Abstract
The choroid plexus is a multifunctional organ that sits at the interface between the blood and cerebrospinal fluid (CSF). It serves as a gateway for immune cell trafficking into the CSF and is in an excellent position to provide continuous immune surveillance by CD4+ T cells, macrophages and dendritic cells and to regulate immune cell trafficking in response to disease and trauma. However, little is known about the mechanisms that control trafficking through this structure. Three cell types within the choroid plexus, in particular, may play prominent roles in controlling the development of immune responses within the nervous system: the epithelial cells, which form the blood-CSF barrier, and resident macrophages and dendritic cells in the stromal matrix. Adhesion molecule and chemokine expression by the epithelial cells allows substantial control over the selection of cells that transmigrate. Macrophages and dendritic cells can present antigen within the choroid plexus and/or transmigrate into the cerebral ventricles to serve a variety of possible immune functions. Studies to better understand the diverse functions of these cells are likely to reveal new insights that foster the development of novel pharmacological and macrophage-based interventions for the control of CNS immune responses.
Keywords: cerebrospinal fluid, pleocytosis, monocytes, macrophages, dendritic cells, T cells, inflammation, adhesion molecules, chemokines
Once considered isolated from the immune system, it is now clear that the central nervous system (CNS) is continuously monitored by immune cells, many of which circulate through the cerebrospinal fluid (CSF). On average, normal CSF contains about 1.12 white blood cells/µl1 with approximately 500,000 immune cells trafficking through the ventricles each day. Within the CNS, specialized tissue interfaces and intrinsic factors play a prominent role in the control of this trafficking and the development of inflammatory responses. These interfaces include the blood-brain barrier, the choroid plexus and the meningeal vasculature. While considerable attention has been paid to immune cell interactions at the blood-brain barrier, fewer studies have focused on the role of the blood-cerebrospinal fluid barrier within the choroid plexus and meninges.2-4 The limited knowledge of the control of immune cell trafficking at the blood-CSF barrier stands in stark contrast to the widespread use of CSF leukocyte pleocytosis in clinical studies to evaluate inflammation in the CNS. Available evidence clearly indicates that the choroid plexus has many unique features such as abundant dendritic cells,5 macrophages6 and patterns of epithelial adhesion molecule expression7 that support continuous immunosurveillance1,8,9 as well as the ability to regulate cell activation and trafficking in response to pathological insults.8,10 However, many outstanding questions remain regarding the regulation and function of immune cell trafficking through the choroid plexus into the cerebral ventricles. How are these cells recruited? What regulates the extent and selectivity of the trafficking? How do the immune cells function after entry into the CSF? In this commentary we discuss the dynamic role of the choroid plexus in the control of CNS immune cell trafficking.
The Choroid Plexus and Meningeal Vasculature as a Gateway to the CNS
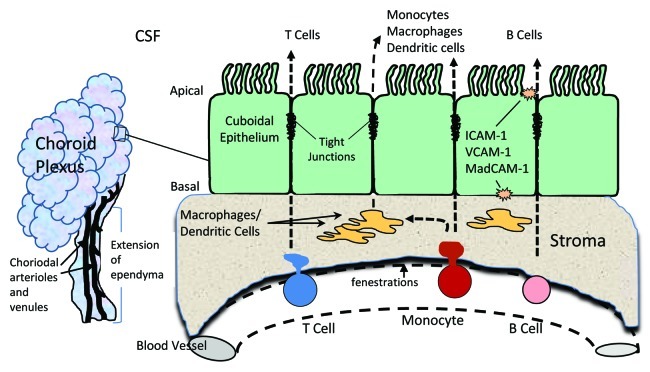
The choroid plexus is a specialized organ with unique properties, the best known of which is the production of CSF. It extends from the ependyma of the lateral, third and fourth ventricles of the brain and is composed of blood vessels surrounded by a stromal matrix and a single layer of cuboidal epithelium (Fig. 1).2 CSF is produced by the cuboidal epithelium at a high rate of 0.2–0.4 ml/min/g of tissue in mammals with a turnover of 3–4 times/day.11-13 To support this production of CSF, the choroid plexus maintains a rate of blood flow that is ~5–10 times greater than other tissues. This high rate of blood flow and the absence of a vascular barrier (fenestrated capillaries with no tight junctions) also provide excellent exposure to circulating immune cells within the choroid plexus. Cells that transmigrate across the endothelium enter a stromal matrix that contains numerous macrophages and dendritic cells poised for interactions. To gain access to the CSF, cells must then traverse the cuboidal epithelium, which expresses tight junctions that form the blood-CSF barrier. While the epithelium serves many functions that have been the focus of excellent reviews,11,14 this commentary will discuss the role of the choroid plexus in the regulation of immune cell trafficking.
