Abstract
Prostate cancer is the second most frequently diagnosed cancer and the sixth leading cause of death from cancer in men. Epithelial-mesenchymal transition (EMT) is a process by which cancer cells invade and migrate, and is characterized by loss of cell-cell adhesion molecules such as E-cadherin and increased expression of mesenchymal proteins such as vimentin; EMT is also associated with resistance to therapy. Snail, a master regulator of EMT, has been extensively studied and reported in cancers such as breast and colon; however, its role in prostate cancer is not as widely reported. The purpose of this review is to put together recent facts that summarize Snail signaling in human prostate cancer. Snail is overexpressed in prostate cancer and its expression and activity is controlled via phosphorylation and growth factor signaling. Snail is involved in its canonical role of inducing EMT in prostate cancer cells; however, it plays a role in non-canonical pathways that do not involve EMT such regulation of bone turnover and neuroendocrine differentiation. Thus, studies indicate that Snail signaling contributes to prostate cancer progression and metastasis and therapeutic targeting of Snail in prostate cancer holds promise in �future.
Keywords: Snail, prostate cancer, epithelial-mesenchymal transition, metastasis, therapy, cell adhesion, neuroendocrine differentiation, bone turnover
Introduction
Prostate cancer incidence and mortality �worldwide
Prostate cancer is the second most frequently diagnosed cancer in men worldwide, the sixth leading cause of cancer death in men and the fifth most common cancer overall.1 The majority of registered cases (three-quarters) occur in developed nations with the highest rates reported in Australia/New Zealand, Western and Northern Europe and Northern America.1 It is believed that part of the reason prostate cancer is so widespread in developed countries is due to increased practice of prostate specific antigen (PSA) testing and subsequent biopsies.1 Relative high rates of prostate cancer has also been found in developing areas such as the Caribbean, South America and sub-Saharan Africa, while the lowest rates are found in South-Central Asia.1 Prostate cancer is the leading cause of cancer and the second leading cause of morbidity in men in North America.2 African American men have the highest incidence and mortality rates as compared with Caucasian men and present with more aggressive disease at the time of �diagnosis.2
Epithelial-mesenchymal �transition (EMT)
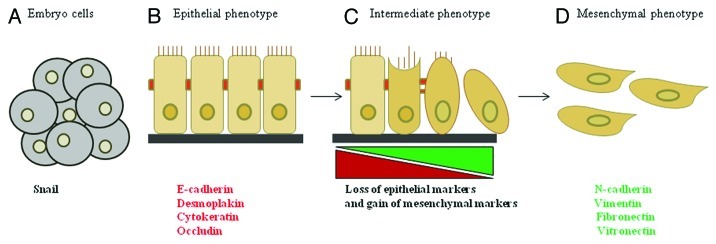
The epithelial-mesenchymal transition (EMT), first described by developmental biologists, is the morphological change that epithelial cells undergo at specific sites during embryonic development, which results in more migratory cells. EMT can be induced by growth factors such as transforming growth factor β (TGF-β), epidermal growth factor (EGF) and transcription factors such as Snail, twist and slug.3 EMT has also been characterized in epithelial cancers, where tumor cells at the invasive front undergo this transition to promote invasion, migration and subsequent metastasis.4,5 Studies have shown that during EMT epithelial cells expressing keratin intermediate filaments (such as cytokeratin), desmosomes (such as desmoplakin) and adherens junction proteins (such as E-cadherin and occludin) repress genes encoding these cell adhesion proteins and modify the type of intermediate filaments expressed (Fig. 1).4,5 This is accompanied by acquisition of mesenchymal markers that includes vimentin and N-cadherin, synthesis of extracellular matrix molecules such as fibronectin and a flattened phenotype (Fig. 1).4,5 These cells subsequently become more migratory, express gelatinases to become more invasive and traverse underlying basement membrane.4,5 Following EMT, the cells may differentiate into other cell types or revert back to an epithelial �cell.3,4,6
Figure 1. Overview of EMT. (A) EMT is a multi-step process, normally occurring during embryogenesis when Snail expression is high. (B) In normal adult cells, polarized epithelial cells express epithelial markers to maintain an adherent phenotype. (C) During cancer progression, colocalization of epithelial and mesenchymal markers indicates an intermediate EMT phenotype due to progressive loss of epithelial markers and gain of mesenchymal markers. (D) Cells that have undergone EMT exhibit a mesenchymal phenotype and high levels of Snail �expression.
There has been much debate as to whether EMT is an in vitro artifact or whether it really occurs in vivo. In support of EMT in vivo, circulating tumor cells from patients with castration-resistant prostate cancer (CRPC) have been found to co-express both epithelial markers such as epithelial cell adhesion molecule (EpCAM) and mesenchymal markers such as vimentin, N-cadherin and O-cadherin, suggesting the presence of EMT intermediate phenotype in vivo.7 A separate study has shown that metastatic tissue from prostate and breast cancer patients co-expresses E-cadherin epithelial marker and mesenchymal markers such as vimentin.8 These studies suggest that there is plasticity between EMT and mesenchymal-epithelial transition (MET) in �vivo.
