Abstract
Background
The household is a recognized community reservoir for Staphylococcus aureus. This study investigated potential risk factors for intra-household S. aureus transmission, including the contribution of environmental contamination.
Methods
We investigated intra-household S. aureus transmission using a sample of multiple member households from a community-based case-control study examining risk factors for CA-MRSA infection conducted in Northern Manhattan. During a home visit, index subjects completed a questionnaire. All consenting household members were swabbed, as were standardized environmental household items. Swabs were cultured for S. aureus. Positive isolates underwent further molecular characterization. Intra-household transmission was defined as having identical strains among two or more household members. Multiple logistic regression was used to identify independent risk factors for transmission.
Results
We enrolled 291 households: 146 index cases, 145 index controls and 687 of their household contacts. The majority of indexes were Hispanic (85%), low income (74%), and female (67%), with a mean age of 31 (range 1–79). The average size of case and control households was 4 people. S. aureus colonized individuals in 62% of households and contaminated the environment in 54% of households. USA300 was the predominant clinical infection, colonizing and environmental strain. Eighty-one households had evidence of intra-household transmission: 55 (38%) case and 26 (18%) control households (P<.01). Environmental contamination with a colonizing or clinical infection strain (aOR: 5.4 [2.9–10.3] P<.01) and the presence of a child under 5 (aOR: 2.3 [1.2–4.5] P = .02) were independently associated with transmission. In separate multivariable models, environmental contamination was associated with transmission among case (aOR 3.3, p<.01) and control households (aOR 27.2, p<.01).
Conclusions
Environmental contamination with a colonizing or clinical infection strain was significantly and independently associated with transmission in a large community-based sample. Environmental contamination should be considered when treating S. aureus infections, particularly among households with multiple infected members.
Introduction
Numerous studies have documented the role of the household as a community reservoir for Staphylococcus aureus [1]–[4]. After a household member becomes infected, high levels of S. aureus colonization and infection often occur among other household members [5]–[8]. These reports have observed that epidemic clones tend to “ping pong” among family members, resulting in a high rate of recurrent infections [9]–[11]. Reducing the frequency of these infections in this setting has proven difficult.
Studies of household transmission of S. aureus, including those that focus on the spread of healthcare-associated methicillin-resistant S. aureus (MRSA) and, more recently, those that examined the spread of community-associated (CA) S. aureus, have identified a number of risk factors associated with transmission. Factors include the presence of an underlying skin condition, the sharing of items, living in a household with a previously infected member, or direct contact such as bathing an infected child [12]–[15]. In contrast with infections in the healthcare setting [16], the limited number of published studies on household transmission suggest that nasal carriage of S. aureus is not always associated with transmission [17].
One potential risk factor for transmission that has received limited attention in community households is the role of environmental contamination with S. aureus. Fomites have been identified as possible vectors of S. aureus transmission and infection in different settings, including the healthcare setting [18] and other community based settings, such as drug user venues [19], [20]. We recently reported that among individuals with a MRSA infection, household environmental contamination with the clinical infection strain was associated with an increased risk of antecedent infection [21]. USA300 accounted for the majority of these infections. The limited success of decolonization strategies to prevent recurrent infections in households has also raised concern regarding the role of environmental contamination in these infections [22], [23].
In light of these observations, we conducted a study to assess colonization and transmission of all S. aureus among the households of CA-MRSA infected case participants and healthy control participants. The study examined potential sociodemographic, health, hygiene, drug use and other household level risk factors for intra-household S. aureus transmission, including the contribution of environmental contamination.
Methods
Ethics Statement
Written informed consent was obtained from each individual before participation. Parental consent was required for the participation of children <18 years old. Pediatric assent was obtained from those capable of providing it. The Institutional Review Board of Columbia University Medical Center approved this study.
Study Population
This analysis was part of a case-control study examining risk factors for CA-MRSA infection in the Northern Manhattan community, as defined by zip codes containing the Columbia University Medical Center catchment area. Data collection took place between January 2009 and May 2011. The overall study design has been previously described [21] and is briefly summarized below.