Figure 1. Left: schematic representation of the choroid plexus showing the multi-lobed structure that extends from the ependyma into the lateral, third and fourth ventricles. The choroidal artery and arterioles entering through the stalk provide a rich supply of blood that circulates through the choroid plexus and then exits via venules. Right: a small segment of epithelium adjacent to a single capillary is depicted. The vascular endothelium is separated from the epithelium by a stromal matrix that contains numerous macrophages and dendritic cells. Trafficking cells easily transmigrate across the fenestrated vascular endothelium, which has no barrier. To reach the cerebrospinal fluid (CSF) in the cerebral ventricles, migrating cells must traverse the stroma and the epithelium, which contains tight junctions that form the blood-CSF barrier. The resident macrophages and dendritic cells in the stroma may also transmigrate across the epithelium into the CSF.
Adhesion Molecule Expression in the Choroid Plexus
Early studies of the choroid plexus began to reveal its potential immunological importance by demonstrating the expression of adhesion molecules on the surface of the choroid plexus epithelium and the ability of the epithelial cells to present antigen to T cells.15 Anatomical studies described macrophages on the surface of the ventricles (epiplexus cells) suggesting that these cells may traffic into the CSF from the choroid plexus.16 Support for this view was obtained from experiments showing that systemically delivered markers that do not cross the blood-brain or blood-CSF barriers did appear in ventricular macrophages.17 Highly efficient communication between the CSF and the immune system was shown by studies demonstrating a potent immune response after delivery of antigens directly into the CSF18 and the development of unusually high systemic viral titers after infusion of immunodeficiency virus into the CSF.19 Numerous studies have subsequently illustrated that the choroid plexus and meninges are uniquely structured to support and regulate immune cell trafficking.
Adhesion molecules are expressed on the epithelium
An important feature of the choroid plexus is the expression of the adhesion molecules VCAM-1, ICAM-1, P-selectin and E-cadherin on the epithelium rather than the vascular endothelium.7,20-24 This suggests that trafficking within the choroid plexus is largely controlled by the epithelium. These adhesion molecules are also expressed along venules at the base of the choroid plexus and within the meninges.7,25 Importantly, ICAM-1, VCAM-1 and P-selectin are constitutively expressed at these sites suggesting an environment that supports continuous trafficking of immune cells and immunosurveillance of the nervous system.9,10 Other adhesion molecules such as MadCAM are expressed de novo in response to specific stimuli such as infections.26 VCAM-1, ICAM-1 and MadCAM are all upregulated in the choroid plexus epithelium in response to autoimmune responses,23 infection23,27 and traumatic brain injury.28 Similar upregulation could be seen in vitro after exposing choroid plexus epithelial cells to proinflammatory molecules, illustrating their direct responsiveness to inflammatory stimuli.12 Regulation also occurs at the stromal venules exiting the choroid plexus, which express P-selectin, E-selectin and ICAM-1 and support leukocyte trafficking.7,25 While it is clear from these studies that choroidal epithelium and the associated vasculature support immune cell trafficking, detailed studies of adhesion molecule expression under different conditions are needed to better appreciate the extent of control at these sites.
