Abstract
The enteric nervous system (ENS) is critically important for many intestinal functions such as peristalsis and secretion. Defects in the embryonic formation of the ENS cause Hirschsprung disease (HSCR) or megacolon, a severe birth defect that affects approximately 1 in 5,000 newborns. One of the least understood aspects of ENS development are the cellular and molecular mechanisms that control chain migration of the ENS cells during their migration into and along the embryonic gut. We recently reported a mouse model of HSCR in which mutant embryos carrying a hypomorphic allele of the Phactr4 gene show an embryonic gastrointestinal defect due to loss of enteric neurons in the colon. We found that Phactr4 modulates integrin signaling and cofilin activity to coordinate the forces that drive enteric neural crest cell (ENCC) migration in the mammalian embryo. In this extra view, we briefly summarize the current knowledge on integrin signaling in ENCC migration and introduce the Phactr protein family. Employing the ENS as a model, we shed some light on the mechanisms by which Phactr4 regulates integrin signaling and controls the cell polarity required for directional ENCC migration in the mouse developing gut.
Keywords: PP1, Phactr4, directional migration, enteric nervous system, β1 integrin
Hirschsprung disease (HSCR) is a common congenital disorder that is characterized by loss of enteric neurons from varying lengths of the gastrointestinal tract. The human disease appears to result from the defective development of the enteric nervous system (ENS) during embryogenesis. ENS development is a complex process starting from the migration of specific populations of neural crest cells, called enteric neural crest cells (ENCCs), into the foregut and their continued migration through the gut mesenchyme in a rostral to caudal direction to populate the entire gastrointestinal tract. During migration, ENCCs proliferate and begin to differentiate into various types of neurons and glial cells. These neurons and glial cells cluster to form the ganglia of the mature ENS, which acts to control many intestinal functions, including peristalsis, gastric and pancreatic secretion, and the immune response.1 The in vivo migratory behavior of ENCCs within the embryonic gut has been studied by time-lapse imaging of fluorescently labeled ENCCs. This has revealed several intriguing features of cell movements. ENCCs migrate as chains of interconnected cells and they show complicated patterns of migration.2,3 Instead of a simple linear movement toward the caudal gut, the cells at the migrating wavefront change frequently in migration speed and direction. Moreover, there appear to be “pioneer” cells at the front of the wavefront that explore the environment and then help to direct the migration of the cells behind them, perhaps providing a cellular scaffold for the trailing cells to migrate along.2,3 The migratory behavior also varies depending on the region of the gut. At the beginning of migration within the foregut and midgut, ENCCs tend to remain interconnected as chains and to move persistently. However, when the ENCCs reach the cecum region, they pause for a period of several hours. Some ENCCs break away from the migratory chains leading to an increased number of solitary cells at the migratory wavefront. During the pause, solitary cells located at the migratory wavefront display increased exploratory behavior while cells further behind the wavefront display more restricted movement.2,3 This region-specific change in cell behavior might be a reflection of the unique property of the cecum as a signaling center with very high concentrations of glial cell-line-derived neurotrophic factor (GDNF) and endothelin 3 (EDN3),4,5 molecules which help regulate the migration of ENCCs. These data also indicate that the distinct microenvironment of the developing gut plays an important role in modulating the migration of ENCCs. Once the ENCCs pass the cecum, the solitary cells rejoin the migratory chains and resume their behavior as a collective group of migratory ENCCs.3,6
ENCC migration is tightly regulated by various processes including interactions between the cell and the extracellular matrix. The strength of the cell-substrate contacts can affect cell migration, i.e., strong interactions can inhibit migration.7,8 ENCCs migrate along the gut mesenchyme, which contains various types of extracellular matrix, and in vitro studies indicate that ENCCs can behave differently in response to different extracellular matrix components.9 Integrins are the major cell-adhesion transmembrane proteins that connect cells to components of the extracellular matrix. Integrins are a large family of proteins consisting of α and β proteins. In mouse, there are 18 α and 8 β subunits, which can form 24 different integrin heterodimers, each of them preferentially binding to a set of ECM substrates.10 Upon binding, integrins activate signaling pathways