Abstract
Enteroaggregative Escherichia coli (EAEC) is an important cause of endemic and epidemic diarrheal disease worldwide. Although not classically considered an inflammatory pathogen in the style of Shigella and Salmonella species, clinical data from patients suggests that inflammatory responses may play an important role during EAEC disease. However, the specific role of inflammation during EAEC pathogenesis has not been investigated in detail. To better understand how EAEC may induce inflammation, we have focused our attention on the intimate interactions between EAEC and the host epithelium and the subsequent induction of host cell signaling events leading to innate immune responses. Here, we discuss our recent findings on the signaling pathway by which EAEC promotes transepithelial migration of polymorphonuclear leukocytes (PMNs), the role of aggregative adherence fimbriae in triggering this event and the implementation of human intestinal xenografts in immunodeficient mice for studying EAEC pathogenesis in vivo. Our findings suggest that EAEC shares conserved mechanisms of inducing PMN recruitment with other intestinal pathogens, providing new insight into the potential pathological consequences of EAEC-induced inflammation.
Keywords: enteroaggregative Escherichia coli, inflammation, 12-lipoxygenase, aggregative adherence fimbriae, human intestinal xenografts
Introduction
Enteroaggregative Escherichia coli (EAEC) is an enteric pathogen increasingly recognized for causing acute and persistent diarrheal illness in developing countries, as well as worldwide foodborne outbreaks.1 While EAEC may in fact be one of the most common bacterial causes of diarrhea, the lack of global routine surveillance systems for detecting EAEC has likely rendered it underreported.2 In the past year, however, focus on EAEC has increased following a major outbreak in Germany in 2011.3
EAEC pathogenesis results from colonization of the intestinal mucosa via a stepwise process of adherence and subsequent biofilm formation, which is followed by toxin release leading to secretion of intestinal fluids.1
Several enteric pathogens, including E. coli pathotypes, are agents of inflammatory diarrhea, the histopathologic hallmark of which is infiltration of polymorphonuclear leukocytes (PMNs).4-7 The role of inflammation during EAEC pathogenesis has only recently been considered, and increasing evidence suggests that inflammatory responses may play a substantial role in EAEC pathology. Clinical studies have documented elevated levels of pro-inflammatory markers, including interleukin (IL)-8, IL-1β and fecal lactoferrin and leukocytes, in EAEC-infected individuals.8-10 Therefore, unraveling the mechanisms underlying EAEC-induced inflammation and dissecting the role of these events in disease are important steps toward advancing the understanding of this emerging pathogen.
Induction of Host Cell Inflammatory Signals by EAEC
Accumulation of PMNs at inflamed sites is a common outcome of colonization of mucosal surfaces by pathogenic bacteria. Intestinal epithelial cells respond by releasing cytokines and distinctive PMN-specific chemoattractants that—in combination with numerous adhesion molecules—recruit PMNs from the blood stream and direct their movement through endothelial and epithelial barriers to the luminal surface. Recruitment of PMNs is the first line of response of the host immune system to bacterial infection, geared toward destruction of invading pathogens. However, the nonspecific neutrophil effectors can cause collateral damage, thus potentially contributing to disease pathology. Moreover, several pathogens have evolved strategies to resist neutrophil killing or even benefit from eliciting inflammation.
In line with other enteric pathogens, previous studies have shown that EAEC infection of polarized intestinal epithelial cells triggers mitogen-activated protein kinase signaling cascades that lead to nuclear factor kappa-B (NFκB) activation, which in turn stimulates the release of an array of pro-inflammatory cytokines, including the potent PMN chemokine interleukin (IL)-8.11,12 Thus, basolaterally released IL-8 likely plays a major role in recruiting PMNs to the subepithelial space in response to EAEC infection.
A recent study from our group shows that EAEC-induced migration of PMNs across the epithelium requires apical secretion of a second, lipid-based PMN chemoattractant (Fig. 1). Specifically, EAEC infection of polarized T84 colonic epithelial cells triggers calcium-independent phospholipase A2 (iPLA2)-mediated release of arachidonic acid from the cell membrane. Through enzymatic action of 12-lipoxygenase (12-LOX), arachidonic acid is then metabolized into hepoxilin A3 (HXA3), a member of the eicosanoid class of lipids with potent PMN chemoattractant properties. EAEC infection also triggers an increase in expression of the apically located membrane ATP-binding cassette (ABC) transporter multidrug resistance-associated protein 2 (MRP2), which subsequently functions as an efflux pump for the vectoral release of HXA3 to the apical surface. Secreted HXA3 then forms a chemotactic gradiant through the tight junctional complex, thus directing paracellular transit of PMNs across the epithelial monolayer to the luminal surface13 (Fig. 1).
