Abstract
The extracellular matrix is constructed beyond the plasma membrane, challenging mechanisms for its control by the cell. In plants, the cell wall is highly ordered, with cellulose microfibrils aligned coherently over a scale spanning hundreds of cells. To a considerable extent, deploying aligned microfibrils determines mechanical properties of the cell wall, including strength and compliance. Cellulose microfibrils have long been seen to be aligned in parallel with an array of microtubules in the cell cortex. How do these cortical microtubules affect the cellulose synthase complex? This question has stood for as many years as the parallelism between the elements has been observed, but now an answer is emerging. Here, we review recent work establishing that the link between microtubules and microfibrils is mediated by a protein named cellulose synthase-interacting protein 1 (CSI1). The protein binds both microtubules and components of the cellulose synthase complex. In the absence of CSI1, microfibrils are synthesized but their alignment becomes uncoupled from the microtubules, an effect that is phenocopied in the wild type by depolymerizing the microtubules. The characterization of CSI1 significantly enhances knowledge of how cellulose is aligned, a process that serves as a paradigmatic example of how cells dictate the construction of their extracellular environment.
Keywords: cellulose microfibrils, cellulose synthase, cellulose synthase interacting protein 1, cortical microtubules, plant cell wall, Pom-Pom2
In Euclid’s geometry, parallel lines cannot meet, but Riemann and others found geometries where they do meet, leading us to spaces both fascinating and useful. A contemporary case of parallel lines whose meeting is impossible but informative is offered by the humble vascular plant. Perhaps of doubtful utility for readers of Cell Adhesion & Migration, this case nevertheless could fascinate them, concerning as it does the problem of information flowing out of the cell, a flow that is a hallmark of living things but far less well understood than is the typical receptor-mediated inward flow. And fascination does beget utility, as non-Euclidean geometries proposed for pure mathematical abstraction have given rise to solutions in physics.
The plant cell is surrounded by a tough yet pliant extracellular matrix, called the cell wall (see Box 1 for definitions of plant-specific terms). While animal cells make an extracellular matrix, few of them are surrounded by it as completely as is nearly every plant cell. The cell wall is so ubiquitously and intimately associated with the plant cell that one is tempted to consider the wall as one more organelle, albeit a large one. Indeed, the cell wall space defines a compartment, known as the apoplast. However, the cell wall is unquestionably outside of the plasma membrane and for that reason extracellular. Far from being a random entangled network, for example like an agarose gel, the cell wall is highly organized (Fig. 1A). The organization makes the cell wall mechanically anisotropic, a property that is required by morphogenesis to channel expansion into anisotropic shapes, such as stems and leaves. How does information from the cell flow across the plasma membrane to constrain the construction of cell wall?
Box 1.
Defining terms.
Apoplast: The continuum formed by cell walls, airspaces, and dead cells, such as the water conducting xylem.
Arabidopsis thaliana: The “fruit fly” of plant biology; that is, a species chosen for utility in research rather than for its economic relevance.
Cell wall: The polysaccharide-rich extracellular matrix that surrounds every plant cell.
Cellulose: The general name for polymers of β 1 → 4 linked glucose associated into a crystalline or semi-crystalline fibril.
Cellulose microfibril: The typical form of cellulose in vascular plants, with a minimal diameter of ~3 nm and an indefinite length.
Cellulose synthase: The multi-peptide complex at the plasma membrane that synthesizes a cellulose microfibril.
Cellulose synthase A (CESA): The polypeptide thought to be responsible for catalyzing the reaction where UDP glucose is added to the growing chain of polymerized glucose.
Cortical array of microtubules: An array of mainly parallel microtubules in the cell cortex associated closely with the plasma membrane, present in essentially all plant cells during interphase.
Rosette: A synonym for the cellulose synthase complex, based on its hexameric appearance in the electron microscope.
