Abstract
Cell migration influences cell-cell interactions to drive cell differentiation and organogenesis. To support proper development, cell migration must be regulated both temporally and spatially. Mesoderm cell migration in the Drosophila embryo serves as an excellent model system to study how cell migration is controlled and influences organogenesis. First, mesoderm spreading transforms the embryo into a multilayered form during gastrulation and, subsequently, cells originating from the caudal visceral mesoderm (CVM) migrate along the entire length of the gut. Here we review our studies, which have focused on the role of fibroblast growth factor (FGF) signaling, and compare and contrast these two different cell migration processes: mesoderm spreading and CVM migration. In both cases, FGF acts as a chemoattractant to guide cells’ directional movement but is likely not the only signal that serves this role. Furthermore, FGF likely modulates cell adhesion properties since FGF mutant phenotypes share similarities with those of cell adhesion molecules. Our working hypothesis is that levels of FGF signaling differentially influence cells’ response to result in either directional movement or changes in adhesive properties.
Keywords: cell migration, adhesion, mesoderm spreading, caudal visceral mesoderm, FGF signaling, Heartless, Pyramus, Thisbe, Drosophila embryo
Introduction
Cell migration is a fundamental developmental process that involves interplay between extracellular signaling molecules, cell surface receptors, and intracellular signal transduction pathways.1 Movement of cells is often directional, with cells sensing the appropriate direction of migration based on recognition of region-specific cues.2 During embryonic development, cell migration is a very influential process as it results in rearrangement of cells from one part of the embryo to another, effectively controlling cell-cell interactions to drive cell differentiation and organogenesis. In vitro studies using cell culture have provided many mechanistic insights into cell migration. However, in vivo studies undeniably provide additional insight into the role of the natural environment.
Many studies in a number of model organisms have provided important knowledge regarding how groups of cells move in a coordinate fashion to influence morphogenesis during development.3,4 In the zebrafish, several signaling pathways including FGF influence collective migration of cells of the lateral line primordium to control both morphogenesis and migration.3,5 In the neural crest of vertebrates, it is clear that communication between cells within the migrating collective is necessary for the group of neural crest cells to move; as a result of these cell-cell interactions, contact-dependent cell polarity through N-cadherin is modulated to regulate cell movements.6 Studies of tracheal cell migration in Drosophila have shown that FGF signaling influences the collective movement of this cell group; cells with the highest levels of FGF activity take the lead position.7 We propose that comparative studies of different systems may provide important insight into general mechanisms that guide collective cell migration.
Strength of the Drosophila System for In Vivo Analyses of Cell Migration
The fruit fly Drosophila melanogaster is a genetically tractable organism that contains many components of mammalian signaling pathways. Drosophila has little genetic redundancy compared with vertebrates, and other strengths of this system include the short generation time (10 d) and relatively quick methods for generating transgenics (four weeks). Therefore, in Drosophila, cellular and genetic approaches can be combined to study biological processes that often provide insights into human dysplasia and disease.8
For example, Drosophila is an excellent system to study how FGF signaling supports development. Only three FGF ligands [Pyramus (Pyr), Thisbe (Ths) and Branchless (Bnl)] and two FGF receptors [Heartless (Htl) and Breathless (Btl)] exist in Drosophila.9 Furthermore, we have shown that only three receptor-ligand complexes are active: Pyr and Ths activate Htl, while Bnl activates Btl.10 In contrast, over 120 FGF-FGFR combinations presumably function in vertebrates.11 In Drosophila, the Htl fibroblast growth factor receptor (FGFR) is encoded by a single exon so it is likely that Pyr and Ths activate the same isoform, making this the first pair of invertebrate FGFs to bind the same FGFR isoform.9 In addition, Pyr and Ths exhibit significant homology to vertebrate FGFs, specifically, to the FGF8 family.12 Given all this information, the Drosophila model system offers a great potential for studying FGF signaling and why ligands often act concurrently.