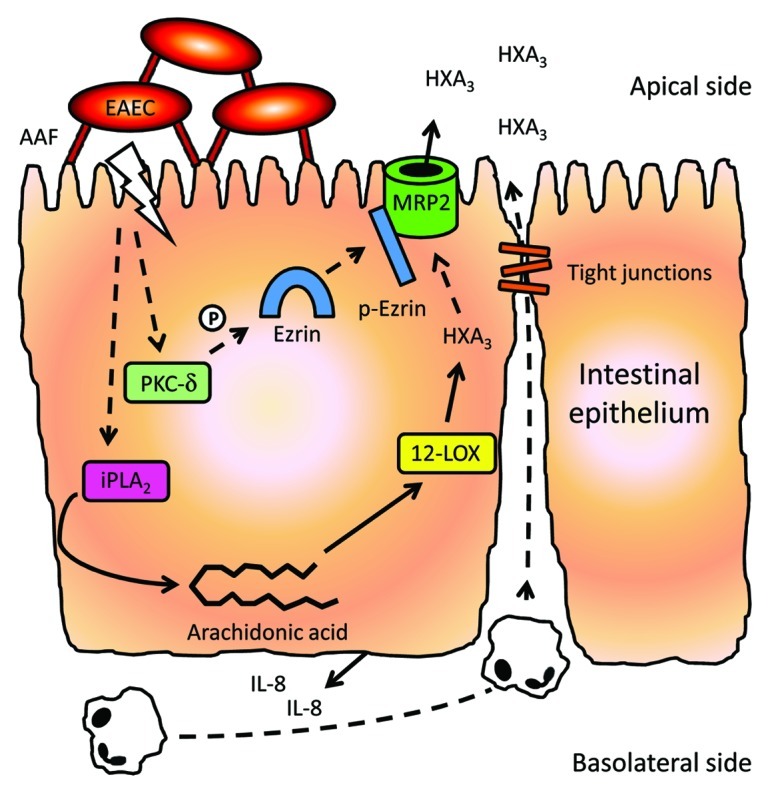
Figure 1. Model of EAEC-induced PMN transepithelial migration. AAF-mediated binding of EAEC to the surface of the intestinal epithelium triggers basolateral release of pro-inflammatory cytokines, e.g., IL-8, thus recruiting PMNs to the subepithelial space. Moreover, AAF binding also induces iPLA2-mediated release of arachidonic acid from the host cell membranes. Through 12-LOX activity, arachidonic acid is then metabolized into HXA3, which is then transported across the apical membrane by MRP2, thus generating a chemotactic gradient of the lipid across the tight junctional complex driving transepithelial migration of PMNs to the apical surface. PKC-δ also plays a key role in these inflammatory events, presumably by activating ezrin which in turn aids in facilitating transport of MRP2 to the apical membrane. Solid lines represent events supported by published results. Hashed lines represent speculations from our group. Modified from Boll et al.13
Increasing evidence suggests that 12-LOX-mediated apical release of HXA3 to promote PMN transepithelial migration is a conserved mechanism by which the intestinal epithelium responds to intruding inflammatory pathogens, including Salmonella enterica serovar Typhimurium (S. Typhimurium), Shigella flexneri, Campylobacter species and EAEC.13-15 Yet, the upstream events by which these pathogens elicit inflammation are very much distinct, reflecting their discrete strategies for promoting infection. As an example, S. Typhimurium and S. flexneri rely on effector proteins, translocated into the host cells by type III secretion systems (T3SS), to interact with host cells, leading to invasion of the epithelium and triggering and manipulation of innate immune responses.16,17 While E. coli pathotypes such as enteropathogenic E. coli and enterohemorrhagic E. coli also employ T3SS-dependent infection strategies, this does not appear to be the case for EAEC. Moreover, unlike S. flexneri and S. Typhimurium, EAEC strains generally do not invade the epithelium, and instead remain anchored in the intestinal mucosa.18 Our recent work shows that EAEC-induced PMN transmigration requires only binding of the bacteria to the apical epithelial surface, an event facilitated by aggregative adherence fimbriae (AAF), the principal adhesins of EAEC.19
The pro-inflammatory properties appear to be conserved among different variants of these adhesins as all four AAF subtypes identified thus far promote PMN transepithelial migration.19 The AAF subunits are phylogenetically related to those of the Afa/Dr family of E. coli adhesins, all of which employ the chaperone-usher pathway for fimbrial assembly.20 Notably, other members of this family, such as the F1845 adhesin and Dr hemagglutinin of diffusely adhering E. coli (DAEC), have also been shown to promote PMN transepithelial migration,6 thus inferring a common strategy of F1845/Dr/AAF-mediated inflammatory responses among these two E. coli pathotypes.