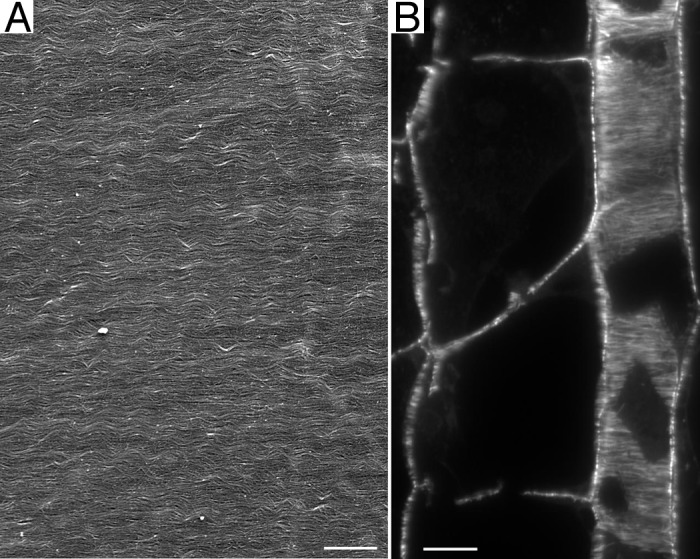
Figure 1. Parallel lines. (A) Cell wall of the inflorescence stem of A. thaliana imaged with field emission scanning electron microscopy. For method, see reference 23. (B) Methacrylate section through the root of A. thaliana stained with an antibody against tubulin. The section plane passes through the middle of a file of wide cells and through the cortex of a file of narrow cells; cortical microtubules are viewed end-on in the former and in face view in the latter. For method, see reference 24. Scale bars: (A) = 600 nm, (B) = 10 µm.
In contrast to the animal cell’s extracellular matrix, the plant cell wall is chiefly polysaccharide. The functional analog of the protein, collagen, is the polysaccharide, cellulose. Structurally, cellulose comprises unbranched polymers of glucose linked side by side in a (partly) crystalline array and stabilized by a network of hydrogen bonds dense enough to give cellulose a tensile strength rivaling steel.1,2 The crystalline array comprises a few dozen of the unbranched polymers, forming a structural unit called the cellulose microfibril. The degree of polymerization of each glucose chain is on the order of ten thousand, similar to the scale of the cell itself. Such a molecule could be packaged into a secretory vesicle only by wizardry and, lacking magic wands, cells synthesize cellulose at the plasma membrane. This is accomplished by a multi-subunit complex called cellulose synthase. At its cytosolic side, this complex reacts with UDP-glucose and extrudes polymerized glucan chains into the extracellular space. Each synthase complex probably makes 18 to 36 chains that somehow associate or crystallize into a microfibril. As the chains are extended, the cellulose synthase moves along the plasma membrane, presumably powered by the energy released from hydrolyzing UDP-glucose or crystallization.
Mechanistic details about the synthesis of cellulose are scarce, primarily because the reaction proceeds with great difficulty in vitro,3 and frustratingly not at all in extracts from the model plant, Arabidopsis thaliana, the species in which most of the genetic evidence relating to cellulose synthesis has been obtained. Genetics have implicated a family of putative glycosyl transferases, called cellulose synthase A (CESA), and it appears that a functional cellulose synthase complex requires three distinct CESA family members.1,2 An antibody against a CESA labels a hexameric structure, termed a rosette, that is abundant in freeze-fracture images of the plasma membrane.4 This, along with genetics and estimations of the lateral dimensions of a microfibril have led to a model where each cellulose synthase is a rosette, containing 36 CESA polypeptides, with each (or possibly each pair) synthesizing a glucose chain. However, we know neither how CESA proteins are organized within a rosette nor what other proteins, if any, are also components of the complex, although sucrose synthase is likely.5
Knowledge about cellulose synthesis has recently been enhanced by the development of a system whereby the movement of the synthase can be imaged in living cells.6 A specific CESA sequence is tagged with a fluorescent protein and introduced into a background where the corresponding native gene has been inactivated mutationally; when imaged through a spinning-disc confocal fluorescence microscope, the tagged cellulose synthase complexes are seen as spots at the plasma membrane. Over time, the spots move (see Fig. 2A and C). The velocity, ~0.3 µm min−1, along with the density of the complexes per unit area, plausibly account for rates of cellulose synthesis measured in bulk. In the absence of in vitro enzymology, imaging the tagged CESA in living cells provides a readout of the reaction rate that is particularly valuable.