Here we discuss two FGF-dependent cell migrations, where in both cases Htl FGFR is expressed in the migrating cells, during Drosophila embryogenesis. First, FGF signaling through Htl FGFR controls how mesoderm cells come in contact with the ectoderm and promotes mesodermal cell movement as one migrating collective.13,14 Second, at a later stage of embryogenesis, Htl-dependent FGF signaling directs a long-distance migration of two cell clusters called caudal visceral mesoderm (CVM), required for proper gut formation.15 These two cell migration events appear quite different: in one case, a tube of cells collapses to a mound of cells, which then spreads into a monolayer such that every cell directly contacts the ectoderm; and in the other case, two distinct groups of cells move coordinately on the left and right sides of the embryonic body from the posterior of the embryo toward the anterior. Nevertheless, FGF signaling supports these two movements in what appears to be a similar manner, supporting both directional movement and also, possibly, modulation of cell adhesion state.10,14-16
We suggest that levels of FGF ligands influence whether FGF signaling acts to regulate chemoattraction (far from the FGF source/low FGF concentration) vs. cell adhesion (close to the FGF source/high FGF concentration). As a cell is attracted to move toward the correct “position,” it would make sense that cell adhesion is upregulated to help the cell remain where it should be. Below we review the relevant data that lead us to propose this model.
Case I: Mesoderm Spreading during Drosophila Gastrulation
Migration of mesoderm cells during gastrulation is an important step for the regional specification of various mesodermal derivatives.17 It has been appreciated for a while that FGF signaling is required to support mesoderm cell movement,13,18-20 but its role in this process was not understood until recently. Htl FGFR is expressed in the migrating mesoderm and two ligands (Pyr and Ths) are expressed in the ectoderm.
To provide insight into the role of FGF in supporting mesoderm spreading during gastrulation, we devised an imaging protocol that allows examination of the movement of hundreds of mesoderm cells deep within Drosophila embryos during gastrulation.21 Embryos with ubiquitously expressed histone H2A-GFP were imaged and nuclei of mesoderm cells were tracked, using methodology that we developed.21 Tracking data was transformed into cylindrical coordinates to fit the body plan of the embryo: collapse of the mesodermal tube and intercalation movements occur in the radial direction; dorsal spreading occurred in the angular (azithumal) direction; whereas a strong movement along the length of the embryo was correlated with germband elongation. These studies showed that movement of mesoderm cells during gastrulation is directed and appears highly organized (e.g., the angular position at the end of the migration process is twice that at the start, for each and every cell).14 Moreover, through live imaging of wild-type embryos, we identified that cell movements relating to collapse, spreading, and monolayer formation are distinct, as they do not overlap temporally.
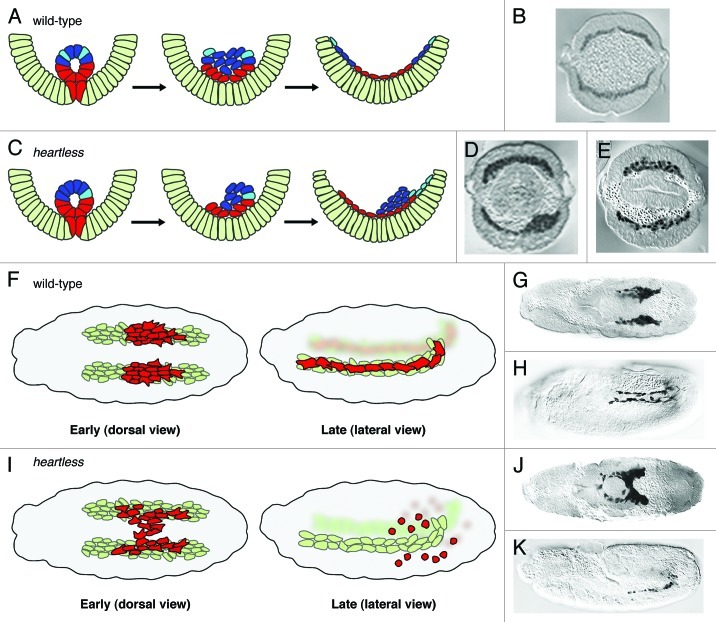
