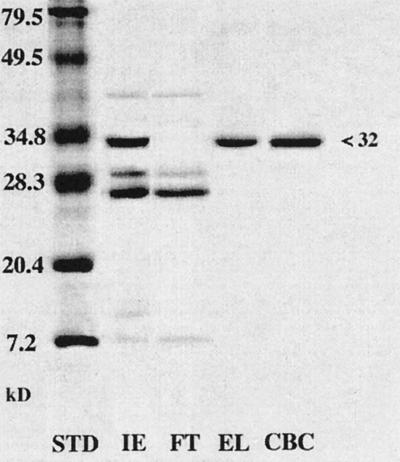
Figure 4.
Ripe grape berries accumulate abundant quantities of CBC. Proteins from different fractions of colloidal chitin chromatography were separated by SDS-PAGE (15% gel) and stained with Coomassie blue R. Molecular mass standards (STD) are indicated on the left. Fractions are as follows: IE, high-pH eluate from CG 50 cation-exchange chromatography of clarified juice; FT, flow-through during column loading; EL, high-pH eluate (chitin-binding proteins); and CBC, CBC purified by HPLC.
Sugar and Antifungal Protein Accumulation Occur Coordinately during Fruit Ripening
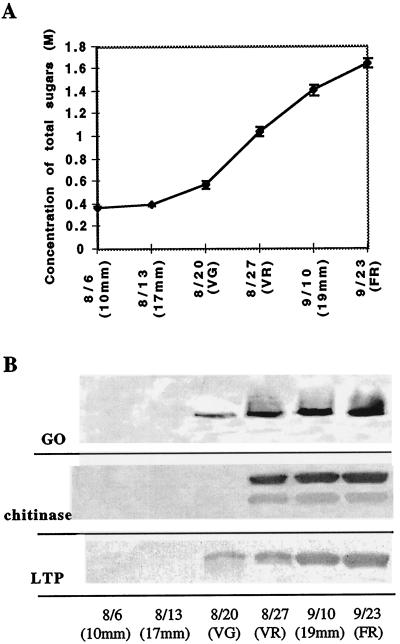
Total sugar levels in berries increased substantially during the onset of ripening (termed “veraison”), coincident with major accumulation of the antifungal proteins (Fig. 5). During this period the molarity of total sugars, which are composed primarily of Glc and Fru (Zoeklein et al., 1996), increased from 0.58 to 1.1 m Glc equivalents. The antifungal proteins represented the predominant proteins in the berries during this period, in part because of reduced abundance of photosynthetic enzymes. The absolute levels of GO and CBC increased to as much as 200 μg g−1 fresh weight of the berry, estimated by comparing total protein extracts with known amounts of purified proteins on SDS-PAGE (data not shown), although accumulation levels varied among different genotypes (Fig. 1B). High levels of the proteins also persisted during senescence.
Figure 5.
Antifungal proteins coordinately accumulate with sugars during grape berry ripening. A, Sugar accumulation during ripening. Grape berry sugar concentration was measured by refractometry, and effective molarity of total sugars was derived from a Glc standard curve. Values are means ± se from five samples of two berries each. B, Abundance of GO, CBC, AC, and LTP during ripening. Shown are immunoblots of total protein (15 μL loaded per lane from an equivalent fresh weight) of ripening berries. Proteins were separated by SDS-PAGE (15% gel) and reacted with tobacco anti-osmotin (Singh et al., 1985) (GO), tobacco anti-chitinase (Yun et al., 1996) (chitinase), or grape LTP antibodies (LTP). Harvest dates are indicated as day/month 10mm, Green berry, 10 mm in diameter; 17mm, green berry, 17 mm in diameter; VG, preveraison berry (green); VR, postveraison berry (red); 19mm, mid-ripe-stage berry, 19 mm in diameter; and FR, fully ripe berry.