to convert signals from outside the cell to inside to regulate many cellular processes including cell adhesion, proliferation, migration and differentiation.11 β1 integrin forms the largest integrin subfamily as it can assemble into heterodimers with 12 different α subunits. In mice, knockout of the β1 integrin gene leads to early embryonic lethality during the peri-implantation period.12,13 β1 integrin is expressed very broadly during murine embryogenesis and studies of ENS development have shown that β1 integrin is expressed both in ENCCs and in gut mesenchyme.14,15 Conditional knockout of β1 integrin in ENCCs results in colonic aganglionosis due to a migration defect occurring primarily during ENCC invasion toward the cecum and postcecum hindgut.9,15 Live imaging analysis of β1 integrin null ENCCs shows that these cells are still able to migrate but their directionality and speed is significantly reduced. Moreover, mutant cells become abnormally aggregated and do not form the typical chain network.9,15 The α subunit(s) involved in ENCC migration are yet unknown. Extracellular matrix ligands that can interact with integrins, such as tenascin-C and fibronectin, are highly enriched in the gut mesenchyme of the cecum and hindgut. In vitro analysis shows that tenascin-C can inhibit the adhesion and migration of ENCCs while fibronectin acts oppositely to promote migration.9 Therefore, it is believed that these two ligands act in opposition to modulate integrin signaling and help to control the region-specific nature of ENCC migration. Recently a new player in ENCC migration has been uncovered called Phactr4, a phosphatase and actin binding protein, that acts to antagonize β1 integrin signaling in regulating in vivo migration of the embryonic ENCCs.16
The Phactr family, originally identified as protein phosphatase 1 (PP1)-interacting proteins, is composed of four members (Phactr1–4).17 Protein sequence analysis suggests that all four proteins also contain three RPEL repeats, which are monomeric G-actin binding motifs.18-20 Indeed, Phactr proteins and actin can be co-immunoprecipitated from the soluble fraction of cell lysates, thus establishing a physical interaction between Phactr proteins and G-actin.17,19,21 Moreover, each Phactr family member when overexpressed leads to a change in cell shape and to the formation of cell protrusions of variable length and direction, highlighting a role in regulating actin cytoskeleton dynamics.22 However, relatively little is known about the in vivo functions of the Phactr family. Starting from a forward genetic screen for embryonic defects in mice, we identified a missense mutation in the PP1-binding motif of Phactr4 (the mutation is named Phactr4humdy). This mutation specifically disrupts binding of PP1 to Phactr4 and causes a misregulation of PP1 activity.21 Analysis of the phenotypes of Phactr4humdy mutants has shown that Phactr4 plays at least two distinct roles in mammalian development. Phactr4humdy mutant embryos display defects in neural tube closure and eye development due to abnormal cell proliferation controlled through Phactr4-PP1-Rb (Rb: retinoblastoma protein) pathway.21 Recently we uncovered a new role of Phactr4 in regulating ENS development.16 Phactr4humdy mutants show a HSCR-like defect due to a novel function of Phactr4 in regulating ENCC migration through Phactr4-PP1-integrin signaling.16 Live imaging analysis of Phactr4humdy ENCCs shows that these cells are highly motile but their directionality is significantly decreased, leading to incomplete ENS colonization of the gut. To migrate long distances throughout the gut mesenchyme, ENCCs must be able to adjust to changing extracellular environments. An open issue, which remains to be explored, is the behavior of wild-type ENCCs, as well as Phactr4humdy mutant ENCCs, in regard to different extracellular matrix molecules.
Phactr4 is present at the focal adhesion complex and it is proposed to serve as a scaffold to bridge actin and PP1 with integrin signaling and cofilin activity to regulate cytoskeletal dynamics required for ENCC directional migration in the mammalian embryo (Fig. 1).16 PP1 is a ubiquitously expressed multifunctional serine/threonine phosphatase with broad roles in various cellular processes such as ENCC migration, neuronal development, cytoskeleton organization, cell division, muscle contraction and metabolism.16,23-25 The activity of PP1 is controlled through its interaction with regulatory subunits that provide subcellular localization and target specificity.26,27 Phactr4 is specifically localized to different subcellular compartments: the lamellipodium, focal adhesions, the cytoplasm and nucleolus (Fig. 2).16 The distinct localization of Phactr4 and its unique structural motifs, of which only disruption of the PP1 binding domain has been functionally tested, indicate that Phactr4 may play multiple roles in regulating various cellular processes in the mammalian embryo and adult.