The fact that these movements occur in a stepwise manner suggested to us that different molecular signals control each step. Our data showed that FGF signaling through the Htl FGFR controls one of the earlier steps, organized collapse of the mesodermal tube onto the underlying ectoderm (Fig. 1A and B).14 This organized and symmetric collapse is crucial for the subsequent movements. This step positions all mesoderm cells close enough to the ectoderm to support the subsequent spreading, perhaps so that they might also receive additional guidance cues. In FGF mutants, tube collapse often occurs randomly, and this likely contributes to the variability of mesoderm spreading defects observed (Fig. 1C). For instance, if the invaginated tube collapses to the right or left, then a more severe “lumpy” mesoderm phenotype is observed (Fig. 1D). However, in a FGF mutant where the tube by chance collapses symmetrically at the midline (as in wild-type), then the mesoderm spreading defect is quite subtle (Fig. 1E).14
Figure 1. Comparison of cell movements in wild-type and heartless mutant embryos. (A and C) Schematic based on published results;14 (B, D and E) cross-sections of anti-Twist staining of wild-type and htl mutant embryos. (A and B) In wild-type, all mesoderm (red/blue/cyan) cells contact the ectoderm (light green) and are able to spread dorsally to form a monolayer. (C) In htl mutants, only the subset of cells (red/cyan) that contact the ectoderm undergoes directed movements. Depending on how the tube collapses, the mutant phenotype can be severe (D) or subtle (E). (F and I) Schematic based on published results;15 CVM reporter croc-lacZ in wild-type (G and H) and htl mutant (J and K) embryos stained with anti-βgal oriented with anterior to the left. (F and G) The dorsal view of wild-type at stage 11 shows the two distinct, symmetrical clusters of CVM cells (red) migrating on the two bands of TVM cells (light green). (F and H) At stage 13, the lateral view reveals complete CVM migration with cells evenly distributed along the TVM. (I and J) In htl mutant embryos, CVM cells are intermixed in early migration. (I and K) Later stages of mutant embryos illustrate CVM cell death and loss of contact with the TVM. (B--D) and (G and J) were reprinted with permission from references 14 and 15, respectively.
Based on our combined approach of live imaging, cell tracking and quantitative analyses, we determined that mesoderm cells move as two behaviorally distinct cell populations in htl mutant embryos. It was not appreciated before our study that a subset of mesoderm cells maintains their ability to migrate coordinately in the absence of FGF signaling—those cells in contact with the ectoderm exhibit a dorsally directed migration as in wild-type (i.e., movement in the angular direction) (Fig. 1C, red cells). In contrast, those cells located at a distance from the ectoderm, which originate from the upper part of the invaginated tube, appear lost and undergo random movements (Fig. 1C, blue cells).14 However, even in mutants, if cells from the upper part of the invaginated tube happen to come close to the ectoderm, those cells are able to join the migrating collective and also to move in a directed fashion (Fig. 1C, cyan cells). Quantitative analysis of cell tracking data was necessary to provide this insight.
In addition, we found that cell intercalation influences spreading,14 but it does so most clearly during cell monolayer formation, the last step of the mesoderm spreading process.16 These final intercalation events simply turn a multi-layered mesoderm organization into a monolayer without any additional dorsally directed movement. This process is not a convergent extension, but more analogous to “zippering.”