Immune Cell Trafficking through the Choroid Plexus
Immune cell trafficking into the CSF under normal conditions
Up to 450,000 white blood cells are normally found within the CSF of healthy adults (0–3 cells/mm3) with a CSF:blood ratio of ~1:2,500 for lymphocytes and 1:2,000 for monocytes.1,7 The composition of these cells is quite different from blood indicating that the cells are highly selected for entry into the brain compartment. Flow cytometry studies designed to define the leukocyte populations in normal CSF have only recently been performed. Patients undergoing surgery for non-neurological conditions had an average of 1.12 leukocytes/µl in CSF with the following relative composition: 8% granulocytes, 68% lymphocytes, 23% monocytes and 2.2% myeloid dendritic cells.1 In contrast to normal blood (58.5% granulocytes, 35% lymphocytes 6.5% monocytes and 0.24% dendritic cells), the relative composition of cells in the CSF was heavily weighted toward dendritic cells (2.2% vs. 0.24%) and monocytes (23% vs. 6.5%). T cells, the most abundant cells in CSF, were enriched moderately relative to blood (68% vs 35%). Notably, most T cells were CD4+ central memory (CD45RA−/CD27/28+) T cells with retention of capacity for homing to secondary lymphoid organs.25 Forty-six percent of these T cells were also CD69+ vs. 4% in blood indicating recent activation.1,25 Like the CD4+ T cells, CD8+ T cells that penetrated into the CSF were mostly activated central memory cells. Natural killer (NK) cells and B cells were very low in normal CSF suggesting that they are not involved in normal immune surveillance of the central nervous system. The functions of the T cells and monocytic cells that routinely circulate in the CSF are not well understood but may involve different functional pathways. Expression of adhesion molecules VCAM-1 and ICAM-1 on the luminal side of the choroid plexus epithelium7 suggests that immune cells in the CSF might traffic back into the choroid plexus where they could interact with resident macrophages/dendritic cells and/or re-enter the blood stream. Alternatively, immune cells could follow the flow of the CSF and exit the CNS compartment into cervical lymph nodes or blood and ultimately contribute to a more general immune response. In each case, macrophages and dendritic cells are in an excellent position to initiate an early innate immune response that primes the nervous system through the secretion of cytokines (inflammatory and anti-inflammatory), chemokines and/or growth factors.
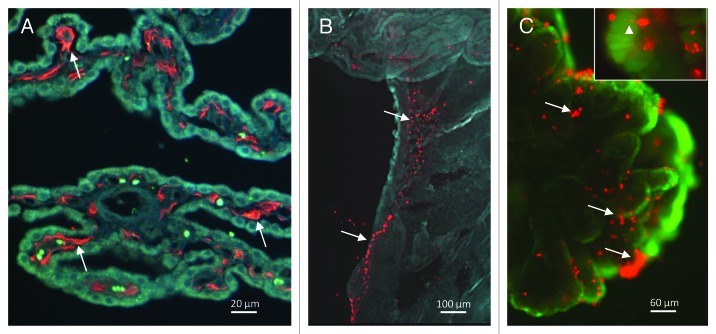
A unique feature of the choroid plexus is the presence of a large population of macrophages and dendritic cells within the stroma (Fig. 2A) and adjacent to blood vessels within the base of the choroid plexus (Fig. 2B). In rat choroid plexus, MHC II+/OX62+ dendritic cells outnumbered CD68+/ED2+ resident tissue macrophages in the stroma with the opposite pattern in the pial leptomeninges.29 In patients with multiple sclerosis (MS), viral encephalitis and HIV infection, intense HLA-DR staining was seen in the choroid plexus stroma, epithelium and epiplexus cells indicating active participation in antigen presentation.5,24 In studies designed to explore the early response to lentiviral infection, lymphocyte trafficking into the choroid plexus was one of the earliest signs of pathology reinforcing the view that this structure may serve as an early warning system to set the stage for subsequent immune interactions.30
Figure 2. (A) Iba1 immunoreactive monocytic cells (arrows) within the stroma of the choroid plexus of a cat infected with feline immunodeficiency virus. (B) Macrophages/dendritic cells labeled with DiI acetylated low density lipoprotein (LDL) in the stalk of a choroid plexus explant imaged at low magnification. Regions with abundant macrophages tend to follow the vasculature within the stalk. (C) Initially absent on the surface of the choroid plexus, large numbers of DiI acetylated LDL labeled cells (arrows) migrate to the surface of the epithelium of a choroid plexus explant cultured in vitro. The epithelium in the background is stained with the live cell stain calcein. Inset: Enlarged view of a single labeled macrophage/dendritic cell (arrowhead) with a process extending between epithelial cells.