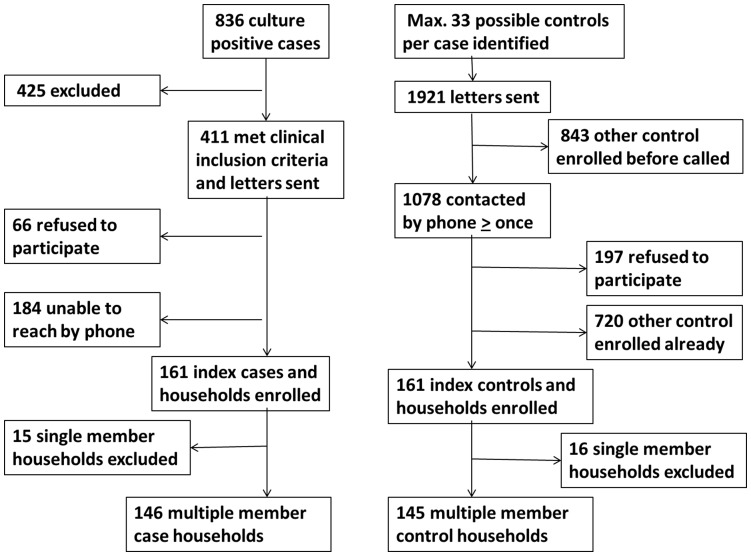
One hundred sixty one index cases with CA-MRSA infections agreed to participate in the study and were enrolled (Figure 1). Index cases were interviewed, on average, 31 days after a positive culture was obtained (sd 19; range 9–116 days). One hundred sixty one age-matched index controls identified from the Columbia University Medical Center dental clinic were enrolled in the study after undergoing the same enrollment procedures as cases. Interviews were carried out with index case and matching index controls, on average, within 28 days of each other (sd 17; range 7–90 days).
Figure 1. Flow chart enrollment of case and control households.
Microbiological Specimen Collection and Molecular Analyses
The clinical infection isolates of index case subjects were retrieved from the clinical microbiology laboratory at Columbia University Medical Center. These isolates were obtained from identified sites of infection. All but one of the clinical infection isolates were located [21]. Anterior nares cultures were collected with sterile pre-moistened swabs (Becton Dickinson Culturette Systems) from all consenting household members, excluding children <1 year old (n = 52). The average number of nasal swabs collected was similar in case and control households (3.5 versus 3.2, P = .21).
A standardized list of environmental items were sampled with pre-moistened swabs in all households: door knobs, TV remote, living room light switch, toy, couch or bed, computer or radio, house phone or index cellular phone, bathroom sink, kitchen appliance handle. The average number of environmental items swabbed was similar in case and control households (8.7 versus 8.8, P = .81).
Culture swabs were incubated overnight at 37°C in high-salt 6.5% broth and plated onto Mannitol Salt Agar (Becton Dickinson) for 48 h at 35°C. Positive mannitol-fermenting yellow colonies were isolated onto 5% Sheep Blood Agar plates (Becton Dickinson) and single colonies were selected for further analysis. S. aureus was identified by coagulase and Protein A detection kit (Murex StaphAurex).
S. aureus positive isolates were genotyped by spa-sequencing using Ridom-staph software [24], [25]. Methicillin-resistance was assessed by presence and type of Staphylococcal Chromosomal Cassette (SCC)mec using multiplex PCR [26], [27]. S. aureus isolates characterized as spa type 8 with or without the presence of SCCmec were categorized as USA300.
S. aureus Transmission Risk Factor Questionnaire
A structured questionnaire was administered to index participants to collect household-level sociodemographic information and assess risk factors for CA-MRSA, including health-related, hygiene-related, and other household-level risk factors. Potentially sensitive information was obtained using audio computer-assisted self-interviewing.
Measures
The main outcome measure used in this study among both case and control households was S. aureus strain similarity within households as a proxy for transmission. Similarity was defined as having identical strains, as determined by spa typing, among two or more household members. Since interviews were conducted and specimens collected shortly after index cases were identified, colonization of a case household member with the clinical infection strain was additionally considered evidence of intra-household transmission, regardless of the current colonization status of the index case.
Environmental contamination with a colonizing or clinical infection strain was defined as one or more household items being contaminated with the same strain as a colonized household member or with the clinical infection strain.
Statistical Analyses
Because the primary objective of our analysis was to assess intra-household transmission, single member households were removed from the data set. Among the 161 case households, there were 15 single member households and among the 161 control households, there were16 single member households. In order to rule out the possibility that age-matching of index cases and controls had a measureable influence on household level variables, matched pair analyses were conducted on a data set that excluded all single member households as well as their age-matched pairs. As anticipated, the results were identical; indicating that matching on the individual level had no impact on household level variables. Therefore, the final sample presented here includes 291 multiple member households: 146 case households and 145 control households.