How Does EAEC Activate the 12-LOX Pathway to Trigger Inflammation?
How AAF-facilitated adherence of EAEC to the epithelium is linked to activation of the 12-LOX pathway is yet to be determined. However, binding of AAF to the extracellular matrix (ECM) proteins fibronectin, laminin and type IV collagen has been demonstrated. It is possible that AAF trigger host signal transduction indirectly by binding to ECM proteins that then interact with host cell receptors such as integrin α5β1.21
ECM protein-mediated integrin signal transduction in epithelial cells has been shown to involve phosphorylation of protein kinase C delta (PKC-δ).22 Notably, EAEC infection of T84 cell monolayers triggers phosphorylation and translocation of PKC-δ to the cell membrane, and blocking of PKC-δ activity strongly attenuates EAEC-induced PMN transmigration.13 The exact role of PKC-δ in EAEC-induced inflammation warrants further investigation. However, a different PKC isoform, PKC-α, has been shown to play a central role in S. Typhimurium-induced PMN transmigration by phosphorylating ezrin which then associates with MRP2 and mediates localization of the membrane transporter to the apical surface.23 We speculate that PKC-δ plays a similar role in the event of EAEC-induced HXA3 secretion, as PKC-δ has been shown to be involved in facilitating translocation of MRP2 to the plasma membrane in rat hepatocytes.24 In addition, PKC-δ activity has been shown to cause disruption of tight junctions of intestinal Caco-2 cells through oxidative injury,25 whereas inhibition of PKC-δ appears to confer enhanced barrier function to the cells by promoting expression and assembly of the tight junction proteins occludin and claudin-1.26 Conversely, EAEC has been shown to cause epithelial barrier disruption in T84 cell monolayers through delocalization of occludin and claudin-1.27 Based on these findings, it is evident that PKC-δ activity may play a role in several aspects of EAEC-induced inflammation.
The findings described above point to a role for ECM proteins and integrins in EAEC adherence and possibly in the elicitation of inflammatory responses. However, as ECM proteins are generally localized to the epithelial basement membrane, other receptors are likely to be involved in at least the early stages of infection, at which point the bacteria are restricted from gaining access to the ECM proteins. Additional unknown AAF receptors may therefore likely play a role in activating the 12-LOX pathway and triggering inflammation.
Eliciting Inflammation as a Pathogenic Strategy to Circumvent and Exploit the Host Immune Response
While host inflammatory responses are intended as a first line of defense, several pathogens have evolved sophisticated ways of subverting these events to promote infection and cause disease. For instance, S. Typhimurium utilizes an electron acceptor generated by the host respiratory burst during inflammation to gain a growth advantage over the intestinal microbiota.28 In another example, S. flexneri benefits from the opening of tight junctions—a direct effect of PMN transmigration—to invade the intestinal epithelium from the basolateral surface.29 Moreover, antimicrobial proteins released from neutrophil granules have been shown to enhance adherence of S. flexneri to epithelial cells during the initial steps of infection.30 In a third example, PMN transmigration induced by Afa/Dr-expressing DAEC has been shown to trigger synthesis of tumor necrosis factor α and IL-1β. This, in turn, upregulates apical surface expression of decay-accelerating factor, the receptor for Afa/Dr adhesins, thus promoting enhanced bacterial colonization.31
Similar to DAEC, our studies show that PMN transepithelial migration enhances subsequent adherence of EAEC to T84 cell monolayers, suggesting that EAEC may also benefit from eliciting inflammation. This enhanced adherence is not due to loss of barrier integrity but rather a direct consequence of post-transmigratory PMN-mediated events that alter host cell signaling.13 Distinguishing them from Afa/Dr adhesins, AAF do not appear to bind to decay-accelerating factor.21 However, adenosine derived from neutrophils that infiltrate the lumen during active intestinal inflammation has been shown to trigger apical secretion of fibronectin from T84 cells.32 Moreover, adenosine facilitates enhanced adherence of EAEC to T84 cell monolayers, likely through AAF-fibronectin binding.21 Thus, enhanced EAEC adherence following PMN transmigration could be mediated by increased availability of ECM proteins on the apical surface. In addition, adenosine secretion stimulates fluid secretion from the epithelium.33 Triggering of inflammation may therefore both enhance colonization of EAEC on mucosal surfaces as well as aid in the diarrheal spreading of the bacteria.