Proteins That Accumulate in Fruits during Ripening Inhibit Growth of Grape-Pathogenic Fungi and This Activity Is Enhanced by Sugars
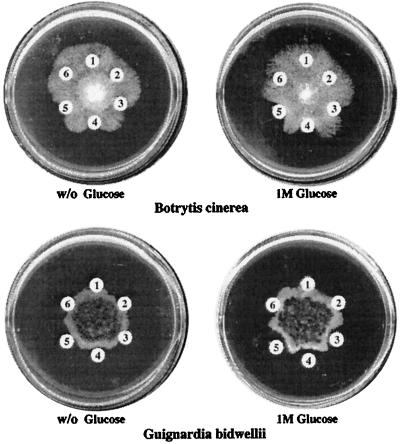
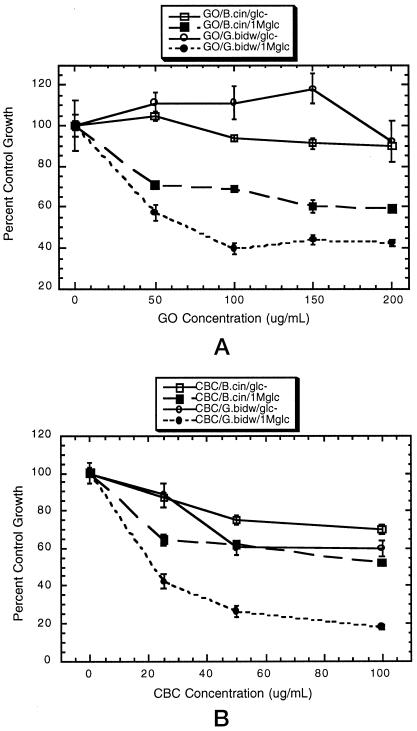
Hyphal growth of grape isolates of Guignardia bidwellii and Botrytis cinerea was inhibited by GO and CBC (Figs. 6 and 7). Virtually no chitinase activity was detected in the GO protein fraction (Table II), indicating that antifungal activity was not attributable to contaminating chitinases. Petri-dish and quantitative assays established that the effects of GO and CBC against B. cinerea and G. bidwellii were enhanced substantially by inclusion of 1 m Glc in the medium (Figs. 6 and 7). This effect was more evident with GO, which had substantially less antifungal activity in the absence of solute (Fig. 7). Fungal-growth inhibition was additive when GO and CBC were assayed in combination; however, no synergism was evident between the two proteins, either in the presence or absence of Glc (Fig. 6). The sugar modulation of antifungal protein activity seemed to be mediated through a nutrition-independent mechanism, because Xyl (a nonmetabolizable osmolyte) also enhanced the activity of the proteins (Table III). The LTP exhibited no antifungal activity against either G. bidwellii or B. cinerea at doses up to 200 μg mL−1 (data not shown).
Figure 6.
Hyphal growth inhibition of G. bidwellii and B. cinerea by GO and CBC is enhanced by Glc, although the sugar does not have antifungal activity. Hyphal plugs were inoculated onto medium without (w/o) or with 1 m Glc. After the fungal colony diameter had reached 1.5 cm, filter paper discs were placed onto medium around the edges of the colony containing: 1, water (no protein added); 2, boiled GO and CBC (100 μg each); 3, GO (100 μg); 4, CBC (100 μg); 5, GO and CBC (50 μg each); and 6, LTP (100 μg).
Figure 7.
GO and CBC antifungal activities are facilitated by Glc. Hyphal growth of G. bidwellii (G.bidw) and B. cinerea (B.cin) was measured at time intervals on medium containing GO or CBC without (glc−) or with 1 m Glc (1Mglc). Values represent mean growth as a percentage of the control ± se, n = 6. Controls consisted of glc− or 1Mglc medium with boiled, denatured protein at the highest concentration. A, GO. B, CBC.
Table II.
Purified GO and LTP fractions are devoid of chitinase activity
| Protein Fraction | Total Protein | Chitinase
|
|
|---|---|---|---|
| Protein equivalent | Percent | ||
| μg | |||
| GO | 100 | <1 | <0.5 |
| 360 | 2.5 | 0.69 | |
| LTP | 100 | <1 | <0.5 |
Chitinase activity in GO and LTP fractions was determined and the chitinase content estimated from the activity.
Table III.
Glc or Xyl are equivalent in their capacity to facilitate antifungal activity of GO and CBC against G. bidwellii
| Protein | 0.5 m
|
|
|---|---|---|
| Glc | Xyl | |
| % | ||
| GO | 25.9 ± 1.3 | 25.5 ± 6.4 |
| CBC | 67.9 ± 1.4 | 56.8 ± 1.4 |
Values expressed are mean percent inhibition (se ± n = 3), in the presence of GO or CBC (100 μg/mL) plus m 0.5 solute, relative to control growth on medium with the solute but without antifungal protein. Data were derived from quantitative assays as described in Methods.