Frequencies are presented for case index and control index participant descriptive data; chi-square tests and t-tests are used for these comparisons. In bivariate analyses comparing case and control households on sociodemographic and risk factor data, logistic regression models controlling for household size were used. In order to identify independent risk factors for intra-household S. aureus transmission, logistic regression models were used for both bivariate and multivariable analyses; these analyses controlled for household size and case-control status. All variables associated with intra-household S. aureus transmission at P<.20 in bivariate analyses were considered for inclusion in the final logistic regression model. To limit the impact of collinearity, correlations between covariates were examined. A sensitivity analysis was conducted to determine whether to include highly correlated variables. Adjusted odds ratios and 95% confidence intervals are presented. All statistical tests were 2-sided and P<.05 was considered statistically significant. Data were analyzed using SAS 9.1 software (SAS Institute Inc., North Carolina).
Results
Study Population Characteristics
Among the 291 index participants enrolled in the study (146 cases and 145 controls), the average age was 31 years (sd 19; range 1 to 79, P = .43). The majority were female (64% of cases versus 71% of controls, P = .23) and Hispanic (84% of cases versus 86% of controls, P = .65) and less than half had graduated high school (46% of case versus 38% of controls, P = .17).
Index participants named a total of 961 household members; of which 687 (71%) participated. The average size of case and control households was 4 people (sd 2; median 4; range 2 to 11). The mean age of household members was 27 years (sd 21; range 0 to 92 years). Fifty-one percent were female. There were no significant differences between case and control household members on any of these sociodemographic characteristics. There was no difference between case and control households in the average number of participating members (2.5 versus 2.2, P = .21). On the individual level, case non-index household members were more likely to provide specimens than control non-index household members [364/482 (76%) versus 323/479 (67%), (P = .04)].
Table 1 presents the distribution of household-level sociodemographics and risk factors among case and control households. Case households were more likely to have a higher income (P = .03) and share towels than control households (P = .05). Although there were significant differences between case and control households for variables associated with index case eligibility, control households also reported high levels of skin or soft-tissue infection (SSTI) (63%), antibiotic use (61%), and hospitalization (26%) in the past 6 months.
Table 1. Bivariate analyses of household-level sociodemographics and risk factors by case-control status.
| Case households | Control households | ||||||
| (N = 146) | (N = 145) | ||||||
| N | (%) | N | (%) | aORa | (95% CI) | P | |
| Sociodemographics | |||||||
| Income < $21,000 | 98 | (68) | 114 | (80) | 0.5 | (0.3–0.9) | .02 |
| Child ≤5 present | 64 | (44) | 56 | (39) | 1.3 | (0.8–2.2) | .31 |
| Pet present | 44 | (30) | 42 | (29) | 1.1 | (0.6–1.8) | .83 |
| Travel to the Dominican Republic in the past 6 months | 38 | (26) | 34 | (23) | 1.1 | (0.7–2.0) | .61 |
| Health and hygiene factors | |||||||
| Surgery in the past 6 months | 26 | (18) | 32 | (22) | 0.8 | (0.4–1.4) | .36 |
| Injects insulin in the past 6 months | 16 | (11) | 7 | (5) | 2.4 | (1.0–6.1) | .06 |
| Home healthcare attendant | 14 | (10) | 11 | (8) | 1.3 | (0.6–3.0) | .54 |
| Shares towels | 38 | (26) | 24 | (17) | 1.8 | (1.0–3.2) | <.05 |
| Shares Razor | 19 | (13) | 12 | (8) | 1.7 | (0.8–3.6) | .19 |
| Crowding (>1 person per room) | 81 | (56) | 91 | (63) | 0.6 | (0.3–1.1) | .10 |
| Contaminated environment with a colonizing orclinical infection strain | 73 | (50) | 43 | (30) | 2.4 | (1.5–3.9) | <.01 |
| Drug use and other risk factors | |||||||
| Illicit drug use in the past 6 months | 6 | (5) | 1 | (1) | 6.2 | (0.7–52.1) | .10 |
| HIV, IDU, MSM, Prison, STD in the past 6 months | 12 | (10) | 7 | (6) | 1.7 | (0.7–4.6) | .27 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; IDU, intravenous drug use; MSM, men who have sex with men; STD, sexually transmitted disease.
a. Logistic regression was used to calculate adjusted OR’s and 95% CI’s, controlling for household size.