The choroid plexus may provide a rich source of macrophages and dendritic cells that traffic into the cerebral ventricles
The extent of macrophage and dendritic cell trafficking from the choroid plexus into the cerebral ventricles may be more robust than generally appreciated. In choroid plexus explants cultured in vitro we have shown that macrophages/dendritic cells labeled with DiI-acetylated low density lipoprotein (LDL) increased in number and migrated to the apical surface of the epithelium8 (Fig. 2C). The rapid expansion of these cells and the migration was highly dependent on the presence of epithelial cells and was not dependent on an experimental chemokine gradient. The direction of migration was preferentially through the epithelial barrier even when little or no barrier was present in the opposite direction, suggesting that the cells are drawn to the epithelium. The migration in the absence of an external challenge was substantial and the expansion and migration of these cells was further enhanced in the presence of immunodeficiency virus.8 This response indicted that robust mechanisms are present in the isolated organ that recruit macrophages across the epithelium under both normal and pathological conditions. The functions of these cells in the CSF are not known. However, in preliminary unpublished studies, the potential potency of trafficking macrophages was demonstrated by the induction of intense and widespread neutrophil trafficking into the brain after infusion of 105 lentivirus-infected choroid plexus-derived macrophages into the lateral ventricle (approximately 50 cells/µl of CSF). Cultured PBMCs or free virus failed to produce a similar response. How these cells induced such a potent and widespread response is not known. These observations raise numerous possibilities for the functions of these macrophages as well as the potential for therapeutic manipulation. For example, monocyte/macrophages are currently being engineered for the delivery of antiretroviral and other drugs to sites within the CNS.31 Natural targeting of these cells to damaged regions guided by secreted chemokines would be expected to provide selective and efficient delivery of therapeutic agents. Based on our observations, we believe that macrophages trafficking into the CSF from the choroid plexus and associated vasculature play a critical role in the evolution of CNS immune responses. A better understanding of the functions of the choroid plexus macrophages may reveal novel opportunities for control of CNS inflammation, neural repair and neurogenesis. Much needs to be done to explore this possibility as many important questions remain to be answered: What is the extent of their influence on recruitment and trafficking of other immune cells? How robust is macrophage trafficking into the ventricles in vivo? What is the functional impact of antigen presentation within the choroid plexus? Do ventricular macrophages enter the brain parenchyma or simply exit the nervous system into blood and lymph nodes?
Immune cell trafficking into the CSF varies greatly in response to disease and trauma
A study of CSF cell populations in 65 patients with various inflammatory diseases of the CNS (MS, neuroborreliosis, bacterial infection, viral infection and malignancies) identified patterns unique to each disease.10,28 The normal cellular composition of CSF and changes in major cell subsets seen in response to various diseases are summarized in Figure 3. These changes may reflect trafficking of immune cells through the blood-brain barrier and meningeal vasculature as well as the choroid plexus although the relative contribution from various sources is still poorly understood. Neuroborreliosis (NB) was associated with increased numbers of activated lymphocytes and CD19−/CD138+ plasma cells in the CSF; viral encephalitis/meningitis (VM) with activated CD4+ lymphocytes and neutrophils; bacterial meningitis (BM) and traumatic brain injury with monocytes and neutrophils, MS with activated CD4+ and CD8+ lymphocytes and plasma cells and malignancies with malignant cells and activated monocytes. In each case, the cells that traffic into the CSF do not reflect the composition of immune cells in blood.10,28,32 Cells within the CSF may also vary over time. For example, the early stages of viral encephalitis and meningitis present with neutrophil infiltration and are followed at later stages by mostly CD3+/CD4+ lymphocytes.10 The unique trafficking patterns led Adam et al.10 to suggest that the cellular composition of CSF may have diagnostic potential. In addition, the trafficking patterns may provide insights into the various ways in which the choroid plexus selects and activates immune cells.