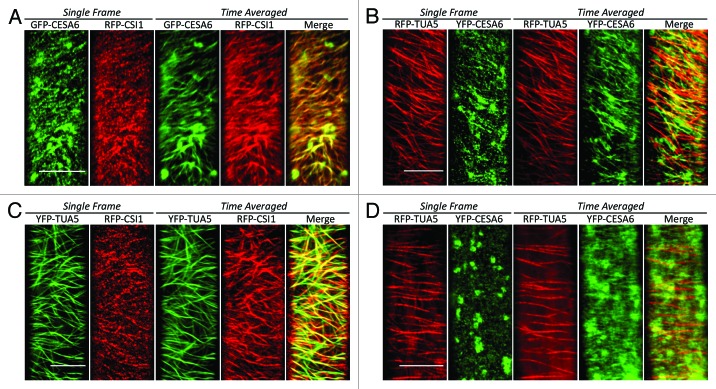
Figure 2. Co-localization of CSI1, CESA complexes, and microtubules. (A) Wild-type seedlings co-expressing GFP-CESA6 and RFP-CSI1: the co-alignment of CSI1 and CESA complexes is evident in the merged time-averaged image. (B) Wild-type seedlings co-expressing YFP-TUA5 and RFP-CSI1: the co-alignment of CSI1 and microtubules is evident in the merged time-averaged image. (C) Wild-type seedlings co-expressing RFP-TUA5 and YFP-CESA6: the co-alignment of CESA complexes and microtubules is evident in the merged time-averaged image. (D) In csi1 seedlings co-expressing YFP-CESA6 and RFP-TUA5, CESA particles are randomly distributed, their time-averaged trajectories are apparently shorter and rarely co-localized with microtubules. Note that the large, roughly circular structures in the GFP-CESA6 (A) and YFP-CESA6 (C) images are Golgi bodies. The time-averaged images are projections of 60 frames (~5 min) acquired at 5 sec intervals. Bars = 10 µm. Methods described further in reference 15.
Insofar as cellulose constitutes about one third of the cell wall mass and is, by far, its longest and stiffest component, the cell goes some way toward guiding the assembly of the cell wall by constraining the orientation in which the cellulose microfibrils form. Given that microfibrils are long and stiff, and synthesized within the confined, essentially two-dimensional space between plasma membrane and extant cell wall, self-assembly probably contributes to the parallel arrangement of microfibrils, driven by entropic and van der Waals forces, which apparently also drive orientation of cytoskeletal filaments.7 However, microfibrils are oriented on the macroscopic scale. For example, in the stem sampled for Figure 1, microfibrils are aligned perpendicular to that stem over a distance on order of a centimeter and throughout thousands of cells. Macroscopic order on this scale is beyond the reach of self-assembly based on molecular forces and thus the cell must supplement intrinsic self-assembly with information specifying cellular polarity.
What better way to specify polarity than through the cytoskeleton? Just inside the plasma membrane, plant cells contain an array of microtubules, called the cortical array.8,9 Like those in animal cells, microtubules in the cortical array turn over rapidly and exhibit dynamic instability; however, unlike most other arrays, the cortical array lacks a microtubule organizing center and the polarity of microtubules within the array is apparently random.
Cortical microtubules are well ordered (Fig. 1B), and their alignment usually parallels that of the cellulose microfibrils. In fact, microtubules were first imaged in plant cells and were hypothesized to orient cellulose microfibrils based on the congruent alignment between these elements, even though microtubules are inside the cell and microfibrils are outside.10,11 Since then, this hypothesis has been confirmed in general but not in detail. The overall claim of the hypothesis is confirmed by countless examples where microtubules parallel microfibrils and countless more where depolymerizing the microtubules impairs the alignment among microfibrils.12 Although there appear to be exceptions, these many examples make clear that microfibrils are oriented with respect to the microtubules. But the hypothesis remains incomplete because the mechanism behind this flow of orientation information has remained obscure.