Molecular Characterization of S. aureus Isolates
There was no difference in the number of individuals colonized with S. aureus in case versus control households [185/510 (36%) versus 157/467 (34%), P = .31]. Among the 185 S. aureus isolates colonizing case household members, we detected 64 different spa-types. Among the 157 S. aureus isolates colonizing control household members, we detected 79 different spa-types. There was no difference in the number of environmental items contaminated with S. aureus in case versus control households [198/1269 (16%) versus 164/1269 (13%), P = .20)]. Among the 198 S. aureus isolates contaminating environmental items in case households, we detected 50 different spa-types. Among the 164 S. aureus isolates contaminating environmental items in control households, we detected 47 different spa-types. USA300 was the most frequently cultured strain among case households. It was cultured from the nares of at least one person in 41 (28%) case households and from at least one environmental object in 36 (25%) case households. In contrast, USA300 was rarely retrieved among control households. It was cultured from the nares of at least one person in only 6 (4%) control households and from at least one environmental object in only 12 (8%) control households.
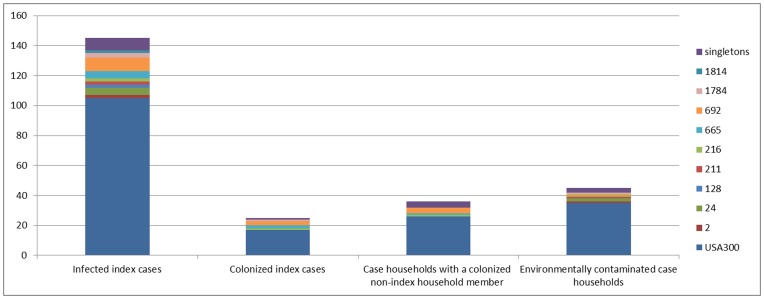
USA300 (n = 105, 72%) was also the most common clinical infection isolate. The remaining 40 (28%) clinical infection isolates belonged to 17 different spa-types. Less than one fifth of index cases (n = 25 or 17%) were nasally colonized with the clinical infection strain at the time of the home visit. A quarter of case households (n = 36 or 25%) had at least one non-index member nasally colonized with the clinical infection strain. USA300 (n = 105) as compared with all other clinical infection strains (n = 40) was not significantly more likely to be found on the index (16% versus 20%, P = .59), a non-index household member (25% versus 25%, P = .95), or in the environment (33% versus 25%, P = .34) (Figure 2).
Figure 2. Distribution of spa types of 145 clinical infection isolates among case households.
Distribution of spa types of 145 clinical infection isolates among infected index cases, colonized index cases, case households with a colonized non-index household member, and environmentally contaminated case households.
S. aureus Colonization, Environmental Contamination, and Transmission
Including the index participants, S. aureus colonization was observed more frequently among the 146 case households than among the 145 control households (69% versus 55%, P = .02). Table 2 presents the distribution of S. aureus colonization among indexes, S. aureus colonization among non-index household members, and S. aureus environmental contamination by case-control status. Overall, MRSA was more likely to be collected among indexes, non-index household members and the environment in case households compared to controls. The difference in MRSA colonization was mainly accounted for by USA300. This finding was expected given the study design.
Table 2. S. aureus colonization and environmental contamination by case-control status.
| Case households | Control households | ||||||
| (N = 146) | (N = 145) | ||||||
| N | (%) | N | (%) | aORa | (95% CI) | P | |
| S. aureus colonization among indexes | |||||||
| Colonized with S. aureus | 41 | (28) | 50 | (35) | 0.7 | (0.5–1.2) | .24 |
| Colonized with MRSA | 25 | (17) | 3 | (2) | 10.3 | (3.0–35.4) | <.01 |
| Colonized with USA300 | 18 | (12) | 3 | (2) | 6.9 | (2.0–24.3) | <.01 |
| Colonized with MSSA | 16 | (11) | 47 | (32) | 0.3 | (0.1–0.5) | <.01 |
| S. aureus colonization among non-index household members | |||||||
| Colonized with S. aureus | 85 | (58) | 54 | (37) | 2.6 | (1.6–4.4) | <.01 |
| Colonized with MRSA | 39 | (27) | 4 | (3) | 13.4 | (4.6–38.9) | <.01 |
| Colonized with USA300 | 28 | (19) | 4 | (3) | 8.2 | (2.8–24.2) | <.01 |
| Colonized with MSSA | 60 | (41) | 51 | (35) | 1.3 | (0.8–2.2) | .26 |
| S. aureus environmental contamination | |||||||
| Contaminated with S. aureus | 84 | (58) | 74 | (51) | 1.3 | (0.8–2.1) | .24 |
| Contaminated with MRSA | 43 | (30) | 6 | (4) | 9.8 | (4.0–24.0) | <.01 |
| Contaminated with USA300 | 36 | (25) | 12 | (8) | 3.7 | (1.8–7.4) | <.01 |
| Contaminated with MSSA | 54 | (37) | 69 | (48) | 0.6 | (0.4–1.0) | .07 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
a. Logistic regression was used to calculate adjusted OR’s and 95% CI’s, controlling for household size.