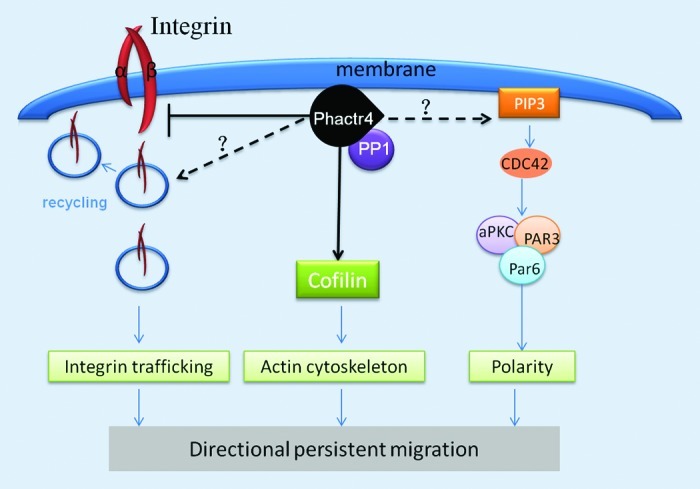
Figure 1. Proposed model of Phactr4 function in regulating directional migration. Phactr4 is localized to the leading edge of the cell membrane where it interacts with PP1 to regulate various signaling pathways required for directional migration. Phactr4 acts through Rho-Cofilin pathway to control the actin cytoskeleton. Phactr4 may also function through the Par Complex (Par3/Par6/aPKC) to determine cell polarity. Phactr4 negatively regulates integrin signaling, and it may control integrin trafficking, which is important for directional migration.
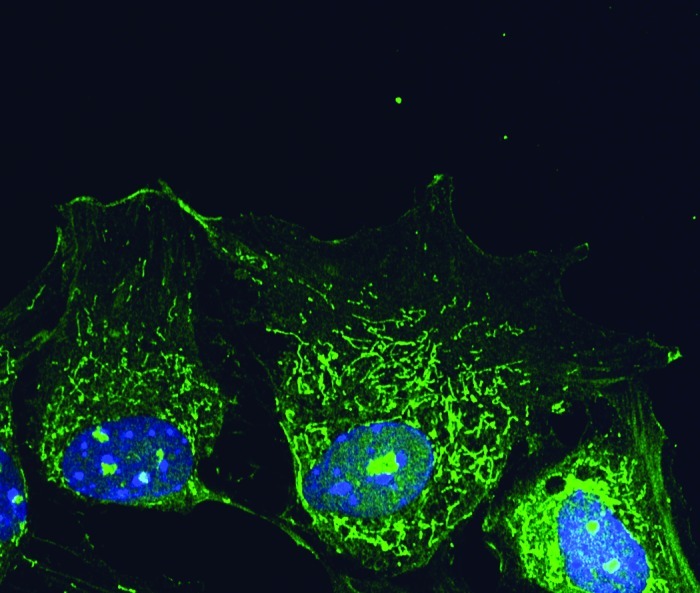
Figure 2. Phactr4 protein localization in the cell. Mouse embryonic fibroblast cells stained with Phactr4 antibody (green) and hoechst (blue). Phactr4 is present in the cell membrane, cytoplasm and nucleolus.