Apart from the role of EMT in promoting invasion and migration, a role for EMT has been found in promoting resistance to therapy. EMT has been associated with resistance to chemotherapy in pancreatic cancer,9,10 while EMT inducers, Snail and Slug, can mediate resistance to radiotherapy and chemotherapy in ovarian cancer cells.11 In addition, Twist, another transcription factor that can induce EMT, mediates resistance to chemotherapy (paclitaxel) in breast cancer cells via upregulation of AKT-2.12 With respect to prostate cancer, Snail has been associated with resistance to cisplatin chemotherapeutic drug and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), as Snail knockdown in DU145 prostate cancer cells sensitizes the cells to cisplatin- and TRAIL-mediated apoptosis.13 Snail transcription factor, a well-known regulator of EMT, is not as well studied in prostate cancer as in other cancers. Therefore, our goal is to summarize recent findings on Snail signaling in human prostate cancer which has not been previously �reported.
Snail Expression and �Regulation
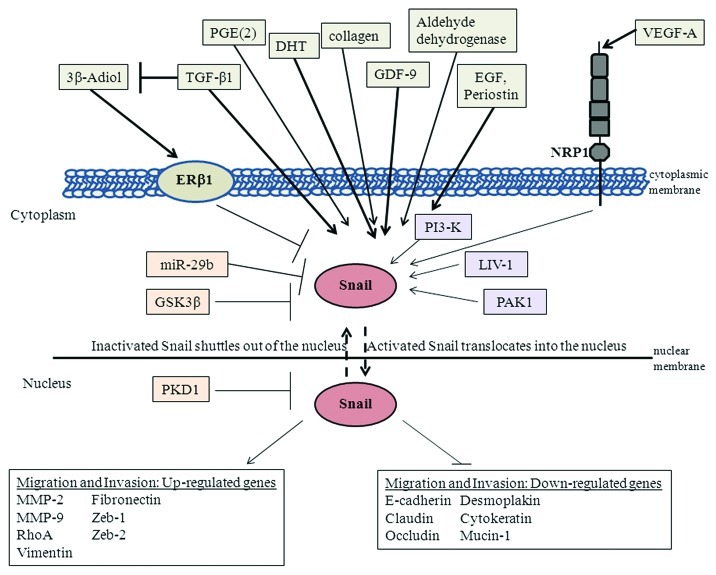
Snail transcription factor, a member of the Snail superfamily, is a zinc finger protein that can mediate EMT through downregulation of cell adhesion molecules such as E-cadherin by binding several E-boxes located in the promotor region.14 Snail can also lead to repression of tight junction proteins like claudin, occludin and zona occludin-1 (ZO-1).15,16 In addition, Snail regulates other epithelial genes such as cytokeratin 18 and Mucin 1.17 Snail can mediate increase in expression of mesenchymal markers such as vimentin, fibronectin, matrix metalloproteinases (MMPs) and RhoA.14,18-20 Snail can also induce other E-cadherin repressors such as Zeb-1 and Zeb-2.21 The overall effect of Snail is that it leads to increased migration and invasion.18,19 Snail has also been shown to confer survival properties either concomitantly with induction of EMT or independent of EMT.22-24 Snail is overexpressed in several cancers; for example, one study has revealed that Snail is sufficient for induction of EMT and promotion of mammary tumor recurrence in vivo, and that Snail expression correlates with a decreased relapse-free survival in women with breast cancer.25 Nuclear Snail has also been associated with tumor progression in ovarian cancer, while remarkably, it was nuclear Snail within stroma that was associated with colon cancer progression.26,27 The various known downstream targets of Snail are depicted �in Figure 2.
Figure 2. Summary of Snail signaling in prostate cancer. Snail can be activated by growth factors such as TGF-β, EGF, VEGF-A and GDF-9; extracellular matrix proteins such as collagen and periostin; hormones such as DHT; kinases such as PI3-K and PAK1; lipid molecules such as PGE(2); enzymes such as aldehyde dehydrogenase 7A1 and LIV-1 zinc finger protein. On the other hand, factors that can negatively regulate Snail include hormones such as 3β-Adiol, which inhibits Snail through ER-β, GSK-3β, PKD1 and miR-29b micro-RNA. Activated Snail can subsequently upregulate or downregulate various genes to increase migratory and invasive �potential.
Snail expression in prostate �cancer
Examination of gene expression profile by microarray has revealed that Snail expression is increased from normal to localized to metastatic prostate cancer.28,29 Another study has determined from immunohistochemical staining of tissue microarray specimens that Snail staining is significantly associated with Gleason grade.30 However, in their study, Snail expression was not correlated with T stage, metastasis at the time of diagnosis, risk of or time to recurrence.30 We have previously shown by immunohistochemistry that Snail expression increases with prostate cancer progression from benign to bone metastatic clinical specimens.31 With respect to mouse models, Snail protein levels increases with tumor progression in PTEN knockout mice that spontaneously developed prostate cancer.32 Snail overexpression in ARCaP prostate cancer cells also resulted in increased tumorigenicity in vivo.33 Therefore, Snail expression appears to increase with prostate cancer �progression.