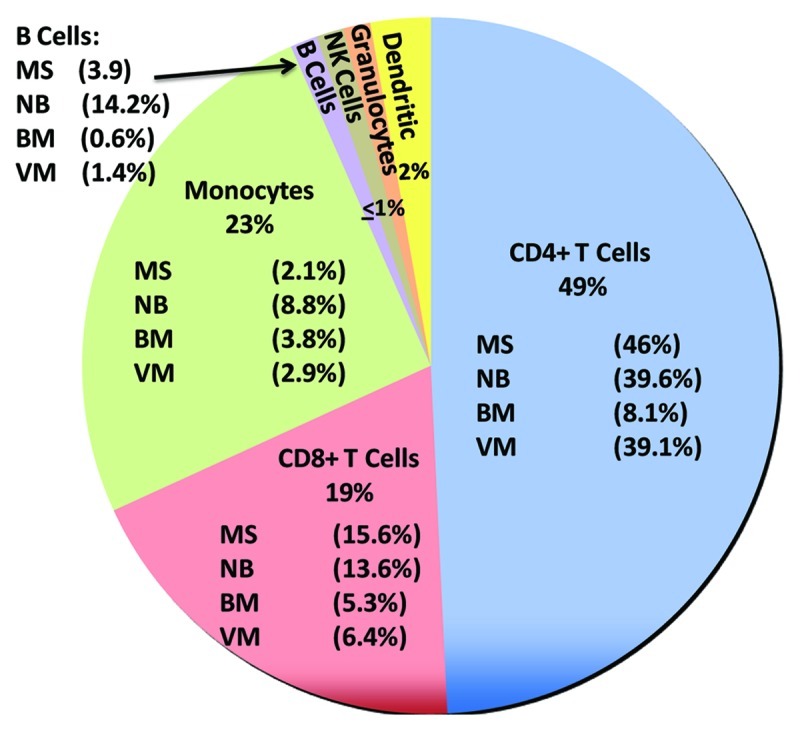
Figure 3. Leukocyte populations in human CSF under normal and pathological conditions. The relative abundance of cells (percentage of total cells) in CSF from patients was summarized from de Graaf et al.,1 Kivisäkk et al.,25 Neuenberg et al.35 and Provencio et al.9 The predominant cells in normal CSF were T cells (49% CD4+, 19% CD8+). Monocytes were the second most abundant leukocyte found in normal CSF (23%) while B cells, granulocytes and dendritic cells were present at low levels. The percentage composition may change in response to various diseases as illustrated (MS, multiple sclerosis; NB, neuroborreliosis; BM, bacterial meningitis; VM, viral meningitis). B cells were increased compared with normal CSF during infections such as NB (14.2% vs. < 1%) and during MS (3.9% vs. < 1%). Because of large increases in lymphocytes and granulocytes in some diseases, monocytes may increase in number but constitute a lower percentage of the total CSF cell population. For example, monocyte numbers increase approximately 500-fold in BM relative to normal CSF but represent only about 3.8% of total CSF cells.
What stimulates the trafficking of immune cells through the choroid plexus?