Apart from AAF, at least one other EAEC virulence factor has been identified as a contributing factor in causing or modulating host immune responses. Pic, a serine protease autotransporter of Enterobacteriaceae (SPATE) found in EAEC, S. flexneri and uropathogenic E. coli, cleaves mucin, induces mucus release and confers a growth advantage in a mouse model of mucosal colonization.34,35 More intriguingly, Pic was recently shown to target several Sialyl Lewis-X-modified glycoproteins on the immune cell surface, including CD43 and CD45. The effects of Pic activity on these leukocytes included impaired chemotaxis and migration of PMNs, activation of the PMN oxidative burst, and activation and apoptosis in T-cells. Moreover, a Pic mutant S. flexneri strain was found to induce a greater inflammatory response than the wild-type strain in a guinea pig keratoconjunctivitis model, implying that the overall effects of Pic are predominantly anti-inflammatory.36 Thus, it is tempting to speculate a dual role for Pic in EAEC pathogenesis in which Pic mediates penetration of the mucosal layers by the bacteria as well as counteracts the inflammatory response induced by AAF-mediated adherence. However, Pic may also act to enhance inflammatory responses by facilitating premature activation of PMNs and subsequent tissue damage or by causing mislocalization of adherent PMNs.36 Thus, whether Pic-mediated immune modulation by EAEC contributes to pro- and/or anti-inflammatory effects in the human intestine remains to be determined.
Human Intestinal Xenografts as a Model for Studying EAEC Pathogenesis
Animal models provide useful means to study aspects of bacterial pathogenesis that cannot be addressed using cell culture-based studies such as the roles of mucus or the commensal flora in intestinal colonization, or how the complex interplay between different host cell types affect immune responses. However, implementation of suitable small animal models for studying EAEC disease has long been hampered by the fact that EAEC appears to be pathogenic only in the human intestinal tract.34,37 This may result from the inability of AAF adhesins to bind to different versions of receptors present on the mucosal surfaces of tested animal species as compared with humans.
To overcome this hurdle, we have recently employed human intestinal xenografts in severe-combined immunodeficient (SCID-HU-INT) mice as a novel model for studying EAEC disease and innate immune responses in vivo. These transplanted xenografts become extensively vascularized, secrete mucus and develop into morphologically normal human intestine.38 The use of SCID-HU-INT mice as an infection model has previously been demonstrated for other enteric pathogens adapted to humans such as enterohemorrhagic E. coli and Shigella species.39,40 We have shown that EAEC induces extensive tissue damage and inflammation in the human intestinal tissues in this model as marked by PMN infiltration, goblet cell depletion and edema formation. Moreover, these pathological markers—particularly inflammatory infiltrates—were strongly correlated with expression of AAF.19 The SCID-HU-INT mouse model offers an exciting opportunity to study other aspects of EAEC pathogenesis as well.