Factors that regulate Snail localization through �phosphorylation
Snail expression and activity can be regulated by various factors. The activity of Snail is controlled by its phosphorylation which also regulates its subcellular localization.34-37 Glycogen synthase kinase-3β (GSK-3β) has been reported to phosphorylate Snail, thus promoting its export from the nucleus and subsequent degradation by the proteosome in the cytosol.35,36 GSK-3β negatively regulates Snail by phosphorylation of Snail at two consensus motifs resulting in β-TRCP-mediated ubiquitination, localization of Snail in the cytosol and its proteasomal degradation.38 Alternatively, Snail phosphorylation by p21-activated kinase 1 (PAK1) on Ser246 leads to its activation by promoting accumulation of Snail in the nucleus and thus its repressor functions.39 Protein kinase D1 (PKD1) phosphorylates Snail on Ser11 which leads to its nuclear export via 14-3-3σ binding and thus inhibition of EMT.40 Therefore, Snail localization and activity can be regulated by various kinases as shown �in Figure 2.
Growth factors that regulate Snail in prostate �cancer
Snail activity can be regulated by several growth factors. TGF-β1, EGF and vascular endothelial growth factor-A (VEGF-A) have all been shown to activate EMT and Snail in prostate cancer cells.31,41-43 Two separate studies have shown that VEGF-A and TGF-β can promote Snail nuclear localization in PC3 prostate cancer cells, while TGF-β and EGF cooperate to induce EMT in ARCaP prostate cancer cells leading to increased migratory potential.31,41,43 The mechanism by which VEGF-A mediates EMT is by binding to its receptor neuropilin-1 (NRP1), which subsequently promotes nuclear localization of Snail.43 A separate study has shown that EGF promotes Snail expression and EMT via the AKT pathway in DU145 and PC3 prostate cancer cells.42 Growth and differentiation factor 9 (GDF-9), a member of the bone morphogenetic protein (BMP) family, induces an activin-like kinase-5 (ALK-5)-mediated EMT in PC3 prostate cancer cells which is associated with increased Snail and N-cadherin, and decreased E-cadherin.44 Thus, several growth factors can mediate EMT through upregulation and/or activation of Snail in prostate cancer �cells (Fig. 2).
Other factors that regulate Snail in prostate �cancer
Interestingly, hypoxia can influence EMT by regulating VEGF-A. Through a cascade of events, hypoxia has been shown to promote hypoxia inducible factor 1 α (HIF-1α) which then leads to transcriptional upregulation of VEGF-A expression and stimulation of the VEGF-A/NRP1 pathway resulting in Snail nuclear localization and EMT in PC3 prostate cancer cells.43 This hypoxia-mediated EMT can be negatively-regulated by estrogen receptor β-1 (ERβ1) repression, as knockdown of ERβ1 stabilized HIF-1α and promoted EMT.43 Within prostate cancer tissue, ERβ expression is diminished in higher gleason grade prostate cancer which supports the notion that ERβ expression is inversely related with tumor progression.45-47 Moreover, it has been shown that 5α-androstane-3β, 17β-diol (3β-Adiol), an ERβ1 specific ligand, can inhibit hypoxia-mediated EMT by destabilizing HIF-1α and repressing VEGF-A.43 Interestingly, TGF-β and hypoxia can diminish ERβ expression and thereby induce �EMT.43
Extracellular matrices can also regulate Snail expression. Type 1 collagen extracellular matrix can upregulate Snail, Slug and PI3KCA and reduce E-cadherin in SKOV3 ovarian cancer cells and PC3 prostate cancer cells.48 Periostin, a secreted extracellular matrix protein, upregulates Snail, decreases E-cadherin and increases invasiveness in prostate cancer cells via AKT �activation.49
A positive correlation between prostaglandin E(2) [PGE(2)] and Snail has been reported in prostate cancer cells, as antagonizing its receptor PGE(2)-induced E-prostanoid-4 receptor (EP4) decreases migration and Snail levels and increases E-cadherin.32 Androgens, specifically dihydrotestosterone (DHT), can activate Snail and EMT in PC3 and LNCaP prostate cancer cells.50 High levels and activity of aldehyde dehydrogenase enzyme 7A1 has been associated with bone metastasis, and knockdown of this enzyme resulted in decreased Snail, Slug and twist and decreased bone metastasis while intra-prostatic growth was not �affected.51
MicroRNA-29b (miR-29b) is lower in prostate cancer cells and tissue as compared with normal epithelial cells, and its overexpression in PC3 prostate cancer cells decreased Snail, Twist and N-cadherin levels, while E-cadherin levels was �increased.52
LIV-1, a zinc transporter protein has been shown to induce EMT by increasing Snail, MMP-2 and -9 activation which results in shedding of heparin binding-epidermal growth factor (HB-EGF).53 This leads to constitutive phosphorylation of epidermal growth factor receptor (EGFR) and subsequent ERK signaling leading to increased prostate cancer metastasis to bone and soft tissue.53 Therefore, Snail can also be regulated by other transcription factors like HIF-1α, extracellular matrix molecules such as collagen, lipid compounds such as PGE(2), hormones such as androgen, microRNA and the zinc transporter �protein, LIV-1 (Fig. 2).