Many studies have examined chemokines within the CSF to begin to explain trafficking and in many cases correlations exist between specific chemokines and CSF immune cells that express the appropriate chemokine receptor. However, some have pointed out the substantial epithelial and stromal barriers that would prevent circulating or newly migrated immune cells from sensing CSF chemokines. How then do cells find their way through these barriers? Studies of neutrophil trafficking have shown that the epithelium may release chemokines in a polarized fashion to attract cells to their surface and facilitate transmigration.33 These observations again point to the epithelium as a critical element in the regulation of immune cell trafficking. Table 1 illustrates the chemokine profile in “normal” CSF from patients with no significant inflammation or detectable neurodegenerative disease (adapted from Meeker et al.34). The CSF profile is dominated by chemokines that attract monocytes, such as CCL-2 (MCP-1), IL-8, fractalkine, MIP-1β, MIP-1δ and MIP-3α. This would be consistent with the in vitro studies described above showing robust movement of macrophages through the epithelium where they could then respond to chemokines circulating in the CSF. The chemokine profile is also consistent with the preferential accumulation of monocytic cells in human CSF as described above. The fate of these cells is unclear. Some may become epiplexus cells residing on the on the wall of the ventricles where they would not easily be detected. Many may also have a dendritic phenotype not visible with standard monocyte markers. More detailed studies focusing on macrophage and dendritic cell phenotypes and functions may provide a better appreciation of the number and fate of cells entering the CSF from the choroid plexus.
Table 1. Chemokines in normal human CSF.
| Chemokine | Common name | Receptor | Relative expression (%) ± sem |
|---|---|---|---|
| CCL2 |
MCP-1 |
CCR2 |
18.022 ± 1.710 |
| CXCL8 |
IL-8 |
CXCR1, CXCR2 |
1.040 ± 0.477 |
| CX3CL1 |
Fractalkine |
CX3CR1 |
0.915 ± 0.204 |
| CCL18 |
PARC, MIP-4 |
- |
0.841 ± 0.235 |
| CCL4 |
MIP-1b |
CCR5 |
0.758 ± 0.435 |
| CCL15 |
MIP-1d |
CCR1, CCR3 |
0.730 ± 0.151 |
| CCL22 |
MDC |
CCR4 |
0.674 ± 0.160 |
| CCL20 |
MIP-3a |
CCR6 |
0.626 ± 0.144 |
| XCL1 |
Lymphotactin |
XCR1 |
0.483 ± 0.069 |
| CCL18 |
I-309 |
CCR8 |
0.453 ± 0.065 |
| CCL24 |
Eotaxin-3 |
CCR3 |
0.426 ± 0.233 |
| CCL3 |
MIP-1a |
CCR5, CCR1 |
0.412 ± 0.252 |
| CCL25 |
TECK |
CCR9 |
0.305 ± 0.068 |
| CXCL11 |
I-TAC |
CXCR3 |
0.243 ± 0.051 |
| CCL19 |
MIP-3b |
CCR7 |
0.203 ± 0.063 |
| CXCL12 |
SDF-1 |
CXCR4 |
0.154 ± 0.080 |
| CCL5 |
RANTES |
CCR1, CCR3, CCR5 |
ND |
| CCL17 |
TARC |
CCR4, CCR8 |
ND |
| CXCL13 |
BCA-1 |
CXCR5 |
N/A |
| CXCL10 | IP-10 | CXCR3 | N/A |
Relative expression of chemokines in CSF was calculated as the % of total protein content on an array of 120 proteins (Ray BioTech). Values below 0.45 were generally not considered to be significantly greater than zero using a p value of 0.01. ND, no detectable signal; N/A, known to be present in CSF but not available on the array.
The chemokine profiles in CSF can change substantially in response to disease and trauma but the origin of these changes is also poorly understood. CCL2 increases and decreased during different stages of MS and perhaps HIV infection. CXCL10 increases in both MS and HIV infection. Macrophages/dendritic cells, epithelium and parenchymal cells may all contribute to the secretion of these and other chemokines into the CSF but the contributions of each are not well understood. This is a relatively unexplored area that needs greater attention to provide a better understanding of how different cells coordinate with the choroid plexus to control immune cell trafficking.