Table 3 presents the distribution of environmental contamination with a colonizing or clinical infection strain and household transmission by case-control status. Environmental contamination with a colonizing or clinical infection strain was observed in 73 case and 43 control households (50% versus 30%, P<.01). Among the 73 case households with environmental contamination with a colonizing or clinical infection strain, 54 (37%) households were contaminated with the same strain as was colonizing a household member, 45 (31%) households had the clinical infection strain present in the environment, and 26 (18%) households had both the environment contaminated with the same strain as was colonizing a household member and the clinical infection strain present in the environment.
Table 3. Environmental contamination with a colonizing or clinical infection strain and intra-household transmission by case-control status.
| Case households | Control households | ||||||
| (N = 146) | (N = 145) | ||||||
| N | (%) | N | (%) | aORa | (95% CI) | P | |
| Environmental contamination with a colonizing or clinical infection strain | |||||||
| Contaminated with a colonizing strain or the clinical infection strain | 73 | (50) | 43 | (30) | 2.4 | (1.5–3.9) | <.01 |
| Contaminated with a colonizing strain | 54 | (37) | 43 | (30) | 1.4 | (0.9–2.3) | .17 |
| Contaminated with the clinical infection strain | 45 | (31) | |||||
| Contaminated with a colonizing strain and the clinical infection strain | 26 | (18) | |||||
| Intra-household transmission | |||||||
| ≥2 household members colonized with identical strains or ≥1non-index household member colonized with the clinical infection strain | 55 | (38) | 26 | (18) | 3.1 | (1.8–5.6) | <.01 |
| ≥2 household members colonized with identical strains | 35 | (24) | 26 | (18) | 1.5 | (0.8–2.8) | .17 |
| ≥1 non-index household member colonized with theclinical infection strain | 36 | (25) | |||||
| ≥2 household members colonized with identical strains and ≥1non-index household member colonized with the clinical infection strain | 16 | (11) | |||||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
a. Logistic regression was used to calculate adjusted OR’s and 95% CI’s, controlling for household size.
Eighty-one households had evidence of S. aureus transmission: 55 (38%) case households and 26 (18%) control households (P<.01). Among the 55 case households with transmission, 35 (24%) households had multiple members colonized with the same strain, 36 (25%) households had the clinical infection strain present among a non-index household member and 16 (11%) households had both.
Risk Factors for Household Transmission
Table 4 presents analyses assessing risk factors for S. aureus transmission. In bivariate models, environmental contamination with a colonizing or clinical infection strain, having a child under 5, having a pet, a household member having had surgery in the past 6 months, and crowding were positively associated with transmission at P<.20. Injecting insulin and travel to the Dominican Republic in the past 6 months were negatively associated with transmission at P<.20. In the final multivariate model, environmental contamination with a colonizing or clinical infection strain (aOR 5.4, P<.01) and the presence of a child less than five years old (aOR 2.3, P = .02) remained independently associated with intra-household transmission.
Table 4. Bivariate and multivariate analyses of risk factors for S. aureus transmission within households.
| Bivariate analysesa,b | Multivariate analysesa | |||||
| aOR2 | (95% CI) | P | aOR3 | (95% CI) | P | |
| Sociodemographics | ||||||
| Income < $21,000 | 0.8 | (0.4–1.4) | .38 | |||
| Child ≤5 present | 2.4 | (1.3–4.4) | .01 | 2.3 | (1.2–4.5) | .02 |
| Pet present | 1.6 | (0.9–2.9) | .13 | 1.8 | (0.9–3.5) | .10 |
| Travel to the Dominican Republic in the past 6 months | 0.6 | (0.3–1.2) | .14 | 0.6 | (0.3–1.3) | .19 |
| Health and hygiene risk factors | ||||||
| Surgery in the past 6 months | 1.9 | (0.9–3.7) | .08 | 2.1 | (1.0–4.5) | .07 |
| Injects insulin in the past 6 months | 0.3 | (0.1–1.2) | .09 | 0.3 | (0.1–1.1) | .07 |
| Home healthcare attendant | 0.8 | (0.3–2.3) | .68 | |||
| Shares towels | 1.4 | (0.7–2.6) | .36 | |||
| Shares Razor | 1.5 | (0.6–3.4) | .36 | |||
| Crowding (>1 person per room)c | 2.1 | (0.9–4.5) | .07 | |||
| Contaminated environment with a colonizing or clinicalinfection strain | 5.1 | (2.8–9.4) | <.01 | 5.4 | (2.9–10.3) | <.01 |
| Drug use and other household level risk factors | ||||||
| Illicit drug use in the past 6 months | 2.5 | (0.5–12.7) | .26 | |||
| HIV, IDU, MSM, Prison, STD | 1.6 | (0.5–4.8) | .41 | |||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; IDU, intravenous drug use; MSM, men who have sex with men; STD, sexually transmitted disease.