Canonical Pathways of Snail in Prostate �Cancer
Snail, cell migration and invasion pathways in prostate �cancer
Snail-mediated EMT has been associated with cell migration in prostate cancer. Several of the factors already mentioned that regulate Snail expression also regulates EMT and cell migration in prostate cancer, including TGF-β1, EGF, VEGF-A, GDF-9 and DHT.31,41-44 A direct role for Snail in EMT-associated cell migration has been shown by overexpression of Snail in ARCaP and LNCaP prostate cancer cells which resulted in an EMT associated with decreased/relocalization of E-cadherin, increased vimentin and increased cell migration on collagen.31,54 The signaling pathway by which Snail promotes cell migration in ARCaP cells is probably via MAPK pathway which was activated upon Snail overexpression, since inhibition of the pathway with MAPK inhibitor leads to a partial reversion of EMT.33,55 Snail has also been linked to increased cell invasion in prostate cancer. TGF-β1 has been shown to induce the expression of Snail and cell invasion in prostate cancer cells.56 Thus, Snail can induce EMT which leads to increased migration and invasion in prostate cancer �cells.
Snail and reactive oxygen �species (ROS) signaling
Human cancer development has been associated with chronic inflammation, and ROS released by inflammatory cells may result in DNA damage.57,58 It has also been reported that spontaneous generation of ROS in tumor tissue was positively correlated with clinical stage in small cell lung cancer and squamous cell carcinoma patients.59 In prostate cancer patient tissue, manganese superoxide dismutase antioxidant enzyme levels have been reported to be lower while nuclear oxidative damage products are higher in metastatic tissue as compared with primary tissue, suggesting that increased ROS due to repression of antioxidants may contribute to DNA damage and prostate cancer.60 ROS has also been suggested to mediate EMT. TGF-β treatment of proximal tubular epithelial cells induced EMT indicated by upregulation of hydrogen peroxide and MAPK/extracellular signal-regulated kinase (ERK) signaling in proximal tubular epithelial cells,61 while MMP-3-transfected mammary epithelial cells underwent EMT associated with increased ROS and Snail; moreover, abrogation of ROS with ROS scavenger, N-acetyl cysteine (NAC), could inhibit EMT.62 This suggests that ROS can mediate induction of EMT by growth factors or MMPs. With respect to prostate cancer, we have published that an ARCaP human prostate cancer EMT cell model established by overexpression of Snail transcription factor displayed increased ROS (both hydrogen peroxide and superoxide species) in vitro and in vivo in mouse xenograft models.31,33,54 This EMT could be partially reverted by hydrogen peroxide scavenger, NAC and MEK inhibitor, UO126. Furthermore, NAC could inhibit MAPK activity suggesting that Snail can signal through ROS and MAPK in part to induce EMT.33 So although ROS has been shown to induce Snail expression in breast cancer cells and thereby induce EMT, we have shown for the first time that Snail can also induce ROS and subsequently EMT in prostate cancer cells and this may be possible through regulation of oxidative stress enzymes such as aldehyde oxidase 1 (AOX1).33 Thus, Snail can induce EMT through upregulation of �ROS.
Non-Canonical Pathways of Snail in Prostate �Cancer
Although the role of Snail in canonical pathways involving EMT has been well studied in cancer, there are also some non-canonical pathways that may not involve EMT. Snail may also have a role in a variety of pathways that may not be directly linked to EMT and these will be discussed �below.
Role of Snail in cell adhesion to extracellular �matrix (ECM)
The role of Snail in cell adhesion is not well studied. Haraguchi et al. have reported that Snail enhanced attachment to the extracellular matrix fibronectin in Mardin-Darby canine kidney (MDCK) epithelial cells.63 However, in prostate cancer cells, our data suggest that Snail represses cell adhesion to both fibronectin and collagen.55 Androgen-independent C4-2 prostate cancer cells, a more aggressive subline of the androgen-dependent LNCaP prostate cancer cells exhibits decreased cell adhesion and increased cell migration to collagen I and fibronectin as compared with LNCaP cells. Stable knockdown of Snail in C4-2 cells increased adhesion and decreased migration, indicating that Snail controls these processes. However, the LNCaP-C4-2 progression model does not represent an EMT model in our hands; C4-2 cells express higher levels of Snail than LNCaP cells, similar high levels of E-cadherin and undetectable vimentin, and Snail knockdown in C4-2 cells did not affect EMT markers55 (data not shown). This would suggest that Snail regulation of adhesion and migration in these cells is independent of EMT. We have also found that ARCaP prostate cancer cells stably transfected with Snail undergoes EMT and displays decreased cell adhesion to fibronectin and collagen I, concomitant with decreased α5 (for fibronectin), α2 (for collagen) and β1 integrin expression.55 ERK phosphorylated form was increased in ARCaP Snail-transfected cells compared with control cells and inhibition of the MAPK pathway with UO126 MEK inhibitor abrogated Snail-mediated decrease in cell adhesion and reinduced α5, α2 and β1 integrin expression.55 Thus Snail regulates cell adhesion via the MAPK signaling pathway in prostate cancer cells. It would also appear that Snail can decrease cell adhesion and increase cell migration through EMT-dependent and -independent �pathways.