DISCUSSION
Antifungal Proteins Are Components of Fungal Resistance Developed during Grape Berry Ripening
The demonstration that the grape berry proteins have antifungal activity, and their abundant and developmentally controlled accumulation in berries implicate a potential role for these proteins in host-plant defense against fruit pathogens. Ripe fruits typically are considered vulnerable to pathogen attack because of their enriched levels of sugars and other nutrients, as well as physical changes that facilitate infection (Swinburn, 1983). Plants with climacteric fruits, such as avocado, tomato, and banana, evolved ethylene during ripening, which fostered infection by Colletotrichum spp. (Flaishman and Kolattukudy, 1994). However, for some years it has been known that grape berries acquire resistance to several pathogens during ripening (Delp 1954; Lafon and Clerjeau 1988; Pearson, 1988), and it has been suggested that an osmotin-like protein may be involved in this acquisition of resistance (Tattersall et al., 1997).
Here we establish that GO and CBC, which are effective antifungal proteins against isolates of the grape berry pathogens, accumulate synchronously at stages of ripening when the fruits become resistant to black rot (G. bidwellii), powdery mildew (Uncinula necator), and downy mildew (Plasmopara viticola) (Delp, 1954; Lafon and Clerjeau, 1988; Pearson, 1988; Ramsdell and Milholland, 1988). The levels of GO and CBC in fruits that acquired resistance were consistent with those that substantially inhibited fungal growth in bioassays. Accumulation of thaumatin in fruits of Thaumatococcus daniellii (van der Wel and Loeve, 1972) and thaumatin-like proteins in those of cherry and V. vinifera (Fils-Lycaon et al., 1996; Tattersall et al., 1997) have been reported; however, thaumatin essentially lacks antifungal activity (Vigers et al., 1991) and antifungal capacity has yet to be established for the cherry and the V. vinifera fruit-expressed PR-5 proteins. Typically, grape berries are susceptible to postharvest infection by B. cinerea, so the effective antifungal activity of the fruit GO and CBC was unpredicted. However, the cv Concord used in this study has been classified as B. cinerea resistant (Reich et al., 1993). Perhaps the proposed defense mechanism is unique to this genotype, or it may mitigate colonization rather than mediate immunity. Supporting this, another grape cultivar (SV 5276, Seyval Blanc), classified as highly susceptible to B. cinerea (Reich et al., 1993), accumulated distinctly lower levels of the antifungal proteins than the cv Concord, at the same fully ripe stage (Fig. 1B).
Induction of Antifungal Proteins during Fruit Ripening
At least two potentially interrelated mechanisms may be involved in the regulation of antifungal protein accumulation during ripening: osmotic due to sugar accumulation, or sugar signaling. Osmotin accumulation was first shown to occur as a function of salt/osmotic adaptation of tobacco cells in culture (Singh et al., 1985). The osmotin gene is responsive to hyperosmolarity and pathogen infection, as well as many inducers that have been implicated as components of the signal cascades leading from perception of these stresses (Kononowicz et al., 1992). The osmolarity in grape berries (>1.5 m Glc equivalents) is far in excess of that required to induce accumulation of osmotin, chitinases, and nsLTPs (Singh et al., 1985; Torres-Schumann et al., 1992; Yun et al., 1996). In fact, potentially inductive osmotic levels (380–580 mm Glc equivalents) occur in fruits coincident with the acquisition of resistance (Fig. 5).
As an alternative, sugars may act as a signal molecule to regulate the expression of antifungal protein genes during fruit ripening. There are numerous examples of sugar-repressed genes, most notably those that encode major C-assimilatory enzymes, because these are not required when sugars are in excess (Sheen, 1990; Cheng et al., 1992; Krapp et al., 1993; Jang and Sheen, 1994). However, sugars also up-regulate many genes in a hexose-specific manner, including those that encode defensive proteins such as protease inhibitor II (Johnson and Ryan, 1990), chalcone synthase (Tsukaya et al., 1991), and PR proteins PR3 (Herbers et al., 1995), PR1b, and PR-Q (Herbers et al., 1996a). Both Glc and Fru (the two major sugars accumulating in grape berries) mediate defense gene induction in tobacco leaves (Herbers et al., 1996b) at levels (100–500 mm) that accumulate in ripening grape berries (Fig. 5). This response seems sugar specific because the osmotic agents sorbitol and PEG 8000 did not mediate gene induction.