Summary
In contrast to the blood-brain barrier, the choroid plexus is structured to interact more dynamically with the immune system including mechanisms to support continuous immunosurveillance and specific responses to disease and injury. Epithelium and a large population of macrophages/dendritic cells appear to be major players in these responses via their capacity for adhesion molecule expression, chemokine secretion, antigen presentation and active surveillance. However, more work needs to be done to better appreciate the influence of the choroid plexus on the development of CNS immune responses. Insights into these mechanisms may provide opportunities to develop therapeutic interventions that control inflammation and repair processes in the CNS.
Acknowledgments
This work was supported by NIH Grant R01-MH063646.
Glossary
Abbreviations:
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EAE
experimental autoimmune encephalomyelitis
- HIV
human immunodeficiency virus
- ICAM
intercellular adhesion molecule
- MadCAM
mucosal addressin cell adhesion molecule
- MS
multiple sclerosis
- VCAM
vascular cell adhesion molecule
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21054
References
- 1.de Graaf MT, Smitt PA, Luitwieler RL, van Velzen C, van den Broek PD, Kraan J, et al. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom. 2011;80:43–50. doi: 10.1002/cyto.b.20542. [DOI] [PubMed] [Google Scholar]
- 2.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–79. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune Pharmacol. 2009;4:462–75. doi: 10.1007/s11481-009-9166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010;7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanly A, Petito CK. HLA-DR-positive dendritic cells of the normal human choroid plexus: a potential reservoir of HIV in the central nervous system. Hum Pathol. 1998;29:88–93. doi: 10.1016/S0046-8177(98)90395-1. [DOI] [PubMed] [Google Scholar]
- 6.Chinnery HR, Ruitenberg MJ, McMenamin PG. Novel characterization of monocyte-derived cell populations in the meninges and choroid plexus and their rates of replenishment in bone marrow chimeric mice. J Neuropathol Exp Neurol. 2010;69:896–909. doi: 10.1097/NEN.0b013e3181edbc1a. [DOI] [PubMed] [Google Scholar]
- 7.Kleine TO, Benes L. Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons. Cytometry A. 2006;69:147–51. doi: 10.1002/cyto.a.20225. [DOI] [PubMed] [Google Scholar]
- 8.Meeker RB, Bragg DC, Poulton W, Hudson L. Transmigration of macrophages across the choroid plexus epithelium in response to the feline immunodeficiency virus. Cell Tissue Res. 2012;347:443–55. doi: 10.1007/s00441-011-1301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provencio JJ, Kivisäkk P, Tucky BH, Luciano MG, Ransohoff RM. Comparison of ventricular and lumbar cerebrospinal fluid T cells in non-inflammatory neurological disorder (NIND) patients. J Neuroimmunol. 2005;163:179–84. doi: 10.1016/j.jneuroim.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Adam P, Sobeka O, Scott CS. Analysis of cerebrospinal fluid cell populations with monoclonal antibodies. Folia Microbiol (Praha) 2007;52:529–34. doi: 10.1007/BF02932115. [DOI] [PubMed] [Google Scholar]
- 11.Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol. 2011;39:186–212. doi: 10.1177/0192623310394214. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology. 2001;57:1763–6. doi: 10.1212/WNL.57.10.1763. [DOI] [PubMed] [Google Scholar]
- 13.Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–70. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathanson JA, Chun LL. Immunological function of the blood-cerebrospinal fluid barrier. Proc Natl Acad Sci U S A. 1989;86:1684–8. doi: 10.1073/pnas.86.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling EA, Kaur C, Lu J. Origin, nature, and some functional considerations of intraventricular macrophages, with special reference to the epiplexus cells. Microsc Res Tech. 1998;41:43–56. doi: 10.1002/(SICI)1097-0029(19980401)41:1<43::AID-JEMT5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Ling EA. Ultrastruct and origin of epiplexus cells in the telencephalic choroid plexus of postnatal rats studied by intravenous injection of carbon particles. J Anat. 1979;129:479–92. [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon LB, Knopf PM, Cserr HF. Ovalbumin is more immunogenic when introduced into brain or cerebrospinal fluid than into extracerebral sites. J Neuroimmunol. 1992;40:81–7. doi: 10.1016/0165-5728(92)90215-7. [DOI] [PubMed] [Google Scholar]