Regulation of bone turnover by �Snail
Snail may be important in bone development as developing mouse limb was shown to express high levels of Snail, specifically in the hypertrophic chondrocytes, and this was important for regulation of chondrocyte differentiation.64 We have shown that receptor activator of NFκB ligand (RANKL), a member of the TNF family that is normally expressed on the cell surface of stromal cells and osteoblasts and mediates osteoclast differentiation and osteolysis or bone resorption,65,66 can be upregulated by Snail overexpression in ARCaP and LNCaP prostate cancer cells.31 We have further published that Snail overexpression is associated with increased osteoclastogenesis in vitro and in vivo.31 Therefore, Snail may be important either for prostate cancer cells to home to the bone or to colonize the bone �microenvironment.
Snail and neuroendocrine �differentiation
Neuroendocrine differentiation (NED) plays a role in both normal and pathological conditions of the prostate. The human prostate epithelial cells is made up of secretory, basal and neuroendocrine (NE) cells.67 The NE cells release several secretory products such as serotonin, calcitonin, bombesin, neuron specific enolase (NSE) and chromogranin A (CgA) that may act in an endocrine, paracrine, or autocrine manner in both normal and disease state which includes induction of tumor cell proliferation.68,69 Clinical studies have suggested that NED increases with tumor progression and the development of androgen refractoriness.70,71 We found that LNCaP prostate cancer cells transfected with Snail displayed increase in the neuroendocrine markers, NSE and CgA, while LNCaP C-33 cells that have been previously published as a neuroendocrine differentiation (NED) model exhibited increased expression levels of Snail protein as compared with LNCaP parental cells.54 Functionally, Snail-mediated NED was associated with increased paracrine cell proliferation.54 Moreover, in these same cells, Snail also promoted EMT.54 Therefore, Snail may promote tumor aggressiveness in the LNCaP cells through multiple processes; induction of EMT may be required to promote migration, while NED may promote tumor proliferation by a paracrine �mechanism.
Therapies Targeting �Snail
Current treatments for prostate �cancer
The second line of treatment for patients who have androgen-independent aggressive prostate cancer is with chemotherapy drugs such as paclitaxel (Taxol), docetaxel (Taxotere), mitoxantrone (Novantrone) or estramustine.72 Estramustine can potently inhibit cell proliferation by targeting the microtubules.73 However, research has shown that these drugs cannot totally control metastatic prostate cancer progression leading to newer therapies in combination with chemotherapy. As newer therapies are being developed for cancer, it is becoming more important to develop approaches that increase the effectiveness of chemotherapy, while decreasing its side effects. Several antioxidants in combination with chemotherapy may not increase its effectiveness in prostate cancer, in fact, some may decrease chemotherapeutic response. For example, epigallocatechin-3-gallate (EGCG) and other polyphenols with 1,2-benzenediol moieties, effectively prevented tumor cell death induced by bortezomib (BZM) in vitro and in vivo in multiple myeloma and glioblastoma.74 However, several antioxidants appear to potentiate the effect of chemotherapy. For example, ellagic acid polyphenol reduced chemotherapy induced toxicity in a phase II trial in hormone refractory prostate cancer.75 Vitamin E enhanced the chemotherapeutic effect of adriamycin in prostate cancer �cells.76
Antagonists/therapies that target Snail �signaling
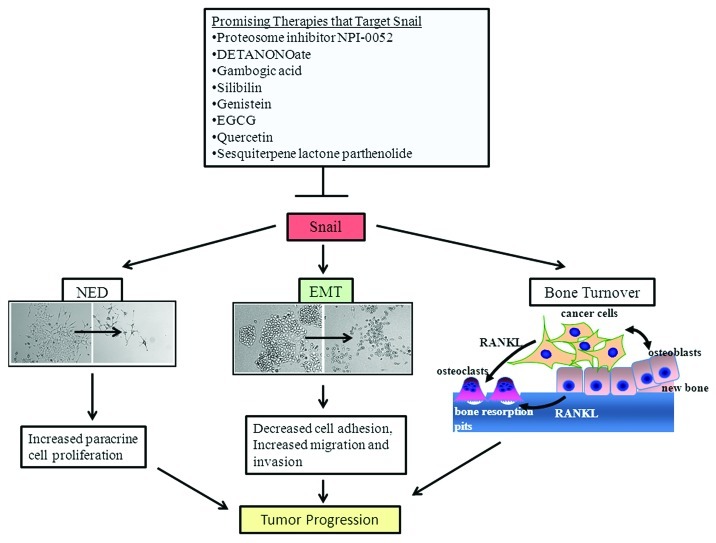
Some therapies that have been developed for prostate cancer appear to target Snail signaling. Proteosome inhibitors have been developed to target the proteosome and induce cancer cell death.77 A novel member of the proteosome inhibitor NPI-0052 (salinosporamide A) isolated from the marine actinomycete Salinispora tropica, is a nonpeptide inhibitor of all the three enzymatic activities of the 20S proteasome.78 It can inhibit Snail mRNA and protein and thereby promote sensitivity to cisplatin- and TRAIL-mediated apoptosis in DU145 prostate cancer cells.13 It has been further shown that Snail inhibition by NPI-0052 allowed for re-expression of the metastasis suppressor, Raf-1 kinase inhibitory protein (RKIP).13 Interestingly, DETANONOate, an agent that increases nitric oxide (NO) levels, decreased Snail expression, increased RKIP levels, inhibited EMT and increased sensitivity to cisplatin- and TRAIL-mediated apoptosis in DU145 and PC3 prostate cancer �cells.13