Cell migration is a highly coordinated process that requires orchestration of a set of cellular movements. To move directionally, cells first have to establish an asymmetric morphology with a specific leading and trailing edge. Polarized intracellular signaling directs the formation of protrusions at the leading edge and integrin-mediated adhesion complexes anchor and stabilize the protrusions. Finally, myosin-mediated contraction and detachment at the lagging edge completes the steps required for cell motility.28,29 Our data suggests that Phactr4 functions as a scaffold protein to provide substrate specificity to PP1. In ENCCs and embryonic fibroblasts, Phactr4 helps to bridge PP1 function to cofilin and β1 integrin to regulate their activities, which are required for directed and stabilized lamellipodial protrusions. However, the exact mechanism underlying Phactr4/PP1 action in regulating integrin signaling is still not clear. Differential trafficking of integrin can affect its signaling and determine the motility strategy of the cell. One consequence of integrin trafficking is the redistribution of integrin at the membrane through integrin internalization as well as recycling back to the membrane. This can contribute to the formation of new adhesions at the leading edge during migration.30 One potential pathway that Phactr4 may act to modulate integrin signaling is by regulating the recycling of specific integrins (Fig. 1). Integrin can be recycled preferentially through different pathways: αvβ3 integrin tends to be recycled by Rab4-dependent short loop pathway31 while α5β1 integrin undergoes Rab11-dependent long loop recycling.32 Perturbation of the Rab4-dependent short loop recycling of αvβ3 integrin promotes the rate of α5β1 integrin recycling and results in random migration in fibroblasts during wound healing.33 In epithelial cells, increased recycling of α5β1 integrin triggers ROCK1 dependent phosphorylation and inactivation of cofilin whose activity is critical for directed migration.33,34 Interestingly, our unpublished studies show that β1 integrin is internalized and recycled to perinuclear vesicles in wild-type embryonic fibroblasts, while in the Phactr4 mutant cells, β1 integrin was observed in a dispersed population of tiny vesicles suggesting that β1 integrin long-loop trafficking is altered (unpublished data). These observations indicate a potential mechanism whereby Phactr4 may modulate integrin trafficking to affect cell migration, a hypothesis that requires future study.
Another parameter that influences migratory behavior is cell polarity. The polarity signaling machinery, the Par (partitioning defective) complex, is composed of Par3, Par6 and atypical protein kinase C (aPKC). The Par complex can locally activate Rho GTPase signaling and coordinate the reorientation of the centrosome and vesicle trafficking to regulate directional persistent migration. An example comes from studies of integrin trafficking, in which the Par complex is thought to direct polarized integrin trafficking and adhesion formation.35 In this case, localization of the Par complex at the leading edge of migrating cells regulates the activity of Numb, which is an adaptor for integrin endocytosis. Numb controls the recycling of β1 integrin behind the leading edge. Direct interaction between Numb and the Par complex promotes Numb phosphorylation which prevents Numb from binding with β1 integrin and thus inhibits integrin internalization. This mechanism connects integrin trafficking on the cell membrane to the Par complex at the leading edge of the cell. It is noted in Phactr4humdy mutant embryonic fibroblasts during wound healing that the reorientation of microtubule organizing center (MTOC) is disturbed as ~60% of the wild-type cells polarize toward the wound whereas only 30% of the mutant cells reorient the MTOC toward the wound, indicating that Phactr4 may also play a role in regulating cell polarity (unpublished data; Fig. 1). Interestingly, Par3 gain of function cells also show defects in polarity and they show a similar morphology to Phactr4 mutant cells.36 The stability of the Par complex can be regulated by Par3 phosphorylation status through PP1.37 Moreover, the yeast homolog of Phactr4, Afr1, has been implicated in specifying bud formation during mating.38,39 From two high-throughput screens for Afr1-interacting proteins in yeast, the polarity proteins Boi1/2 were shown to interact with Afr1.40,41 Boi1/2 are not conserved across species but they contain conserved protein domains such as the Pleckstrin homology (PH) domain and Src homology 3 domain (SH3).42 PH domain proteins are known to interact with phosphatidylinositol lipids such as PtdIns-4,5-P2 (PIP2) and PtdIns-3,4,5-P3 (PIP3) which are important mediators of directional migration in response to external guidance molecules.43,44 Taken together, these data suggest different possible mechanisms by which Phactr4 may act to regulate polarity, such as through the Par complex or through PIP2, PIP3 signaling. To address these possibilities, proteomics studies in combination with biochemical studies can help to reveal the proteins that interact with Phactr4 in mammalian cells in order to better understand the role of Phactr4 in directional migration.
Phactr4 is also localized within nuclei, in particular to the nucleolus but its function there remains unknown. Phactr4 is composed of three repeats of RPEL motifs, which were initially identified as a monomeric G-actin binding domain in myocardin-related transcription factor A (MAL).18,45 MAL is a transcriptional coactivator that interacts with serum-response factor (SRF), a nuclear transcription factor that activates many cytoskeleton-related and mitogen-responsive genes.46 The changes in actin dynamics (assembly and disassembly) can affect MAL’s interaction with G-actin through its RPEL motif, and thus alter its subcellular localization (cytoplasm or nucleus). At high G-actin states, MAL is retained inactive in the cytoplasm by forming reversible complexes with G-actin. At low levels of G-actin, MAL is released and translocates into the nucleus to active SRF-dependent transcription.18,20,47 The nuclear shuttling of MAL and activation of SRF serve to further activate the expression of genes that regulate actin dynamics, thus forming a positive feedback loop. Given the nuclear and cytoplasmic localization of Phactr4, it is highly possible that Phactr4 activity is regulated in a similar fashion through direct binding with G-actin through the RPEL motif. It is possible that the regulated nuclear translocation endows upon Phactr4 specific nuclear functions, perhaps including the regulation of gene transcription, an open question at this time.