FGF signaling is required to guide cell movement radially toward the ectoderm.14,16 First, our data suggest that expression of Thisbe specifically in ventral regions of the ectoderm controls collapse.16 FGF signaling through Thisbe likely promotes directional movement of mesoderm cells in the tube toward the ectodermal source of this ligand, to ensure symmetrical collapse of the invaginated tube.16 Protrusions have been observed that extend from cells located in the tube toward the ventral ectoderm, using electron microscopy,13 which argues for a chemoattractive mechanism supporting collapse. Second, our recent analysis suggested that expression of both ligands, which collectively encompasses the entire ectoderm, influences efficient monolayer formation (in the radial direction) at the final stage of this mesoderm spreading process. FGF mutants (Fig. 1E) and integrin mutants exhibit defects in monolayer formation.16 This shared phenotype supports the view that this last stage in the process requires modulation of mesoderm cell adhesion to the substrate to support monolayer formation. It had been proposed that a straightforward FGF chemoattraction guides dorsally directed movements in the angular direction, since the localized expression pattern of the FGF ligand Pyramus resides within the dorsal ectoderm.10 However, cells are able to migrate dorsally even in pyr mutants,16 indicating that Pyr does not provide cues necessary for dorsally directed migration.
These new data lead us to propose that FGFs have a distinct function at low vs. high concentrations: at low concentration they act in a chemoattractive manner to direct cell movement/orientation required for symmetrical collapse of the invaginated mesoderm tube; whereas at high concentration they act to increase cell adhesiveness to support short-range intercalation movements required for monolayer formation.
Case II: Caudal Visceral Mesoderm Migration Required for Gut Formation
Using a similar approach, we also recently investigated the role of Heartless, Pyramus and Thisbe in supporting migration of another group of cells in the Drosophila embryo, CVM cells.15,22 While a role for CVM cell migration in gut formation has been appreciated, little is known about how these cells accomplish their migration, the longest migration in all of Drosophila embryogenesis.23-25
The CVM migration consists of distinct steps. First, the CVM cluster at the posterior end separates into two symmetric groups: left and right. Subsequently, these two groups of ~30 cells each undergo coordinate and directed movement toward the anterior of the embryo (Fig. 1F, early and 1G). The migration ensues over six hours and throughout the entire course the two separate groups migrate synchronously. This process is necessary to position CVM cells along the entire length of the developing gut (Fig. 1F, late and 1H). Lastly, at the end of their migration, CVM cells fuse with fusion-competent myoblasts to form the longitudinal muscles that ensheath the gut.26
Our working hypothesis has been that CVM migration, like mesoderm spreading, is a multi-step process as different inputs likely influence cells’ movement during the course of their long-distance migration. To start, our studies have focused on the role of FGF signaling in guiding this migration as (1) in FGF mutants the longitudinal visceral muscle fibers, which arise from CVM cells, are absent15,27 and (2) FGF signaling components are expressed in the CVM and the trunk visceral mesoderm (TVM). Htl is expressed in the migrating CVM cells,27 and its ligands Pyramus and Thisbe are expressed within TVM, a substratum (“track”) upon which CVM cells migrate.12,15 The TVM is present as two bands on either side of the embryo, with each band serving as a track for the migration of one cluster of CVM cells.
To obtain insight into the role of FGF signaling during CVM migration, in a recent study,15 we investigated whether FGF guides directional movement of CVM cells, as these cells stay closely associated with the TVM (Fig. 1F). Our results suggest that FGF signaling functions in a chemoattractive manner to guide CVM cell migration and also supports cells’ survival. In the absence of FGF signaling, cells from the right and left sides of the embryo veer off course, detach from their respective TVM, and converge at the midline. In some cases, cells cross over completely to the alternate side, which is a phenotype not observed in wild-type (Fig. 1I, early and 1J). In addition, overexpression of Pyr and/or Ths FGFs at an ectopic location, at the ventral midline, redirects CVM cells toward this source of ligand. Furthermore, most CVM cells eventually die in FGF mutants.15,27 While this might relate to some checkpoint mechanism that ensures that cells that have gone off-track are eliminated, our data support the view that FGF signaling also likely supports cell survival directly (Fig. 1I, late and 1K). Ectopic expression of ligands at a distance can rescue cell viability even if migration remains “off-track.”15
However, even in the absence of FGF signaling, CVM cells still initiate anteriorly-directed forward movement, albeit somewhat misdirected and slow (Fig. 1I, late and 1K). While CVM cells in the FGF mutants are disorganized, perhaps through lack of adhesive properties, they ultimately move forward as long as they are kept alive. Therefore, FGF-independent signals likely exist that also guide anterior movement.