Natural products have also shown some promise toward targeting Snail signaling in prostate cancer. Gambogic acid (GA), a xanthonoid resin from Garcinia hurburyi tree, was able to inhibit tumor necrosis factor α (TNF-α)-induced invasion of PC3 cells in part via downregulation of Snail expression.79 Another study has reported that the nontoxic phytochemical, silibilin, found in herbs such as milk thistle, decreased the levels of Snail and Slug, inhibited EMT and decreased migratory and invasive potential in PC3 and C4-2B prostate cancer cells.80 Genistein, an isoflavonoid derived from soy products, when fed to transgenic adenocarcinoma mouse prostate model (TRAMP/FVB) mice, decreased the incidence of poorly differentiated cancer at the prostatic intra-epithelial neoplasia (PIN) stage.81 This was associated with inhibition of Akt activation, restoration of GSK-3β and downregulation of Snail.81 EGCG, a polyphenol flavonoid isolated from green tea, inhibited Snail, EMT, migration and invasion in CD44+, CD133+ cancer stem cell population isolated from PC3 and LNCaP prostate cancer cells.82 It also increased apoptosis, and its effects on cancer stem cells could be potentiated by co-treatment with quercetin, another dietary bioflavonoid.82 Sesquiterpene lactone parthenolide (PTL), an extract from the plant feverfew (Tanacetum parthenium), was cytotoxic to cancer stem cells isolated from prostate cancer cell lines (DU145, PC3, VCAP and LAPC4) as well as primary prostate cancer stem cells.83 PTL affected several signaling pathways such as focal adhesion kinase (FAK) and MAPK and also altered binding of transcription factors such as Snail, signal transducer and activator of transcription 3 (STAT3) and p53.83 Therefore, targeting Snail may effectively prevent prostate cancer progression and improve sensitivity to �chemotherapy.
Conclusions
Although Snail signaling is not as well studied in prostate cancer as in other cancers, several studies are emerging that shows that it plays a prominent role in tumor progression through induction of EMT and other processes such as NED and bone turnover (Fig. 3). A number of therapy modalities that affect Snail signaling have also been reported (Fig. 3). As more studies emerge, it will become clear that Snail may be an attractive therapeutic target both in primary and metastatic prostate �cancer.
Figure 3. Effects of Snail signaling in prostate cancer and promising therapeutic targets. Snail gene is involved in canonical pathways that induce EMT associated with a transition from an epithelial to mesenchymal morphology, decreased cell adhesion and increased invasion and migration. Snail is also involved in non-canonical pathways such as bone turnover in which cancer cells produce RANKL that stimulates osteoclast maturation and increased bone resorption and NED in which cancer cells acquire long neurite processes and produce factors that promote paracrine cell proliferation. Several promising therapies that can antagonize Snail are �shown.
Acknowledgments
This work was supported by NIH grants 1P20MD002285 (V.O.M.) and �G12RR03062 (V.O.M.).
Glossary
Abbreviations:
- EMT
epithelial mesenchymal �transition
- TGF-β
transforming growth �factor β
- EGF
epidermal growth �factor
- MET
mesenchymal-epithelial �transition
- MAPK
mitogen activated protein �kinase
- TRAIL
tumor necrosis factor-related apoptosis-inducing �ligand
- ERK
extracellular signal-regulated �kinase
- ZO-1
zona �occludin-1
- GSK-3β
glycogen synthase �kinase-3β
- PAK1
p21-activated kinase �1
- PKD1
protein kinase �D1
- ECM
extracellular �matrix
- MDCK
mardin-darby canine kidney �cells
- shRNA
short hairpin �RNA
- VEGF-A
vascular endothelial growth �factor-A
- NRP1
neuropilin-1
- GDF-9
growth and differentiation factor �9
- HIF-1α
hypoxia inducible factor �1 α
- ERβ1
estrogen �receptor β-1
- NED
neuroendocrine �differentiation
- RANKL
receptor activator of NFκB �ligand
- ROS
reactive oxygen �species
- GA
gambogic �acid
- EGCG
epigallocatechin-3-gallate
- PTL
sesquiterpene lactone �parthenolide
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21687
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Williams H, Powell IJ. Epidemiology, pathology, and genetics of prostate cancer among African Americans compared with other ethnicities. Methods Mol Biol. 2009;472:439–53. doi: 10.1007/978-1-60327-492-0_21. [DOI] [PubMed] [Google Scholar]
- 3.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 4.Boyer B, Vallés AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol. 2000;60:1091–9. doi: 10.1016/S0006-2952(00)00427-5. [DOI] [PubMed] [Google Scholar]
- 5.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 6.Savagner P, Vallés AM, Jouanneau J, Yamada KM, Thiery JP. Alternative splicing in fibroblast growth factor receptor 2 is associated with induced epithelial-mesenchymal transition in rat bladder carcinoma cells. Mol Biol Cell. 1994;5:851–62. doi: 10.1091/mbc.5.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao Y, Wu Q, Acquafondata M, Dhir R, Wells A. Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer Microenviron. 2012;5:19–28. doi: 10.1007/s12307-011-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–8. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–7. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–68. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 12.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–87. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 13.Baritaki S, Yeung K, Palladino M, Berenson J, Bonavida B. Pivotal roles of snail inhibition and RKIP induction by the proteasome inhibitor NPI-0052 in tumor cell chemoimmunosensitization. Cancer Res. 2009;69:8376–85. doi: 10.1158/0008-5472.CAN-09-1069. [DOI] [PubMed] [Google Scholar]