In conclusion, the present work has discovered a new and exciting function of Phactr4 in regulating directed migration during ENS development and has opened many future directions to explore the role of Phactr4 in other cellular processes. Depending on the cellular context and the proteins with which Phactr4 interacts, Phactr4 can either function through β1 integrin-ROCK-cofilin in directing ENCC migration or through PP1-Rb pathway to control neural progenitor proliferation during neural tube closure. The unique domain structure of Phactr4 and its distinct subcellular localization further suggests that Phactr4 may play roles in various processes, many of which still remain to be explored.
Acknowledgments
This work was supported by the Department of Pediatrics and L.N. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21266
References
- 1.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–79. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 2.Druckenbrod NR, Epstein ML. Behavior of enteric neural crest-derived cells varies with respect to the migratory wavefront. Dev Dyn. 2007;236:84–92. doi: 10.1002/dvdy.20974. [DOI] [PubMed] [Google Scholar]
- 3.Young HM, Bergner AJ, Anderson RB, Enomoto H, Milbrandt J, Newgreen DF, et al. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol. 2004;270:455–73. doi: 10.1016/j.ydbio.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–16. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- 5.Leibl MA, Ota T, Woodward MN, Kenny SE, Lloyd DA, Vaillant CR, et al. Expression of endothelin 3 by mesenchymal cells of embryonic mouse caecum. Gut. 1999;44:246–52. doi: 10.1136/gut.44.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Druckenbrod NR, Epstein ML. The pattern of neural crest advance in the cecum and colon. Dev Biol. 2005;287:125–33. doi: 10.1016/j.ydbio.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Monier-Gavelle F, Duband JL. Cross talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by beta1 and beta3 integrins in migrating neural crest cells. J Cell Biol. 1997;137:1663–81. doi: 10.1083/jcb.137.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufour S, Beauvais-Jouneau A, Delouvée A, Thiery JP. Differential function of N-cadherin and cadherin-7 in the control of embryonic cell motility. J Cell Biol. 1999;146:501–16. doi: 10.1083/jcb.146.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breau MA, Dahmani A, Broders-Bondon F, Thiery JP, Dufour S. Beta1 integrins are required for the invasion of the caecum and proximal hindgut by enteric neural crest cells. Development. 2009;136:2791–801. doi: 10.1242/dev.031419. [DOI] [PubMed] [Google Scholar]
- 10.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 12.Fässler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 13.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–95. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 14.Pietri T, Eder O, Breau MA, Topilko P, Blanche M, Brakebusch C, et al. Conditional beta1-integrin gene deletion in neural crest cells causes severe developmental alterations of the peripheral nervous system. Development. 2004;131:3871–83. doi: 10.1242/dev.01264. [DOI] [PubMed] [Google Scholar]
- 15.Breau MA, Pietri T, Eder O, Blanche M, Brakebusch C, Fässler R, et al. Lack of beta1 integrins in enteric neural crest cells leads to a Hirschsprung-like phenotype. Development. 2006;133:1725–34. doi: 10.1242/dev.02346. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Kim TH, Niswander L. Phactr4 regulates directional migration of enteric neural crest through PP1, integrin signaling, and cofilin activity. Genes Dev. 2012;26:69–81. doi: 10.1101/gad.179283.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen PB, Greenfield AT, Svenningsson P, Haspeslagh DC, Greengard P. Phactrs 1-4: A family of protein phosphatase 1 and actin regulatory proteins. Proc Natl Acad Sci U S A. 2004;101:7187–92. doi: 10.1073/pnas.0401673101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–42. doi: 10.1016/S0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 19.Sagara J, Arata T, Taniguchi S. Scapinin, the protein phosphatase 1 binding protein, enhances cell spreading and motility by interacting with the actin cytoskeleton. PLoS One. 2009;4:e4247. doi: 10.1371/journal.pone.0004247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guettler S, Vartiainen MK, Miralles F, Larijani B, Treisman R. RPEL motifs link the serum response factor cofactor MAL but not myocardin to Rho signaling via actin binding. Mol Cell Biol. 2008;28:732–42. doi: 10.1128/MCB.01623-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TH, Goodman J, Anderson KV, Niswander L. Phactr4 regulates neural tube and optic fissure closure by controlling PP1-, Rb-, and E2F1-regulated cell-cycle progression. Dev Cell. 2007;13:87–102. doi: 10.1016/j.devcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Favot L, Gillingwater M, Scott C, Kemp PR. Overexpression of a family of RPEL proteins modifies cell shape. FEBS Lett. 2005;579:100–4. doi: 10.1016/j.febslet.