Sugars and Antifungal Proteins Interact to Mediate Host Plant Defense against Phytopathogens
Our results indicate that the antifungal efficacy of GO and CBC is enhanced by concentrations of Glc that occur in fruits as resistance to phytopathogens is acquired. The antipathogenic effect of potential metabolic interactions between sugars and the pathogens apparently involves nutrition-independent mechanisms, since Glc and Xyl, at equivalent osmolarities have the same effect, and Xyl is not metabolized by either G. bidwellii or B. cinerea (neither of these organisms was able to grow on Xyl as a sole C source). The previous concept that sugars furnish an “osmotic challenge” also seems unlikely, since fungal growth rates on medium supplemented with either 1 m Glc or 1 m Xyl were as high or higher than those on basal medium (data not shown). Consequently, the fungi were able to adjust osmotically and generate sufficient turgor for hyphal growth.
A more likely mechanism for the observed enhancement of antifungal protein activity by sugars might be a disruption of fungal gene regulation caused by sugar repression (Ronne, 1995). The most notable examples of sugar-repressed genes, which are relevant in this context, are those that encode pectate lyase in Fusarium solani (Guo et al., 1995) and cellulase in Trichoderma reesei (Ilmen et al., 1996). These enzymes are involved in host colonization by the phytopathogens. Sugar repression of genes involved in pathogenesis or virulence might be facilitated by GO because members of the PR-5 family are presumed to cause formation of membrane pores and this would enhance intracellular uptake of sugars. The maize PR-5 zeamatin presumably facilitated uptake of nikkomycin Z into Candida albicans cells (Vigers et al., 1991).
Another potential mechanism by which hexoses could enhance antifungal activity of GO and CBC is based on solute-mediated preservation of native protein structure, since sugars are well known to stabilize proteins against denaturation (Lee and Timasheff, 1981; Timasheff and Arakawa, 1988). At physiological temperatures, proteins are preferentially hydrated. Glc and other sugars, and polyhydric alcohols, are preferentially excluded from the hydration surface, a process that requires free energy. Unfolding of proteins is energetically unfavorable because solute exclusion would be required for a larger surface area (Arakawa and Timasheff, 1982). Presumably, the hexoses in ripening grape berries would enhance the function of the antifungal proteins by facilitating active conformation in the physiological solution. A chemical basis for sugar enhancement of antifungal activity is very plausible because GO and CBC are proteins with very different structures and functions.
ACKNOWLEDGMENTS
We thank Dr. Meena Narasimhan and Dr. Keyan Zhu-Salzman for their critical review of the manuscript.
Abbreviations:
- AC
acidic chitinase
- CBC
chitin-binding chitinase (basic)
- GO
grape osmotin
- LTP
lipid-transfer protein
- nsLTP
nonspecific LTP
- PR
pathogenesis-related
Footnotes
This research was partially supported by a grant from the Purdue Agricultural Research Program. This is publication no. 15,479 of the Purdue University Agricultural Experiment Station.
LITERATURE CITED
- Abad L, D'Urzo M, Liu D, Narasimhan M, Reuveni M, Zhu J, Niu X, Singh N, Hasegawa P, Bressan R. Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci. 1996;118:11–23. [Google Scholar]
- Arakawa T, Timasheff SN. Stabilization of protein structure by sugars. Biochemistry. 1982;21:6536–6544. doi: 10.1021/bi00268a033. [DOI] [PubMed] [Google Scholar]
- Cheng C, Acedo GN, Cristinsin M, Conkling MA. Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc Natl Acad Sci USA. 1992;89:1861–1864. doi: 10.1073/pnas.89.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutos-Thevenot P, Jouenne T, Maes O, Guerbette F, Grosbois M, La Caer JP, Boulay M, Deloire A, Kader JC, Guern J. Four 9-kDa proteins excreted by somatic embryos of grapevine are isoforms of lipid-transfer proteins. Eur J Biochem. 1993;217:885–889. doi: 10.1111/j.1432-1033.1993.tb18317.x. [DOI] [PubMed] [Google Scholar]
- Delp C. Effect of temperature and humidity on the grape powdery mildew fungus. Phytopathology. 1954;44:615–626. [Google Scholar]