a. Multiple logistic regression was used for analyses, controlling for household size and case-control status.
b. All variables significant at P<.20 in bivariate analyses were considered for inclusion in the final multiple logistic regression model to calculate adjusted OR’s and 95% CI’s.
c. Although P<.20, not included in the final model because of high correlation with household size.
To further explore the role of environmental contamination as a contributing factor to intra-household S. aureus transmission, the data were stratified by case-control status and analyzed separately. In separate multivariable models, environmental contamination with a colonizing or clinical infection strain was significantly and independently associated with S. aureus transmission among both case households (aOR 3.3, p<.01) and control households (aOR 27.2, p<.01).
Discussion
This study is among the first to demonstrate that environmental contamination plays an important role in the household spread of S. aureus. Environmental contamination with a colonizing or clinical infection strain was significantly and independently associated with transmission in a large community-based sample.
There are now several studies that support the contribution of the environment to transmission and infection. In an earlier household-based investigation of the Northern Manhattan community, we found that environmental contamination with the clinical infection strain was associated with an increased risk of antecedent MRSA infections [21]. Additionally, a recent trial designed to reduce the incidence of recurrent CA-S. aureus infections using household decolonization strategies was only partially successful [23]. In this study both mupirocin and chlorhexidine were used to eradicate colonization from the nares as well as other body sites. Subjects in households where all members underwent decolonization still had a 28% and 38% rate of recurrent infections at 3 and 6 months, respectively. As noted by the authors, environmental contamination could help explain the failure of the intervention to prevent recurrent infection in the households [23]. Our finding provides additional evidence to support the hypothesis of Miller and Diep [28] suggesting that fomites constitute a risk for CA-S. aureus infections. Fomites potentially serve as reservoirs, constituting a source for re-colonization or infection of household members that, in turn, increases the risk of transmission to other household members.
We also found that environmental contamination with a colonizing or clinical infection strain was a risk factor for transmission separately among both case households and control households. This finding suggests that the environment is an important reservoir for household transmission of S. aureus, among a large diversity of strain types, and not exclusively among epidemic strains, such as USA300.
As documented in earlier studies, this community-based investigation demonstrated high levels of S. aureus transmission among case and control households [12], [15], [17], [29]. Although there were significant differences between case and control households for variables associated with index case eligibility, control households also reported high levels of skin or soft-tissue infection (63%), antibiotic use (61%), and hospitalization (26%) in the past 6 months. This is in addition to the high levels of environmental contamination. These results therefore demonstrate the high burden of S. aureus among households in general. This high burden of S. aureus and the high levels of antibiotic use, also observed previously in this community [17], [21], may potentially select for the high proportion of CA-MRSA infections being caused by epidemic strains, in this case USA300, among this community.
Among the numerous potential risk factors that we assessed other than environmental contamination (sociodemographic, health, hygiene, drug use and other household level risk factors), only the presence of a child under 5 was significantly and independently associated with transmission. The study design also allowed us to assess the role of infection on S. aureus transmission. Transmission among case households was increased when the clinical infection strain was included (P<.01). Recent antibiotic treatment presumably accounted for the low proportion of indexes that were colonized with the clinical infection strain. The lowered nasal colonization, in addition to the absence of cultures from other body sites, likely resulted in an underestimation of the true burden of S. aureus carriage [17], [29], [30]. Miller et al., recently reported 50% of household members were colonized when multiple body sites were cultured [29]. However, looking exclusively at the spread of the clinical infection strain does not capture all of transmission. As we observed here, strains discordant from the clinical infection isolate were commonly found among case household members and transmission also occurred among control households, although at a significantly lower rate. Overall, the greater level of S. aureus colonization and transmission among case households suggests that CA-MRSA infection presents an additional S. aureus burden among households with an infection.