Indeed, the most popular model for how microtubules influence cellulose posits no mechanism at all.9,12 Instead, the model envisions the microtubules as a kind of molecular guard rail, constraining movement of the cellulose synthase sterically but not interacting with the complex specifically. Although this model accounts for the parallelism, it leaves an important function (ordering the microfibrils) essentially to chance. Moreover, the “guard-rail” model makes no provision for activity of the microtubules in addition to ordering the microfibrils. Cells are parsimonious; along with specifying orientational order, microtubules might have a concomitant role in assisting with cellulose synthesis itself.13 That is, while there is no requirement for microtubules per se for a plant to make cellulose, microtubules could condition the properties of the synthesized material.
But at last, a mechanism is being discovered. Three papers published nearly simultaneously characterize the first protein known to link cortical microtubules and cellulose microfibrils.14-16 Previously, the protein, named cellulose synthase-interacting protein (CSI1), had been fished out of a yeast two-hybrid screen for proteins that interact with CESA; the protein is present at the plasma membrane and moves in linear trajectories resembling those of CESA, and loss-of-function mutants synthesize less crystalline cellulose.17 The new work extends our knowledge of CSI1 and links it to the cortical microtubules.
To begin with, two papers confirm that CSI1 and cellulose synthase are associated intimately.14,15 In the original publication,17 CSI1 and CESA were co-localized almost completely and both moved bi-directionally, at all but indistinguishable velocities; these findings are reproduced in the new work but with a distinct CESA family member (Fig. 2A). Furthermore, an inhibitor of cellulose synthesis, which depletes the membrane of CESA complexes, likewise depletes CSI-positive material. These results leave little doubt as to the close association between CSI1 and CESA.
But a putative linker between CESA complexes and microtubules needs also to interact with microtubules; such an interaction is substantiated in several ways by the new work.14-16 First, CSI1 binds microtubules in vitro, with a dissociation constant, ~1 µM, that is similar to that of canonical microtubule-associated proteins, such as MAP2.15 Microtubule binding is plausible, insofar as CSI1 contains multiple armadillo repeats, motifs known to bestow microtubule-binding activity on several proteins. Second, CSI1 moves along the plasma membrane in trajectories that closely overlay microtubules (Fig. 2B). Third, when microtubules are depolymerized, CSI1 spots at the plasma membrane are reduced in number and intensity, although they do not disappear altogether.14,15 Finally, under some conditions, loss-of-function mutants for CSI1 (a background denoted here as csi1 but also known as pom-pom2) have morphological phenotypes associated with aberrant cortical microtubules,14,16 some of which can be ameliorated by transgenic expression of an animal microtubule-associated protein (MAP4) that is absent from plants. Taken together, this evidence shows that CSI1, although discovered based on its relation to cellulose synthase,17 associates with microtubules, pivotally supporting its hypothetical role as a messenger service from cytosol to cell wall.
Perhaps the most interesting results in the new papers are those concerning the velocity and directionality of CESA movement. In the csi1 background, CESA complexes move at about 70% percent of the rate of the wild type, and when microtubules are depolymerized, the reduction in velocity is similar.15 Further attesting to CSI1 mediating a link between microtubules and CESA, in the csi1 background, CESA trajectories lose their close association with microtubules14,15 (Fig. 2C and D) and deviate somewhat from their linear character observed in the wild type. Notably similar wandering CESA trajectories occur when microtubules are depolymerized, again fingering CSI1 as the microtubule-microfibril go-between. These results imply that as far as the movement of the CESA complex is concerned, the loss of microtubules and the loss of CSI1 are equivalent, making a powerful case for the function of CSI1 being to inform the cellulose synthase complex about the disposition of the microtubules.
Why does depolymerizing the microtubules require 10 to 16 h to slow down the CESA complexes but just 1 h or so to remove microtubules? This discrepancy implies that CSI1 affects CESA movement indirectly. Why does the loss of a microtubule binding protein slow down the CESA complex? Naïvely, we might suppose microtubule-binding activity to act as a brake and the removal thereof would accelerate the movement even as it lost directional control. Finally, is the purview of CSI1 solely guidance or does it extend to synthesis? That is, do microfibrils built in the absence of CSI1 have assembly defects? It would be useful to reconstitute at least part of this motility system in vitro so that this molecular anatomy can be dissected.