We propose that FGF signaling supports several roles throughout the six hours that CVM cells undergo their long-range migration. Initially, wild-type FGF signaling acts in a chemoattractive manner to recruit CVM cells onto the TVM tracks, upon which cells migrate. In the FGF mutants, cells veer off-course and cross over at the midline; this never happens in wild-type embryos.15 However, expression of ligands is found along the length of the TVM, so it is not clear how FGF ligands would support chemotactic movement toward the anterior in the absence of a gradient. Processing or some other modification of ligands may support graded FGF activity to support forward movement of cells once at the TVM. Alternatively, it is possible that FGF signaling, in this context with CVM cells at the TVM, acts as a “permissive” signal to allow cells to effectively sense other signals that may influence anteriorly-directed movement. For example, once CVM cells reach the TVM the role of FGF may be to simply keep CVM cells “on track,” possibly through regulation of cell adhesion properties,28 so that they remain in range to receive other guidance cues.
How Do Cell Collectives Migrate in a Coordinated Fashion?
Whereas one signal may suffice to guide migration of small groups of cells, more complex mechanisms likely safeguard proper migration of larger groups of cells. In addition to external signals influencing direction of migration, it is probable that cells within each group must coordinate with each other to ensure that each migrating collective moves in a directed fashion.
Coordination between cells in a migrating collective may require physical association between them, either stable or transient, and/or chemical signaling. During the mesoderm spreading process, cells are closely associated and likely linked by adherens junctions as well as gap junctions.29 However, these structures remain to be defined in terms of their components (which involve a number of different proteins), their prevalence and dynamics and their role in supporting cell movement. Nevertheless, these junctions certainly have important roles during morphogenesis.30 Cell-cell interactions also occur between neighboring CVM cells, but cells within the migrating collective appear loosely associated. As CVM cells also interact closely with the TVM, we hypothesize that CVM-TVM cell-cell interactions play a significant role in supporting CVM cells' anteriorly directed movement. More careful analysis of the physical associations of homotypic (mesoderm-mesoderm) as well as heterotypic (mesoderm-ectoderm or CVM-TVM) cell-cell interactions should provide important insights. In addition, synchronous migration of the two CVM clusters is abolished in FGF mutants, suggesting a possible novel role for FGF in long-range cell-cell communication. Potential influences to be investigated include regulation of cell adhesion properties, direction of movement, orientation/number of cell projections, cell division and/or cell viability. The complexity of collective migration is highlighted here as each of these features involves multiple proteins and layers of regulation.
Distinct and Overlapping Functions of FGF Ligands
While a very impressive analysis of all vertebrate FGF-FGFR interactions was recently completed in which the binding specificities of ligand-receptor interactions were examined in tissue culture,11 how this relates to in vivo processes, for the most part, is undetermined. Our studies have focused on obtaining this exact information, to define specific roles for each Drosophila FGF in vivo.10,14-16 We have demonstrated that Pyramus and Thisbe ligands have both overlapping as well as distinct functions within the Drosophila early embryo: (1) Thisbe controls collapse of the invaginated mesoderm during gastrulation; (2) both ligands are required to form a proper mesoderm monolayer as the end result of mesoderm spreading during gastrulation; (3) subsequently, primarily Pyramus alone is required for differentiation of dorsal mesoderm lineages; and (4) lastly, both ligands work together to support migration of CVM cells later in embryogenesis.