The epidemic strain USA300 was responsible for the excess burden of S. aureus in case households, accounting for the majority of CA-MRSA infections and environmental contaminants, a trend also noted in our earlier report [21]. Miller et al. recently found that infection of the household index with USA300 was an independent predictor of transmission [29]. These results suggest that USA300, in addition to its enhanced ability to cause infections, is an efficient colonizer of body sites as well as an environmental survivor. Taken together, the data suggest that household transmission of S. aureus is relatively common, regardless of the S. aureus strain. USA300, however, is unique in terms of its heightened invasiveness combined with its transmissibility.
There are a number of limitations to this study. These results are representative of a single predominantly Hispanic community in Northern Manhattan where the majority of index participants were female and may have limited generalizability. Second, this is a retrospective, observational study that uses a proxy variable as evidence of probable household transmission. Therefore, neither the directionality nor the source of transmission may be ascertained and the shared strains potentially indicate a shared exposure. Lastly, this study did not assess the impact of colonization of other body sites and, as noted above, colonization of other body sites is common [17], [29], [30]. Underestimating the colonization of S. aureus, and hence transmission, likely also underestimated the importance of environmental contamination as a reservoir. Although environmental contamination may provide a household reservoir and contribute to transmission, it may also serve as a surrogate marker of efficient colonization of multiple body sites [29], [31].
Notwithstanding these limitations, our findings suggest that environmental contamination plays a significant role in intra-household transmission. Environmental decontamination should be considered when treating CA-MRSA infections, particularly among households with multiple infected members. In light of the predominant role of the epidemic clone USA300, an additional strategy to prevent recurrent infections may be to target those epidemic strains that are both highly transmissible and more successful as pathogens that cause infections. This latter approach would allow a more efficient preventative strategy to be developed.
Acknowledgments
We thank Dr. Ron Myers, Columbia University, School of Dentistry, New York, for his help in implementing the study.
Funding Statement
This work was supported by the National Institutes of Health [AI077690 to FL and AI090013 to ACU) and the Paul A. Marks scholarship (to ACU) by Columbia University Medical Center Institutional grants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wagenvoort J, Toenbreker H, Nurmohamed A, Davies B (1997) Transmission of methicillin-resistantStaphylococcus aureus within a household. European Journal of Clinical Microbiology & Infectious Diseases 16: 399–400. [DOI] [PubMed] [Google Scholar]
- 2. L'Heriteau F, Lucet JC, Scanvic A, Bouvet E (1999) Community-acquired methicillin-resistant Staphylococcus aureus and familial transmission. JAMA 282: 1038–1039. [DOI] [PubMed] [Google Scholar]
- 3. Busato CR, Carneiro Leao MT, Gabardo J (1998) Staphylococcus aureus Nasopharyngeal Carriage Rates and Antimicrobial Susceptibility Patterns Among Health Care Workers and Their Household Contacts. Braz J Infect Dis 2: 78–84. [PubMed] [Google Scholar]
- 4. Huijsdens XW, van Santen-Verheuvel MG, Spalburg E, Heck ME, Pluister GN, et al. (2006) Multiple cases of familial transmission of community-acquired methicillin-resistant Staphylococcus aureus . J Clin Microbiol 44: 2994–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zafar U, Johnson LB, Hanna M, Riederer K, Sharma M, et al. (2007) Prevalence of nasal colonization among patients with community-associated methicillin-resistant Staphylococcus aureus infection and their household contacts. Infect Control Hosp Epidemiol 28: 966–969. [DOI] [PubMed] [Google Scholar]
- 6. Hugo Johansson P, Gustafsson EB, Ringberg H (2007) High prevalence of MRSA in household contacts. Scandinavian Journal of Infectious Diseases 39: 764–768. [DOI] [PubMed] [Google Scholar]
- 7. Lautenbach E, Tolomeo P, Nachamkin I, Hu B, Zaoutis TE (2010) The impact of household transmission on duration of outpatient colonization with methicillin-resistant Staphylococcus aureus. Epidemiology and Infection 138: 683–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ho PL, Cheung C, Mak GC, Tse CW, Ng TK, et al. (2007) Molecular epidemiology and household transmission of community-associated methicillin-resistant Staphylococcus aureus in Hong Kong. Diagnostic microbiology and infectious disease 57: 145–151. [DOI] [PubMed] [Google Scholar]