EAEC as an Emerging and Adaptable Pathogen: The 2011 German Outbreak
The need for an increased understanding of EAEC pathogenesis is emphasized by the major recent outbreak that took place in Germany in May–June 2011, which was caused by a highly virulent Shiga-toxin (Stx)-producing EAEC O104:H4 strain. Over 4,000 cases of diarrhea were reported during this outbreak, of which 22% of patients developed hemolytic-uremic syndrome (HUS), and 54 patients succumbed to the infection.3 This strain exhibited unusually high proportions of adults affected and ratio of HUS cases, as compared with previous outbreaks of Stx-producing enterohemorrhagic E. coli that typically caused more severe disease in children and the elderly and with an average rate of HUS of about 4%.41 In contrast to enterohemorrhagic E. coli, the 2011 German outbreak strain possesses EAEC-specific virulence factors, including AAF, as wells as the three SPATE proteases Pic, SepA and SigA. Thus, the severity of clinical outcomes following infection during this outbreak suggests that the EAEC background conferred enhanced virulence to this strain.3,42
Stx-induced systemic complications require transit of the toxin across the intestinal epithelium upon release from its bacterial host. Epithelial barrier disruption—caused either by the pathogen itself or by infiltrating PMNs responding to infection—is a potential route of paracellular Stx uptake.43 Indeed, PMN migration induced by Stx-producing E. coli has been shown to enhance apical-to-basolateral translocation of Stx across polarized T84 monolayers.44 Adding to the potential impact of inflammation on HUS development, H2O2 production by recruited PMNs has been shown to activate stress responses leading to induction of Stx prophages and thus Stx production.45 Moreover, based on findings from clinical studies, Exeni et al. have suggested that the intensity of PMN activation during infection with Stx-producing E. coli and the speed of onset of PMN impairment is proportionate to the severity of systemic disease.46
Given the ability of EAEC prototype strains to induce both epithelial barrier disruption and PMN transmigration, host inflammation mediated by EAEC virulence factors is likely to play a key role in conferring enhanced virulence to the Stx-producing O104:H4 outbreak strain.
Concluding Remarks
Many enteric pathogens have evolved the ability to engage host cells in complex interactions that trigger inflammatory responses. Our recent work shows that intestinal cells respond to EAEC infection by releasing an arachidonic acid-derived eicosanoid generated through 12-LOX activity that causes PMN transepithelial migration. Distinguishing it from other inflammatory pathogens, EAEC-induced 12-LOX activation requires only binding of the EAEC-defining AAF adhesins. This emphasizes the concept of 12-LOX-mediated signaling as a conserved mechanism by which the intestinal epithelium instigates PMN recruitment to battle enteric pathogens. Reflecting co-evolution, several pathogens have in turn developed sophisticated ways to evade innate immune responses or even benefit from them. Recent studies by ours and other groups suggest that this is also the case for EAEC.
While our studies here provide important new insight into the role of inflammation in EAEC pathogenesis, there is still a lot to be learned. For example, we have yet to determine the “missing link” between AAF-mediated adherence to host cells and induction of the 12-LOX pathway. Moreover, the overall implications of inflammation in EAEC pathology requires further investigation.
Advances in understanding the interplay between EAEC and host cells contributes to our overall understanding of its pathogenesis and provides useful information that may ultimately help in preventing and/or treating disease caused by this emerging pathogen.
Acknowledgments
We would like to thank Dr Rose Szabady and Dr Ana Maldonado-Contreras for critical reading of the manuscript. This work was supported by National Institutes of Health Grants DK56754 and DK33506 to B.A.M.
Glossary
Abbreviations:
- AAF
aggregative adherence fimbriae
- SCID-HU-INT mice
human intestinal xenografts in severe-combined immunodeficient mice
- EAEC
enteroaggregative E. coli
- HXA3
hepoxilin A3
- 12-LOX
12-lipoxygenase
- MRP2
multidrug resistance-associated protein 2
- PKC-δ
protein kinase C-δ
- PLA2
phospholipase A2
- PMN
polymorphonuclear leukocyte
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21241
References
- 1.Harrington SM, Dudley EG, Nataro JP. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol Lett. 2006;254:12–8. doi: 10.1111/j.1574-6968.2005.00005.x. [DOI] [PubMed] [Google Scholar]
- 2.Chattaway MA, Dallman T, Okeke IN, Wain J. Enteroaggregative E. coli O104 from an outbreak of HUS in Germany 2011, could it happen again? J Infect Dev Ctries. 2011;5:425–36. doi: 10.3855/jidc.2166. [DOI] [PubMed] [Google Scholar]