In our most recent study of CVM cell migration,15 we found that ectopic expression of Pyramus and Thisbe together (at the ventral midline in embryos lacking endogenous ligand expression) caused a severe migration defect: CVM cells were essentially recruited to the ectopic site and then stalled. This result brings up the interesting possibility that the combined activity of both ligands is distinct from having either one, because expression of each ligand individually did not support this effect. This led us to propose that FGF ligand heterodimers can support a distinct function possibly through differences in binding affinity, stability, and/or recruitment of cofactors. FGF ligand homodimers bound to FGFR were crystallized and the structure obtained suggested that heterdimeric binding is also possible.31
Why particular developmental processes depend on Pyramus and/or Thisbe is not understood. These molecules may activate distinct intracellular signaling downstream of Htl-activation to support different cell behaviors, for example cell migration vs. cell differentiation.32 However, it does not appear that Pyramus and Thisbe have dedicated functions. For instance, Pyramus supports differentiation of dorsal somatic mesoderm lineages in the embryo by supporting cell differentiation (i.e., transcriptional response),10,33 while supporting cell migration for glia associated with neuronal development of the eye at later stages.32 Alternatively, FGFs may exhibit different range of action or be subject to different regulation. Regarding this last point, we have determined that these ligands are differentially cleaved and that the C-terminus of Thisbe may function to inhibit activity.34 Drosophila, with a total of three FGF ligands compared with 22+ genes in vertebrates, is an attractive model system to investigate the individual activities of FGFs.
Could FGF Signaling Support Cell Movement by Regulating Cells’ Adhesive State?
Cell adhesivity may influence cell-cell interactions to help cells move as a single migrating collective, affecting homotypic interactions and/or the ability of cells to interact with the substratum upon which they migrate.
Our analysis, tracking nuclei, examined the mesoderm spreading process following collapse and suggested mutant cells were more loosely associated with each other. Results showed that movements of cells that originate from the upper part of the tube, and thus do not contact the ectoderm, were misdirected (appearing random) and encompassed far larger distances than normal.14 Furthermore, when Pyramus or Thisbe ligands are ectopically expressed in the mesoderm (essentially increasing FGF signaling), tracking analyses have found that all mesoderm cell movement is halted (McMahon and Stathopoulos, unpublished observation), perhaps through increased adhesion. Our data, following collapse, is consistent with the view that lack of FGF activity results in weak mesoderm-mesoderm cell-cell associations, possibly “rescued” by contact with the ectoderm, whereas too much FGF signaling supports cell-cell associations that are too strong and actually hinder motility (Fig. 2). Another study has shown that at an earlier stage in the absence of FGF signaling, EMT is delayed due to defects in E-cadherin redistribution,35 which is a molecule that can influence cell adhesion properties. An interesting future direction would be to investigate whether mesoderm and ectoderm cells’ adhesion state changes during the various steps of this mesoderm migration process (i.e., EMT, collapse, spreading and monolayer formation).
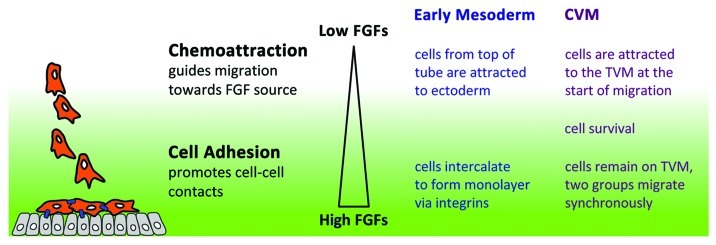
Figure 2. Model of FGF’s dual function. Data from recent studies suggest that FGFs are able to function differentially in a concentration dependent manner. The concentration of FGFs is governed by the proximity of responding cells (orange) to the source (gray cells producing FGFs). At a distance, where levels are low, FGFs works as chemoattractant such that cells become polarized and migrate directionally. Once cells are closer to the source, the higher levels of FGFs promote cell adhesion (blue lines).
Along these lines, when both ligands are expressed in combination (but not individually) within the CVM, cell movement is halted. For the few cells that are able to “break free” from the collective, they appear to migrate just fine. One interpretation of this result is that ectopic expression of ligands results in cessation of movement as CVM cells become too “adherent” to each other. It will be of great interest to examine how the cell adhesion properties of these stalled cells are altered by overexpression of both ligands.