- Eaton F, Rigler N. Influence of carbohydrate levels and root-surface microfloras on Phymatotrichium root rot in cotton and maize plants. J Agric Res. 1946;72:137–161. [Google Scholar]
- Fils-Lycaon BR, Wiersma PA, Eastwell KC, Sautiere P. A cherry protein and its gene, abundantly expressed in ripening fruit, have been identified as thaumatin-like. Plant Physiol. 1996;111:269–273. doi: 10.1104/pp.111.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaishman MA, Kolattukudy PE. Timing of fungal invasion using host's ripening hormone as a signal. Proc Natl Acad Sci USA. 1994;91:6579–6583. doi: 10.1073/pnas.91.14.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Gonzalez-Candelas L, Kolattukudy PE. Cloning of a novel constitutively expressed pectate lyase gene pelB from Fusarium solani f. sp. pisi (Nectria haematococca, mating type VI) and characterization of the gene product expressed in Pichia pastoris. J Bacteriol. 1995;177:7070–7077. doi: 10.1128/jb.177.24.7070-7077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Frommer W, Metraux J, Sonnewald U. Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell. 1996a;8:793–803. doi: 10.1105/tpc.8.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Metraux J, Sonnewald U. Salicylic acid-dependent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett. 1996b;397:239–244. doi: 10.1016/s0014-5793(96)01183-0. [DOI] [PubMed] [Google Scholar]
- Herbers K, Monke G, Badur R, Sonnewald U. A simplified procedure for the subtractive cloning of photoassimilate-responding genes: isolation of cDNAs encoding a new class of pathogenesis-related proteins. Plant Mol Biol. 1995;29:1027–1038. doi: 10.1007/BF00014975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbert J, Hoppe P, Smith A. Some factors affecting infection with and spread of Diplodia seae in host tissue. Phytopathology. 1935;25:1113–1114. [Google Scholar]
- Horsfall J. The story of a nonconformist. Annu Rev Phytopathol. 1975;13:1–13. doi: 10.1146/annurev.py.13.090175.000245. [DOI] [PubMed] [Google Scholar]
- Horsfall J, Dimond A. Interactions of tissue sugar, growth substances and disease susceptibility. Z Pflanzenkr Pflanzenschutz. 1957;64:415–421. [Google Scholar]
- Ilmen M, Thrane C, Penttila M. The glucose repressor gene cre1 of Trichoderma: isolation and expression of a full-length and a truncated mutant form. Mol Gen Genet. 1996;251:451–460. doi: 10.1007/BF02172374. [DOI] [PubMed] [Google Scholar]
- Jang J, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Ryan CA. Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol. 1990;14:527–536. doi: 10.1007/BF00027498. [DOI] [PubMed] [Google Scholar]
- Kononowicz AK, Nelson DE, Singh NK, Hasegawa PM, Bressan RA. Regulation of the osmotin gene promotor. Plant Cell. 1992;4:513–524. doi: 10.1105/tpc.4.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Hofmann B, Schaefer C, Stitt M. Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the “sink regulation” of photosynthesis? Plant J. 1993;3:817–828. [Google Scholar]
- Laemmli UK. Nature. 1970;277:680–684. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafon R, Clerjeau M (1988) Downy mildew. In RC Pearson, AC Goheen, eds, Compendium of Grape Diseases. American Phytopathological Society, St. Paul, MN, pp 11–13
- Lee JC, Timasheff SN. The stabilization of proteins by sucrose. J Biol Chem. 1981;256:7193–7201. [PubMed] [Google Scholar]
- Lotan T, Ori N, Fluhr R. Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell. 1989;1:881–887. doi: 10.1105/tpc.1.9.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains E. The relation of some rusts to the physiology of their hosts. Am J Bot. 1917;4:179–220. [Google Scholar]
- Meyer B, Houlne G, Pozueta-Romero J, Schantz M, Schantz R. Fruit-specific expression of a defensin-type gene family in bell pepper. Plant Physiol. 1996;112:615–622. doi: 10.1104/pp.112.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AD, Wahleithner JA, Lund M, Bonnett HT. Chitinase, beta-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell. 1990;2:673–684. doi: 10.1105/tpc.2.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RC (1988) Powdery mildew. In RC Pearson, AC Goheen, eds, Compendium of Grape Diseases. American Phytopathological Society, St. Paul, MN, pp 9–11