- 3.Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med. 2011;365:709–17. doi: 10.1056/NEJMoa1106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormick BA, Siber AM, Maurelli AT. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect Immun. 1998;66:4237–43. doi: 10.1128/iai.66.9.4237-4243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bétis F, Brest P, Hofman V, Guignot J, Bernet-Camard MF, Rossi B, et al. The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infect Immun. 2003;71:1068–74. doi: 10.1128/IAI.71.3.1068-1074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savkovic SD, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–7. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steiner TS, Lima AA, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg DE, Jiang ZD, Steffen R, Verenker MP, DuPont HL. Markers of inflammation in bacterial diarrhea among travelers, with a focus on enteroaggregative Escherichia coli pathogenicity. J Infect Dis. 2002;185:944–9. doi: 10.1086/339617. [DOI] [PubMed] [Google Scholar]
- 10.Cennimo D, Abbas A, Huang DB, Chiang T. The prevalence and virulence characteristics of enteroaggregative Escherichia coli at an urgent-care clinic in the USA: a case-control study. J Med Microbiol. 2009;58:403–7. doi: 10.1099/jmm.0.005793-0. [DOI] [PubMed] [Google Scholar]
- 11.Harrington SM, Strauman MC, Abe CM, Nataro JP. Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell Microbiol. 2005;7:1565–78. doi: 10.1111/j.1462-5822.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- 12.Khan K, Konar M, Goyal A, Ghosh S. Enteroaggregative Escherichia coli infection induces IL-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-kappaB and AP-1 in INT-407 cells. Mol Cell Biochem. 2010;337:17–24. doi: 10.1007/s11010-009-0282-3. [DOI] [PubMed] [Google Scholar]
- 13.Boll EJ, Struve C, Sander A, Demma Z, Krogfelt KA, McCormick BA. Enteroaggregative Escherichia coli promotes transepithelial migration of neutrophils through a conserved 12-lipoxygenase pathway. Cell Microbiol. 2012;14:120–32. doi: 10.1111/j.1462-5822.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mumy KL, Bien JD, Pazos MA, Gronert K, Hurley BP, McCormick BA. Distinct isoforms of phospholipase A2 mediate the ability of Salmonella enterica serotype typhimurium and Shigella flexneri to induce the transepithelial migration of neutrophils. Infect Immun. 2008;76:3614–27. doi: 10.1128/IAI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy H, Cogan T, Humphrey T. Direction of neutrophil movements by Campylobacter-infected intestinal epithelium. Microbes Infect. 2011;13:42–8. doi: 10.1016/j.micinf.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Srikanth CV, Mercado-Lubo R, Hallstrom K, McCormick BA. Salmonella effector proteins and host-cell responses. Cell Mol Life Sci. 2011;68:3687–97. doi: 10.1007/s00018-011-0841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa M, Handa Y, Ashida H, Suzuki M, Sasakawa C. The versatility of Shigella effectors. Nat Rev Microbiol. 2008;6:11–6. doi: 10.1038/nrmicro1814. [DOI] [PubMed] [Google Scholar]
- 18.Nataro JP, Hicks S, Phillips AD, Vial PA, Sears CL. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun. 1996;64:4761–8. doi: 10.1128/iai.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boll EJ, Struve C, Sander A, Demma Z, Nataro JP, McCormick BA, et al. The fimbriae of enteroaggregative Escherichia coli induce epithelial inflammation in vitro and in a human intestinal xenograft model. J Infect Dis. 2012;206:714–22. doi: 10.1093/infdis/jis417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Servin AL. Pathogenesis of Afa/Dr diffusely adhering Escherichia coli. Clin Microbiol Rev. 2005;18:264–92. doi: 10.1128/CMR.18.2.264-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farfan MJ, Inman KG, Nataro JP. The major pilin subunit of the AAF/II fimbriae from enteroaggregative Escherichia coli mediates binding to extracellular matrix proteins. Infect Immun. 2008;76:4378–84. doi: 10.1128/IAI.00439-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh MA, Kang ES, Lee SA, Lee EO, Kim YB, Kim SH, et al. PKCdelta and cofilin activation affects peripheral actin reorganization and cell-cell contact in cells expressing integrin alpha5 but not its tailless mutant. J Cell Sci. 2007;120:2717–30. doi: 10.1242/jcs.003566. [DOI] [PubMed] [Google Scholar]
- 23.Agbor TA, Demma ZC, Mumy KL, Bien JD, McCormick BA. The ERM protein, ezrin, regulates neutrophil transmigration by modulating the apical localization of MRP2 in response to the SipA effector protein during Salmonella Typhimurium infection. Cell Microbiol. 2011;13:2007–21. doi: 10.1111/j.1462-5822.2011.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schonhoff CM, Gillin H, Webster CR, Anwer MS. Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology. 2008;47:1309–16. doi: 10.1002/hep.22162. [DOI] [PubMed] [Google Scholar]