- 14.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 15.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–67. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 16.Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci. 2004;117:1675–85. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- 17.Guaita S, Puig I, Franci C, Garrido M, Dominguez D, Batlle E, et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem. 2002;277:39209–16. doi: 10.1074/jbc.M206400200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang AL, Wang QS, Zhong YH, Chen G, Xi L, Xie CH, et al. [Effect of transcriptional factor snail on epithelial-mesenchymal transition and tumor metastasis] Ai Zheng. 2005;24:1301–5. [PubMed] [Google Scholar]
- 19.Jordà M, Olmeda D, Vinyals A, Valero E, Cubillo E, Llorens A, et al. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci. 2005;118:3371–85. doi: 10.1242/jcs.02465. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama K, Kamata N, Fujimoto R, Tsutsumi S, Tomonari M, Taki M, et al. Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int J Oncol. 2003;22:891–8. [PubMed] [Google Scholar]
- 21.Takkunen M, Grenman R, Hukkanen M, Korhonen M, García de Herreros A, Virtanen I. Snail-dependent and -independent epithelial-mesenchymal transition in oral squamous carcinoma cells. J Histochem Cytochem. 2006;54:1263–75. doi: 10.1369/jhc.6A6958.2006. [DOI] [PubMed] [Google Scholar]
- 22.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–61. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Alvarez C, Blanco MJ, Pérez R, Rabadán MA, Aparicio M, Resel E, et al. Snail family members and cell survival in physiological and pathological cleft palates. Dev Biol. 2004;265:207–18. doi: 10.1016/j.ydbio.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Emadi Baygi M, Soheili ZS, Schmitz I, Sameie S, Schulz WA. Snail regulates cell survival and inhibits cellular senescence in human metastatic prostate cancer cell lines. Cell Biol Toxicol. 2010;26:553–67. doi: 10.1007/s10565-010-9163-5. [DOI] [PubMed] [Google Scholar]
- 25.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Tuhkanen H, Soini Y, Kosma VM, Anttila M, Sironen R, Hämäläinen K, et al. Nuclear expression of Snail1 in borderline and malignant epithelial ovarian tumours is associated with tumour progression. BMC Cancer. 2009;9:289. doi: 10.1186/1471-2407-9-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francí C, Gallén M, Alameda F, Baró T, Iglesias M, Virtanen I, et al. Snail1 protein in the stroma as a new putative prognosis marker for colon tumours. PLoS One. 2009;4:e5595. doi: 10.1371/journal.pone.0005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, et al. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–6. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 29.Beach S, Tang H, Park S, Dhillon AS, Keller ET, Kolch W, et al. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene. 2008;27:2243–8. doi: 10.1038/sj.onc.1210860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heebøll S, Borre M, Ottosen PD, Dyrskjøt L, Orntoft TF, Tørring N. Snail1 is over-expressed in prostate cancer. APMIS. 2009;117:196–204. doi: 10.1111/j.1600-0463.2008.00007.x. [DOI] [PubMed] [Google Scholar]
- 31.Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C, et al. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res. 2008;18:858–70. doi: 10.1038/cr.2008.84. [DOI] [PubMed] [Google Scholar]
- 32.Kim HN, Narayanan NK, Lasano S, Narayanan B. Modulation of PGE2-induced EP4 expression on snail signaling and the impact on epithelial-mesenchymal transition: significance of EP4 antagonism. Anticancer Res. 2011;31:4347–57. [PubMed] [Google Scholar]
- 33.Barnett P, Arnold RS, Mezencev R, Chung LW, Zayzafoon M, Odero-Marah V. Snail-mediated regulation of reactive oxygen species in ARCaP human prostate cancer cells. Biochem Biophys Res Commun. 2011;404:34–9. doi: 10.1016/j.bbrc.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domínguez D, Montserrat-Sentís B, Virgós-Soler A, Guaita S, Grueso J, Porta M, et al. Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol Cell Biol. 2003;23:5078–89. doi: 10.1128/MCB.23.14.5078-5089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280:11740–8. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 36.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–40. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 2005;65:3179–84. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 38.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–40. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 2005;65:3179–84. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 40.Du C, Zhang C, Hassan S, Biswas MH, Balaji KC. Protein kinase D1 suppresses epithelial-to-mesenchymal transition through phosphorylation of snail. Cancer Res. 2010;70:7810–9. doi: 10.1158/0008-5472.CAN-09-4481. [DOI] [PubMed] [Google Scholar]
- 41.Zhau HE, Odero-Marah V, Lue HW, Nomura T, Wang R, Chu G, et al. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis. 2008;25:601–10. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29:4947–58. doi: 10.1038/onc.2010.240. [DOI] [PubMed] [Google Scholar]