Fascinating. But even granting that Earth’s biomass is mostly cellulosic, what is the lesson for the animal biologist? Like the plant cell wall, the animal cell extracellular matrix, whether bone, shell, spicule or tendon, is highly oriented. Where does the order come from? Not entirely from self-assembly, because while this is powerful on the small (sub-cellular) scale, the matrix is organized on a macroscopic scale and requires input from the organism. Although not that well elucidated, the order might come, as in plants, from the cytoskeleton, albeit from actin rather than microtubules.
In biomineralization, the general term used to describe constructing extracellular matrix rich in inorganic compounds, as in bone or shell, secreted proteins can serve as templates for crystallization and vesicles can be delivered selectively to seed crystallization appropriately,18,19 but in general it is not known how large-scale order is imposed or whether the cytoskeleton is involved. One exception is patterning of the silica-rich extracellular matrix (frustule) of the diatom, which depends on the actin cytoskeleton;20 while diatoms are as diverged from animals as are plants, this study stands as a paradigm for how the cell controls the growth of external, mineralized structures.
For aligning protein-rich matrices in vertebrates, actin is known to be involved. Actin helps build collagen-rich tendons, partly through actin-rich structures (fibripositors) in the cell cortex that guide the formation of fibrils21 but also by giving rise to forces that condition the developing tendon.22 That actin guides collagen assembly (and maybe biomineralization) but microtubules guide cellulose assembly should not devalue this comparison. In the evolution of plants and animals, microtubules and actin have, to a large extent, switched places. In animals, microtubules are the mainline tracks for organelles, while actin presides over the cell cortex and regulates whole-cell motility; conversely in plants, actin filaments are the organelle tracks, while microtubules dominate the cortex and regulate cell expansion. But in both kingdoms, information flows from a set of organized cytoplasmic filaments, across the membrane, to regulate the structure and activity of the extracellular matrix.
Therefore, the growing understanding of how cortical microtubules align cellulose microfibrils has valuable lessons for us all. As exemplified by the studies reviewed here on CSI1, we need to intercept information flowing out from many kinds of cell and to many kinds of matrix to understand how organisms draw parallel lines, even if they never meet.
Acknowledgments
Work in the Baskin laboratory on cellulose and the control of growth anisotropy is supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the US Department of Energy through Grant DE-FG-03ER15421. Work in the Gu laboratory is supported by grants from National Science Foundation (1121375), and The Center for LignoCellulose Structure and Formation, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science under Award Number DE-SC0001090.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21121
References
- 1.Endler A, Persson S. Cellulose synthases and synthesis in arabidopsis. Mol Plant. 2011;4:199–211. doi: 10.1093/mp/ssq079. [DOI] [PubMed] [Google Scholar]
- 2.Somerville C. Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol. 2006;22:53–78. doi: 10.1146/annurev.cellbio.22.022206.160206. [DOI] [PubMed] [Google Scholar]
- 3.Guerriero G, Fugelstad J, Bulone V. What do we really know about cellulose biosynthesis in higher plants? J Integr Plant Biol. 2010;52:161–75. doi: 10.1111/j.1744-7909.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- 4.Kimura S, Laosinchai W, Itoh T, Cui X, Linder CR, Brown RMJ., Jr. Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant Vigna angularis. Plant Cell. 1999;11:2075–86. doi: 10.1105/tpc.11.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii S, Hayashi T, Mizuno K. Sucrose synthase is an integral component of the cellulose synthesis machinery. Plant Cell Physiol. 2010;51:294–301. doi: 10.1093/pcp/pcp190. [DOI] [PubMed] [Google Scholar]