- Pierpoint W. The major proteins in extracts of tobacco leaves that are responding hypersensitively to virus-infection. Phytochemistry. 1983;22:2691–2697. [Google Scholar]
- Ramsdell DC, Milholland RD (1988) Black rot. In RC Pearson, AC Goheen, eds, Compendium of Grape Diseases. American Phytopathological Society, St. Paul, MN, pp 15–17
- Reich BI, Pool RM, Peterson DV, Martens M, Henick-Kling T (1993) Wine and Juice Grape Varieties for Cool Climates. Information Bulletin 233. Cornell Cooperative Extension Publication, Geneva, NY
- Robinson SP, Jacobs AK, Dry IB. A class IV chitinase is highly expressed in grape berries during ripening. Plant Physiol. 1997;114:771–778. doi: 10.1104/pp.114.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronne H. Glucose repression in fungi. Trends Genet. 1995;11:12–17. doi: 10.1016/s0168-9525(00)88980-5. [DOI] [PubMed] [Google Scholar]
- Salzman RA, Bressan RA, Hasegawa PM, Ashworth EN, Bordelon BP. Programmed accumulation of LEA-like proteins during desiccation and cold acclimation of overwintering grape buds. Plant Cell Environ. 1996;19:713–720. [Google Scholar]
- Salzman RA, Paino d'Urzo M, Hasegawa PM, Bressan RA, Bordelon BP. Characterization of an antifungal osmotin-like glycoprotein from grape Vitis (L.) spp. (abstract no. 893) Plant Physiol. 1994;105:S-161. [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NK, Handa AK, Hasegawa PM, Bressan RA. Proteins associated with adaptation of cultured tobacco cells to NaCl. Plant Physiol. 1985;79:126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn TR (1983) Post-Harvest Pathology of Fruits and Vegetables. Academic Press, New York, pp 1–21
- Tattersall DB, van Heeswijck R, Bordier Hoj P. Identification and characterization of a fruit-specific, thaumatin-like protein that accumulates at very high levels in conjunction with the onset of sugar accumulation and berry softening in grapes. Plant Physiol. 1997;114:759–769. doi: 10.1104/pp.114.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timasheff SN, Arakawa T. Stabilization of protein structure by solvents. In: Creighton TE, editor. Protein Structure and Function: A Practical Approach. Oxford, UK: IRL Press; 1988. pp. 331–345. [Google Scholar]
- Torres-Schumann S, Godoy J, Pintor-Toro J. A probable lipid transfer protein gene is induced by NaCl in stems of tomato plants. Plant Mol Biol. 1992;18:749–757. doi: 10.1007/BF00020016. [DOI] [PubMed] [Google Scholar]
- Tsukaya H, Oshima T, Naito S, Chino M, Komeda Y. Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol. 1991;97:1414–1421. doi: 10.1104/pp.97.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler C, Slusarenko A, Ward E, Ryals J. Acquired resistance in Arabidopsis. Plant Cell. 1992;4:645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderPlank J. Sink-induced loss of resistance, high sugar disease processes and biotrophy. In: Vanderplank JE, editor. Disease-Resistance in Plants, Ed 2. London: Academic Press; 1984. pp. 107–121. [Google Scholar]
- van der Wel H, Loeve K. Isolation and characterization of thaumatin I and II, the sweet tasting proteins from Thaumatococcus daniellii Benth. J Biochem. 1972;31:221–225. doi: 10.1111/j.1432-1033.1972.tb02522.x. [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Roberts WK, Selitrennikoff CP. A new family of plant antifungal proteins. Mol Plant Microbe Interact. 1991;4:315–323. doi: 10.1094/mpmi-4-315. [DOI] [PubMed] [Google Scholar]
- Yun D, Paino D'Urzo M, Abad L, Takeda S, Salzman R, Chen Z, Lee H, Hasegawa P, Bressan R. Novel osmotically induced antifungal chitinases and bacterial expression of an active recombinant isoform. Plant Physiol. 1996;111:1219–1225. doi: 10.1104/pp.111.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Huesing JE, Shade RE, Bressan RA, Hasegawa PM, Murdock LL. An insecticidal N-acetylglucosamine-specific lectin gene from Griffonia simplicifolia (Leguminosae) Plant Physiol. 1996;110:195–202. doi: 10.1104/pp.110.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeklein BW, Fugelsang KC, Gump BH, Nury FS. Wine Analysis and Production. New York: Chapman & Hall; 1996. [Google Scholar]