- 9. Jones TF, Creech CB, Erwin P, Baird SG, Woron AM, et al. (2006) Family outbreaks of invasive community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 42: e76–78. [DOI] [PubMed] [Google Scholar]
- 10. Huang YC, Ho CF, Chen CJ, Su LH, Lin TY (2007) Nasal carriage of methicillin-resistant Staphylococcus aureus in household contacts of children with community-acquired diseases in Taiwan. Pediatr Infect Dis J 26: 1066–1068. [DOI] [PubMed] [Google Scholar]
- 11. Cook HA, Furuya EY, Larson E, Vasquez G, Lowy FD (2007) Heterosexual transmission of community-associated methicillin-resistant Staphylococcus aureus . Clin Infect Dis 44: 410–413. [DOI] [PubMed] [Google Scholar]
- 12. Mollema FP, Richardus JH, Behrendt M, Vaessen N, Lodder W, et al. (2010) Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol 48: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucet JC, Paoletti X, Demontpion C, Degrave M, Vanjak D, et al. (2009) Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med 169: 1372–1378. [DOI] [PubMed] [Google Scholar]
- 14. Calfee DP, Durbin LJ, Germanson TP, Toney DM, Smith EB, et al. (2003) Spread of methicillin-resistant Staphylococcus aureus (MRSA) among household contacts of individuals with nosocomially acquired MRSA. Infect Control Hosp Epidemiol 24: 422–426. [DOI] [PubMed] [Google Scholar]
- 15. Nerby JM, Gorwitz R, Lesher L, Juni B, Jawahir S, et al. (2011) Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus . Pediatr Infect Dis J 30: 927–932. [DOI] [PubMed] [Google Scholar]
- 16. von Eiff C, Becker K, Machka K, Stammer H, Peters G (2001) Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 344: 11–16. [DOI] [PubMed] [Google Scholar]
- 17. Miller M, Cook HA, Furuya EY, Bhat M, Lee MH, et al. (2009) Staphylococcus aureus in the community: colonization versus infection. PLoS One 4: e6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dancer SJ (2009) The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 73: 378–385. [DOI] [PubMed] [Google Scholar]
- 19. Latkin C, Mandell W, Vlahov D, Oziemkowska M, Celentano D (1996) People and places: behavioral settings and personal network characteristics as correlates of needle sharing. J Acquir Immune Defic Syndr Hum Retrovirol 13: 273–280. [DOI] [PubMed] [Google Scholar]
- 20. Gwizdala RA, Miller M, Bhat M, Vavagiakis P, Henry C, et al. (2011) Staphylococcus aureus colonization and infection among drug users: identification of hidden networks. Am J Public Health 101: 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uhlemann AC, Knox J, Miller M, Hafer C, Vasquez G, et al. (2011) The environment as an unrecognized reservoir for community-associated methicillin resistant Staphylococcus aureus USA300: a case-control study. PLoS One 6: e22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fritz SA, Camins BC, Eisenstein KA, Fritz JM, Epplin EK, et al. (2011) Effectiveness of measures to eradicate Staphylococcus aureus carriage in patients with community-associated skin and soft-tissue infections: a randomized trial. Infect Control Hosp Epidemiol 32: 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fritz SA, Hogan PG, Hayek G, Eisenstein KA, Rodriguez M, et al. (2012) Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis 54: 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, et al. (1999) Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol 37: 3556–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harmsen D, Claus H, Witte W, Rothganger J, Turnwald D, et al. (2003) Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 41: 5442–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milheirico C, Oliveira DC, de Lencastre H (2007) Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus . Antimicrob Agents Chemother 51: 3374–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Milheirico C, Oliveira DC, de Lencastre H (2007) Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus: 'SCCmec IV multiplex'. J Antimicrob Chemother 60: 42–48. [DOI] [PubMed] [Google Scholar]
- 28. Miller LG, Diep BA (2008) Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46: 752–760. [DOI] [PubMed] [Google Scholar]
- 29. Miller LG, Eells SJ, Taylor AR, David MZ, Ortiz N, et al. (2012) Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis 54: 1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee CJ, Sankaran S, Mukherjee DV, Apa ZL, Hafer CA, et al. (2011) Staphylococcus aureus oropharyngeal carriage in a prison population. Clin Infect Dis 52: 775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harbarth S, Liassine N, Dharan S, Herrault P, Auckenthaler R, et al. (2000) Risk factors for persistent carriage of methicillin-resistant Staphylococcus aureus . Clin Infect Dis 31: 1380–1385. [DOI] [PubMed] [Google Scholar]