- 25.Banan A, Farhadi A, Fields JZ, Zhang LJ, Shaikh M, Keshavarzian A. The delta-isoform of protein kinase C causes inducible nitric-oxide synthase and nitric oxide up-regulation: key mechanism for oxidant-induced carbonylation, nitration, and disassembly of the microtubule cytoskeleton and hyperpermeability of barrier of intestinal epithelia. J Pharmacol Exp Ther. 2003;305:482–94. doi: 10.1124/jpet.102.047308. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr. 2009;139:965–74. doi: 10.3945/jn.108.100867. [DOI] [PubMed] [Google Scholar]
- 27.Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect Immun. 2010;78:4958–64. doi: 10.1128/IAI.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–9. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perdomo JJ, Gounon P, Sansonetti PJ. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Invest. 1994;93:633–43. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eilers B, Mayer-Scholl A, Walker T, Tang C, Weinrauch Y, Zychlinsky A. Neutrophil antimicrobial proteins enhance Shigella flexneri adhesion and invasion. Cell Microbiol. 2010;12:1134–43. doi: 10.1111/j.1462-5822.2010.01459.x. [DOI] [PubMed] [Google Scholar]
- 31.Bétis F, Brest P, Hofman V, Guignot J, Kansau I, Rossi B, et al. Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins. Infect Immun. 2003;71:1774–83. doi: 10.1128/IAI.71.4.1774-1783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walia B, Castaneda FE, Wang L, Kolachala VL, Bajaj R, Roman J, et al. Polarized fibronectin secretion induced by adenosine regulates bacterial-epithelial interaction in human intestinal epithelial cells. Biochem J. 2004;382:589–96. doi: 10.1042/BJ20040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, et al. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–5. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington SM, Sheikh J, Henderson IR, Ruiz-Perez F, Cohen PS, Nataro JP. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect Immun. 2009;77:2465–73. doi: 10.1128/IAI.01494-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson IR, Czeczulin J, Eslava C, Noriega F, Nataro JP. Characterization of pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect Immun. 1999;67:5587–96. doi: 10.1128/iai.67.11.5587-5596.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz-Perez F, Wahid R, Faherty CS, Kolappaswamy K, Rodriguez L, Santiago A, et al. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc Natl Acad Sci U S A. 2011;108:12881–6. doi: 10.1073/pnas.1101006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang G, Pulimood AB, Mathan MM, Mathan VI. Enteroaggregative Escherichia coli infection in a rabbit model. Pathology. 2001;33:341–6. [PubMed] [Google Scholar]
- 38.Savidge TC, Morey AL, Ferguson DJ, Fleming KA, Shmakov AN, Phillips AD. Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation. 1995;58:361–71. doi: 10.1046/j.1432-0436.1995.5850361.x. [DOI] [PubMed] [Google Scholar]
- 39.Golan L, Gonen E, Yagel S, Rosenshine I, Shpigel NY. Enterohemorrhagic Escherichia coli induce attaching and effacing lesions and hemorrhagic colitis in human and bovine intestinal xenograft models. Dis Model Mech. 2011;4:86–94. doi: 10.1242/dmm.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Jin L, Champion G, Seydel KB, Stanley SL., Jr Shigella infection in a SCID mouse-human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect Immun. 2001;69:3240–7. doi: 10.1128/IAI.69.5.3240-3247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohawk KL, O’Brien AD. Mouse models of Escherichia coli O157:H7 infection and shiga toxin injection. J Biomed Biotechnol. 2011;2011:258185. doi: 10.1155/2011/258185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, et al. HUS Investigation Team Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–80. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 43.Schüller S. Shiga toxin interaction with human intestinal epithelium. Toxins (Basel) 2011;3:626–39. doi: 10.3390/toxins3060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurley BP, Thorpe CM, Acheson DW. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immun. 2001;69:6148–55. doi: 10.1128/IAI.69.10.6148-6155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner PL, Acheson DW, Waldor MK. Human neutrophils and their products induce Shiga toxin production by enterohemorrhagic Escherichia coli. Infect Immun. 2001;69:1934–7. doi: 10.1128/IAI.69.3.1934-1937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Exeni RA, Ferńndez GC, Palermo MS. Role of polymorphonuclear leukocytes in the pathophysiology of typical hemolytic uremic syndrome. ScientificWorldJournal. 2007;7:1155–64. doi: 10.1100/tsw.2007.172. [DOI] [PMC free article] [PubMed] [Google Scholar]