- 6.Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312:1491–5. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- 7.Popp D, Robinson RC. Supramolecular cellular filament systems: how and why do they form? Cytoskeleton. 2012;69:71–87. doi: 10.1002/cm.21006. [DOI] [PubMed] [Google Scholar]
- 8.Ehrhardt DW, Shaw SL. Microtubule dynamics and organization in the plant cortical array. Annu Rev Plant Biol. 2006;57:859–75. doi: 10.1146/annurev.arplant.57.032905.105329. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd C. Dynamic microtubules and the texture of plant cell walls. Intern Rev Cell Devel Biol 2011; 287-329. [DOI] [PubMed] [Google Scholar]
- 10.Hepler PK, Newcomb EH. Microtubules and fibrils in the cytoplasm of coleus cells undergoing secondary wall deposition. J Cell Biol. 1964;20:529–32. doi: 10.1083/jcb.20.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledbetter MC, Porter KR. A “microtubule” in plant cell fine structure. J Cell Biol. 1963;19:239–50. doi: 10.1083/jcb.19.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baskin TI. On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma. 2001;215:150–71. doi: 10.1007/BF01280311. [DOI] [PubMed] [Google Scholar]
- 13.Wasteneys GO. Progress in understanding the role of microtubules in plant cells. Curr Opin Plant Biol. 2004;7:651–60. doi: 10.1016/j.pbi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Bringmann M, Li E, Sampathkumar A, Kocabek T, Hauser MT, Persson S. POM-POM2/cellulose synthase interacting1 is essential for the functional association of cellulose synthase and microtubules in arabidopsis. Plant Cell. 2012;24:163–77. doi: 10.1105/tpc.111.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Lei L, Somerville CR, Gu Y. Cellulose synthase interactive protein 1 (CSI1) links microtubules and cellulose synthase complexes. Proc Natl Acad Sci U S A. 2012;109:185–90. doi: 10.1073/pnas.1118560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei Y, Gao HB, Yuan M, Xue HW. The arabidopsis ARCP protein, CSI1, which is required for microtubule stability, is necessary for root and anther development. Plant Cell. 2012;24:1066–80. doi: 10.1105/tpc.111.095059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Y, Kaplinsky N, Bringmann M, Cobb A, Carroll A, Sampathkumar A, et al. Identification of a cellulose synthase-associated protein required for cellulose biosynthesis. Proc Natl Acad Sci U S A. 2010;107:12866–71. doi: 10.1073/pnas.1007092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonucci E. Calcification and silicification: a comparative survey of the early stages of biomineralization. J Bone Miner Metab. 2009;27:255–64. doi: 10.1007/s00774-009-0061-y. [DOI] [PubMed] [Google Scholar]
- 19.Weiner S, Addadi L, Clarke BE, Fratzl P. Crystallization pathways in biomineralization. Annu Rev Mater Res. 2011;41:21–40. doi: 10.1146/annurev-matsci-062910-095803. [DOI] [Google Scholar]
- 20.Tesson B, Hildebrand M. Extensive and intimate association of the cytoskeleton with forming silica in diatoms: control over patterning on the meso- and micro-scale. PLoS One. 2010;5:e14300. doi: 10.1371/journal.pone.0014300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canty EG, Starborg T, Lu Y, Humphries SM, Holmes DF, Meadows RS, et al. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J Biol Chem. 2006;281:38592–8. doi: 10.1074/jbc.M607581200. [DOI] [PubMed] [Google Scholar]
- 22.Kalson NS, Holmes DF, Kapacee Z, Otermin I, Lu Y, Ennos RA, et al. An experimental model for studying the biomechanics of embryonic tendon: Evidence that the development of mechanical properties depends on the actinomyosin machinery. Matrix Biol. 2010;29:678–89. doi: 10.1016/j.matbio.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marga F, Grandbois M, Cosgrove DJ, Baskin TI. Cell wall extension results in the coordinate separation of parallel microfibrils: evidence from scanning electron microscopy and atomic force microscopy. Plant J. 2005;43:181–90. doi: 10.1111/j.1365-313X.2005.02447.x. [DOI] [PubMed] [Google Scholar]
- 24.Baskin TI, Wilson JE. Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 1997;113:493–502. doi: 10.1104/pp.113.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]