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 23.Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–56. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 24.Oliver CJ, Terry-Lorenzo RT, Elliott E, Bloomer WA, Li S, Brautigan DL, et al. Targeting protein phosphatase 1 (PP1) to the actin cytoskeleton: the neurabin I/PP1 complex regulates cell morphology. Mol Cell Biol. 2002;22:4690–701. doi: 10.1128/MCB.22.13.4690-4701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez A, Brautigan DL, Mumby M, Lamb NJ. Protein phosphatase type-1, not type-2A, modulates actin microfilament integrity and myosin light chain phosphorylation in living nonmuscle cells. J Cell Biol. 1990;111:103–12. doi: 10.1083/jcb.111.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–84. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–45. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/S0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 29.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 30.Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 31.Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–43. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11:1392–402. doi: 10.1016/S0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 33.White DP, Caswell PT, Norman JC. alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007;177:515–25. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danen EH, van Rheenen J, Franken W, Huveneers S, Sonneveld P, Jalink K, et al. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol. 2005;169:515–26. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Mishima A, Suzuki A, Enaka M, Hirose T, Mizuno K, Ohnishi T, et al. Over-expression of PAR-3 suppresses contact-mediated inhibition of cell migration in MDCK cells. Genes Cells. 2002;7:581–96. doi: 10.1046/j.1365-2443.2002.00540.x. [DOI] [PubMed] [Google Scholar]
- 37.Traweger A, Wiggin G, Taylor L, Tate SA, Metalnikov P, Pawson T. Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3. Proc Natl Acad Sci U S A. 2008;105:10402–7. doi: 10.1073/pnas.0804102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bharucha JP, Larson JR, Konopka JB, Tatchell K. Saccharomyces cerevisiae Afr1 protein is a protein phosphatase 1/Glc7-targeting subunit that regulates the septin cytoskeleton during mating. Eukaryot Cell. 2008;7:1246–55. doi: 10.1128/EC.00024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konopka JB, DeMattei C, Davis C. AFR1 promotes polarized apical morphogenesis in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:723–30. doi: 10.1128/mcb.15.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong AH, Drees B, Nardelli G, Bader GD, Brannetti B, Castagnoli L, et al. A combined experimental and computational strategy to define protein interaction networks for peptide recognition modules. Science. 2002;295:321–4. doi: 10.1126/science.1064987. [DOI] [PubMed] [Google Scholar]
- 41.Tonikian R, Xin X, Toret CP, Gfeller D, Landgraf C, Panni S, et al. Bayesian modeling of the yeast SH3 domain interactome predicts spatiotemporal dynamics of endocytosis proteins. PLoS Biol. 2009;7:e1000218. doi: 10.1371/journal.pbio.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bender L, Lo HS, Lee H, Kokojan V, Peterson V, Bender A. Associations among PH and SH3 domain-containing proteins and Rho-type GTPases in Yeast. J Cell Biol. 1996;133:879–94. doi: 10.1083/jcb.133.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–49. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151:1269–80. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mouilleron S, Guettler S, Langer CA, Treisman R, McDonald NQ. Molecular basis for G-actin binding to RPEL motifs from the serum response factor coactivator MAL. EMBO J. 2008;27:3198–208. doi: 10.1038/emboj.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–65. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Posern G, Sotiropoulos A, Treisman R. Mutant actins demonstrate a role for unpolymerized actin in control of transcription by serum response factor. Mol Biol Cell. 2002;13:4167–78. doi: 10.1091/mbc.02-05-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]