- 19.Liu P, Hudson LC, Tompkins MB, Vahlenkamp TW, Colby B, Rundle C, et al. Cerebrospinal fluid is an efficient route for establishing brain infection with feline immunodeficiency virus and transfering infectious virus to the periphery. J Neurovirol. 2006;12:294–306. doi: 10.1080/13550280600889567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figarella-Branger D, Lepidi H, Poncet C, Gambarelli D, Bianco N, Rougon G, et al. Differential expression of cell adhesion molecules (CAM), neural CAM and epithelial cadherin in ependymomas and choroid plexus tumors. Acta Neuropathol. 1995;89:248–57. doi: 10.1007/BF00309340. [DOI] [PubMed] [Google Scholar]
- 21.Wolburg K, Gerhardt H, Schulz M, Wolburg H, Engelhardt B. Ultrastructural localization of adhesion molecules in the healthy and inflamed choroid plexus of the mouse. Cell Tissue Res. 1999;296:259–69. doi: 10.1007/s004410051287. [DOI] [PubMed] [Google Scholar]
- 22.Carrithers MD, Visintin I, Kang SJ, Janeway CA., Jr. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 2000;123:1092–101. doi: 10.1093/brain/123.6.1092. [DOI] [PubMed] [Google Scholar]
- 23.Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol. 1996;148:1819–38. [PMC free article] [PubMed] [Google Scholar]
- 24.Vercellino M, Votta B, Condello C, Piacentino C, Romagnolo A, Merola A, et al. Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: a neuropathological study. J Neuroimmunol. 2008;199:133–41. doi: 10.1016/j.jneuroim.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 25.Kivisäkk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–94. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelhardt B, Wolburg-Buchholz K, Wolburg H. Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech. 2001;52:112–29. doi: 10.1002/1097-0029(20010101)52:1<112::AID-JEMT13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Deckert-Schlüter M, Schlüter D, Hof H, Wiestler OD, Lassmann H. Differential expression of ICAM-1, VCAM-1 and their ligands LFA-1, Mac-1, CD43, VLA-4, and MHC class II antigens in murine Toxoplasma encephalitis: a light microscopic and ultrastructural immunohistochemical study. J Neuropathol Exp Neurol. 1994;53:457–68. doi: 10.1097/00005072-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol. 1997;61:279–85. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- 29.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–62. doi: 10.1002/(SICI)1096-9861(19990322)405:4<553::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Ryan G, Grimes T, Brankin B, Mabruk MJ, Hosie MJ, Jarrett O, et al. Neuropathology associated with feline immunodeficiency virus infection highlights prominent lymphocyte trafficking through both the blood-brain and blood-choroid plexus barriers. J Neurovirol. 2005;11:337–45. doi: 10.1080/13550280500186445. [DOI] [PubMed] [Google Scholar]
- 31.Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108:2827–35. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichiyama T, Kajimoto M, Matsushige T, Shiraishi M, Suzuki Y, Furukawa S. Mononuclear cell subpopulations in CSF and blood of children with bacterial meningitis. J Infect. 2009;58:28–31. doi: 10.1016/j.jinf.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Szmydynger-Chodobska J, Strazielle N, Zink BJ, Ghersi-Egea JF, Chodobski A. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1503–16. doi: 10.1038/jcbfm.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeker RB, Poulton W, Markovic-Plese S, Hall C, Robertson K. Protein changes in CSF of HIV-infected patients: evidence for loss of neuroprotection. J Neurovirol. 2011;17:258–73. doi: 10.1007/s13365-011-0034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuenburg JK, Furlan S, Bacchetti P, Price RW, Grant RM. Enrichment of activated monocytes in cerebrospinal fluid during antiretroviral therapy. AIDS. 2005;19:1351–9. doi: 10.1097/01.aids.0000181008.39514.ee. [DOI] [PubMed] [Google Scholar]