In addition, in subsequent studies of the mesoderm spreading process, we have found that FGF mutant phenotypes share similarity with those of genes that influence cell adhesion—the Rap1 GTPase and the βPS integrin Myospheroid.16 While the Rap1 GTPase influences both collapse and monolayer formation (similar to FGF), the integrin Myospheroid is specifically required for the final step of this process, monolayer formation. It is possible that proper monolayer formation requires a substantial increase in cell-cell adhesion between mesoderm and ectoderm.
Our working hypothesis is that FGF signaling serves multiple roles to support cell movement. At lower levels, FGF ligands may serve as chemoattractants but once levels are raised, for instance when migrating cells approach the ligand source, then a secondary function of FGF signaling acts to increase cell-cell adhesion properties (Fig. 2). While a role for FGF signaling in modulating cell adhesion to support cell movement remains unclear in the field, experiments using the Drosophila model system have the potential to provide necessary insight. The relative ease of genetic manipulation and live imaging of Drosophila shows promise for the study of the complex and dynamic processes that relate to collective cell migration.
Acknowledgments
We thank members of the Stathopoulos lab for helpful discussions and, specifically, Amy McMahon for providing some images. This work was supported by postdoctoral fellowships from the American Heart Association to Y.B. and from the Moore Foundation CISR.CACR Bio-Grant to S.K. and by grant 1-FY11-423 from the March of Dimes to A.S.
Glossary
Abbreviations:
- FGFR
fibroblast growth factor receptor
- Htl
Heartless
- Btl
Breathless
- FGF
fibroblast growth factor
- Pyr
Pyramus
- Ths
Thisbe
- Bnl
Branchless
- CVM
caudal visceral mesoderm
- TVM
trunk visceral mesoderm
- EMT
epithelial-mesenchymal transition
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21103
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Rørth P. Whence directionality: guidance mechanisms in solitary and collective cell migration. Dev Cell. 2011;20:9–18. doi: 10.1016/j.devcel.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 4.Montell DJ. The social lives of migrating cells in Drosophila. Curr Opin Genet Dev. 2006;16:374–83. doi: 10.1016/j.gde.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Aman A, Piotrowski T. Cell-cell signaling interactions coordinate multiple cell behaviors that drive morphogenesis of the lateral line. Cell Adh Migr. 2011;5:499–508. doi: 10.4161/cam.5.6.19113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, et al. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghabrial AS, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–9. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- 8.Bier E, Bodmer R. Drosophila, an emerging model for cardiac disease. Gene. 2004;342:1–11. doi: 10.1016/j.gene.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Tulin S, Stathopoulos A. Extending the family table: Insights from beyond vertebrates into the regulation of embryonic development by FGFs. Birth Defects Res C Embryo Today. 2010;90:214–27. doi: 10.1002/bdrc.20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadam S, McMahon A, Tzou P, Stathopoulos A. FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development. 2009;136:739–47. doi: 10.1242/dev.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stathopoulos A, Tam B, Ronshaugen M, Frasch M, Levine M. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 2004;18:687–99. doi: 10.1101/gad.1166404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson R, Vogelsang E, Leptin M. FGF signalling and the mechanism of mesoderm spreading in Drosophila embryos. Development. 2005;132:491–501. doi: 10.1242/dev.01603. [DOI] [PubMed] [Google Scholar]
