Abstract
Mouse A9 cells, L-cell-derived mutants deficient in hypoxanthine phosphoribosyltransferase (HPRT; IMP:pyrophosphate phosphoribosyltransferase, EC 2.4.2.8) were found to be incapable of binding 125I-labeled epidermal growth factor (EGF) to the cell surface. The A9 cells were fused with human diploid fibroblasts (WI-38) possessing EGF-binding ability, and human-mouse cell hybrids (TA series) were isolated after hypoxanthine/aminopterin/thymidine/ouabain selection. Analyses of isozyme markers and chromosomes of four representative clones of TA hybrids indicated that the expression of EGF-binding ability is correlated with the presence of human chromosome 7 or 19. Four subclones were isolated from an EGF-binding-positive line, TA-4, and segregation of EGF-binding was found to be concordant with the expression of human mitochondrial malate dehydrogenase (MDHM; L-malate:NAD+ oxidoreductase, EC 1.1.1.37), a marker for chromosome 7, but not with glucosephosphate isomerase (GPI; D-glucose-6-phosphate ketol-isomerase, EC 5.3.1.9), a marker for chromosome 19. Furthermore, evidence from 27 clones of AUG hybrids that were produced between A9 and another human fibroblast line, GM1696, carrying an X/7 chromosome translocation indicated that EGF-binding ability segregates together with human MDHM and two X-linked markers, HPRT and glucose-6-phosphate dehydrogenase (G6PD; D-glucose-6-phosphate:NADP+ 1-oxidoreductase, EC 1.1.1.49), that are located on the translocation chromosome 7p+. These results permit assignment of the gene, designated EGFS, which is associated with the expression of EGF-binding ability, to human chromosome 7 and its localization to the p22-qter region. Because the EGF receptor is reported to be a glycoprotein the EGFS could be either a structural gene(s) for receptor protein or a gene(s) for modifying the receptor protein through glycosylation.
Keywords: hormone, gene mapping, gene regulation
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Influence of lectins on the binding of 125I-labeled EGF to human fibroblasts. Biochem Biophys Res Commun. 1977 Nov 21;79(2):545–552. doi: 10.1016/0006-291x(77)90192-9. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Miyakawa T., Fox C. F., Pruss R. M., Aharonov A., Herschman H. R. Specific radiolabeling of a cell surface receptor for epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2790–2794. doi: 10.1073/pnas.74.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Nexø E., Hollenberg M. D. Isolation of the human placenta receptor for epidermal growth factor-urogastrone. Nature. 1979 Feb 1;277(5695):403–405. doi: 10.1038/277403a0. [DOI] [PubMed] [Google Scholar]
- Kahan B., DeMars R. Localized Derepression on the Human Inactive X Chromosone in Mouse-Human Cell Hybrids. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1510–1514. doi: 10.1073/pnas.72.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- McKusick V. A., Ruddle F. H. The status of the gene map of the human chromosomes. Science. 1977 Apr 22;196(4288):390–405. doi: 10.1126/science.850784. [DOI] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. A review of enzyme polymorphism, linkage and electrophoretic conditions for mouse and somatic cell hybrids in starch gels. J Histochem Cytochem. 1973 Dec;21(12):1066–1081. doi: 10.1177/21.12.1066. [DOI] [PubMed] [Google Scholar]
- Oger J., Arnason B. G., Pantazis N., Lehrich J., Young M. Synthesis of nerve growth factor by L and 3T3 cells in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1554–1558. doi: 10.1073/pnas.71.4.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt R. M., Pastan I. Decreased binding of epidermal growth factor to BALB/c 3T3 mutant cells defective in glycoprotein synthesis. Nature. 1978 Mar 2;272(5648):68–70. doi: 10.1038/272068a0. [DOI] [PubMed] [Google Scholar]
- Roth J. Assay of peptide hormones using cell receptors: application to insulin and to human growth hormone. Methods Enzymol. 1975;37:66–82. doi: 10.1016/s0076-6879(75)37006-7. [DOI] [PubMed] [Google Scholar]
- Sauer F., Greenstein R. M., Reardon P., Riddick D. H. Secondary amenorrhea associated with balanced X-autosome translocation. Obstet Gynecol. 1977 Jan;49(1):101–104. [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Giles R. E., Kucherlapati R. S., Shimizu Y., Ruddle F. H. Somatic cell genetic assignment of the human gene for mitochondrial NADP-linked isocitrate dehydrogenase to the long arm of chromosome 15. Somatic Cell Genet. 1977 Jan;3(1):47–60. doi: 10.1007/BF01550986. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Shimizu Y., Kucherlapati R. S., Ruddle F. H. Immunochemical detection of human enzymes in hybrid cells. Cell. 1976 Jan;7(1):123–130. doi: 10.1016/0092-8674(76)90262-2. [DOI] [PubMed] [Google Scholar]
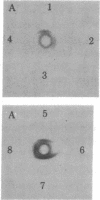
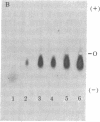
- Shimizu N., Shimizu Y., Ruddle F. H. Assignment of the human mitochondrial NAD-linked malate dehydrogenase gene to the p22 leads to qter region of chromosome 7. Cytogenet Cell Genet. 1978;22(1-6):441–445. doi: 10.1159/000130992. [DOI] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]