- 14.McMahon A, Supatto W, Fraser SE, Stathopoulos A. Dynamic analyses of Drosophila gastrulation provide insights into collective cell migration. Science. 2008;322:1546–50. doi: 10.1126/science.1167094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadam S, Ghosh S, Stathopoulos A. Synchronous and symmetric migration of Drosophila caudal visceral mesoderm cells requires dual input by two FGF ligands. Development. 2012;139:699–708. doi: 10.1242/dev.068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon A, Reeves GT, Supatto W, Stathopoulos A. Mesoderm migration in Drosophila is a multi-step process requiring FGF signaling and integrin activity. Development. 2010;137:2167–75. doi: 10.1242/dev.051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–7. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- 18.Gisselbrecht S, Skeath JB, Doe CQ, Michelson AM. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 1996;10:3003–17. doi: 10.1101/gad.10.23.3003. [DOI] [PubMed] [Google Scholar]
- 19.Beiman M, Shilo BZ, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996;10:2993–3002. doi: 10.1101/gad.10.23.2993. [DOI] [PubMed] [Google Scholar]
- 20.Murray MJ, Saint R. Photoactivatable GFP resolves Drosophila mesoderm migration behaviour. Development. 2007;134:3975–83. doi: 10.1242/dev.005389. [DOI] [PubMed] [Google Scholar]
- 21.Supatto W, McMahon A, Fraser SE, Stathopoulos A. Quantitative imaging of collective cell migration during Drosophila gastrulation: multiphoton microscopy and computational analysis. Nat Protoc. 2009;4:1397–412. doi: 10.1038/nprot.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin; New York: Springer, 1997. [Google Scholar]
- 23.Kusch T, Reuter R. Functions for Drosophila brachyenteron and forkhead in mesoderm specification and cell signalling. Development. 1999;126:3991–4003. doi: 10.1242/dev.126.18.3991. [DOI] [PubMed] [Google Scholar]
- 24.Urbano JM, Domínguez-Giménez P, Estrada B, Martín-Bermudo MD. PS integrins and laminins: key regulators of cell migration during Drosophila embryogenesis. PLoS One. 2011;6:e23893. doi: 10.1371/journal.pone.0023893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismat A, Schaub C, Reim I, Kirchner K, Schultheis D, Frasch M. HLH54F is required for the specification and migration of longitudinal gut muscle founders from the caudal mesoderm of Drosophila. Development. 2010;137:3107–17. doi: 10.1242/dev.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H-H, Zaffran S, Frasch M. Development of the Larval Visceral Musculature. In: Sink H, ed. Muscle Development in Drosophila. New York, N.Y.: Landes Bioscience, 2006:62-78. [Google Scholar]
- 27.Mandal L, Dumstrei K, Hartenstein V. Role of FGFR signaling in the morphogenesis of the Drosophila visceral musculature. Dev Dyn. 2004;231:342–8. doi: 10.1002/dvdy.20088. [DOI] [PubMed] [Google Scholar]
- 28.Reim I, Hollfelder D, Ismat A, Frasch M. The FGF8-related signals Pyramus and Thisbe promote pathfinding, substrate adhesion, and survival of migrating longitudinal gut muscle founder cells. Dev Biol. 2012;368:28–43. doi: 10.1016/j.ydbio.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–96. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- 30.Harris TJ, Sawyer JK, Peifer M. How the cytoskeleton helps build the embryonic body plan: models of morphogenesis from Drosophila. Curr Top Dev Biol. 2009;89:55–85. doi: 10.1016/S0070-2153(09)89003-0. [DOI] [PubMed] [Google Scholar]
- 31.Plotnikov AN, Hubbard SR, Schlessinger J, Mohammadi M. Crystal structures of two FGF-FGFR complexes reveal the determinants of ligand-receptor specificity. Cell. 2000;101:413–24. doi: 10.1016/S0092-8674(00)80851-X. [DOI] [PubMed] [Google Scholar]
- 32.Franzdóttir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klämbt C. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–61. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- 33.Klingseisen A, Clark IB, Gryzik T, Müller HA. Differential and overlapping functions of two closely related Drosophila FGF8-like growth factors in mesoderm development. Development. 2009;136:2393–402. doi: 10.1242/dev.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tulin S, Stathopoulos A. Analysis of Thisbe and Pyramus functional domains reveals evidence for cleavage of Drosophila FGFs. BMC Dev Biol. 2010;10:83. doi: 10.1186/1471-213X-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark IB, Muha V, Klingseisen A, Leptin M, Müller HA. Fibroblast growth factor signalling controls successive cell behaviours during mesoderm layer formation in Drosophila. Development. 2011;138:2705–15. doi: 10.1242/dev.060277. [DOI] [PMC free article] [PubMed] [Google Scholar]