- 43.Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–32. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bokobza SM, Ye L, Kynaston H, Jiang WG. Growth and differentiation factor 9 (GDF-9) induces epithelial-mesenchymal transition in prostate cancer cells. Mol Cell Biochem. 2011;349:33–40. doi: 10.1007/s11010-010-0657-5. [DOI] [PubMed] [Google Scholar]
- 45.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/S0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Jones FE. The ErbB signaling network is coordinately expressed and activated in the mouse prostate. Prostate. 2004;60:68–75. doi: 10.1002/pros.20042. [DOI] [PubMed] [Google Scholar]
- 47.Royuela M, de Miguel MP, Bethencourt FR, Sánchez-Chapado M, Fraile B, Arenas MI, et al. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol. 2001;168:447–54. doi: 10.1677/joe.0.1680447. [DOI] [PubMed] [Google Scholar]
- 48.Cheng JC, Leung PC. Type I collagen down-regulates E-cadherin expression by increasing PI3KCA in cancer cells. Cancer Lett. 2011;304:107–16. doi: 10.1016/j.canlet.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Kim CJ, Sakamoto K, Tambe Y, Inoue H. Opposite regulation of epithelial-to-mesenchymal transition and cell invasiveness by periostin between prostate and bladder cancer cells. Int J Oncol. 2011;38:1759–66. doi: 10.3892/ijo.2011.997. [DOI] [PubMed] [Google Scholar]
- 50.Zhu ML, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J. 2010;24:769–77. doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Pelger RC, van der Pluijm G. The aldehyde dehydrogenase enzyme 7A1 is functionally involved in prostate cancer bone metastasis. Clin Exp Metastasis. 2011;28:615–25. doi: 10.1007/s10585-011-9395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ru P, Steele R, Newhall P, Phillips NJ, Toth K, Ray RB. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther. 2012;11:1166–73. doi: 10.1158/1535-7163.MCT-12-0100. [DOI] [PubMed] [Google Scholar]
- 53.Lue HW, Yang X, Wang R, Qian W, Xu RZ, Lyles R, et al. LIV-1 promotes prostate cancer epithelial-to-mesenchymal transition and metastasis through HB-EGF shedding and EGFR-mediated ERK signaling. PLoS One. 2011;6:e27720. doi: 10.1371/journal.pone.0027720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKeithen D, Graham T, Chung LW, Odero-Marah V. Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate. 2010;70:982–92. doi: 10.1002/pros.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neal CL, Mckeithen D, Odero-Marah VA. Snail negatively regulates cell adhesion to extracellular matrix and integrin expression via the MAPK pathway in prostate cancer cells. Cell Adh Migr. 2011;5:249–57. doi: 10.4161/cam.5.3.15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mu Y, Sundar R, Thakur N, Ekman M, Gudey SK, Yakymovych M, et al. TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun. 2011;2:330. doi: 10.1038/ncomms1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85:473–83. doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ames BN. Mutagenesis and carcinogenesis: endogenous and exogenous factors. Environ Mol Mutagen. 1989;14(Suppl 16):66–77. doi: 10.1002/em.2850140614. [DOI] [PubMed] [Google Scholar]
- 59.Zieba M, Suwalski M, Kwiatkowska S, Piasecka G, Grzelewska-Rzymowska I, Stolarek R, et al. Comparison of hydrogen peroxide generation and the content of lipid peroxidation products in lung cancer tissue and pulmonary parenchyma. Respir Med. 2000;94:800–5. doi: 10.1053/rmed.2000.0825. [DOI] [PubMed] [Google Scholar]
- 60.Oberley TD, Zhong W, Szweda LI, Oberley LW. Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate. 2000;44:144–55. doi: 10.1002/1097-0045(20000701)44:2<144::AID-PROS7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 61.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–75. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 62.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–7. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haraguchi M, Okubo T, Miyashita Y, Miyamoto Y, Hayashi M, Crotti TN, et al. Snail regulates cell-matrix adhesion by regulation of the expression of integrins and basement membrane proteins. J Biol Chem. 2008;283:23514–23. doi: 10.1074/jbc.M801125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seki K, Fujimori T, Savagner P, Hata A, Aikawa T, Ogata N, et al. Mouse Snail family transcription repressors regulate chondrocyte, extracellular matrix, type II collagen, and aggrecan. J Biol Chem. 2003;278:41862–70. doi: 10.1074/jbc.M308336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 67.Hansson J, Abrahamsson PA. Neuroendocrine pathogenesis in adenocarcinoma of the prostate. Ann Oncol. 2001;12(Suppl 2):S145–52. doi: 10.1093/annonc/12.suppl_2.S145. [DOI] [PubMed] [Google Scholar]
- 68.di Sant’Agnese PA. Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer. 1992;70(Suppl):254–68. doi: 10.1002/1097-0142(19920701)70:1+<254::AID-CNCR2820701312>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 69.Shah GV, Rayford W, Noble MJ, Austenfeld M, Weigel J, Vamos S, et al. Calcitonin stimulates growth of human prostate cancer cells through receptor-mediated increase in cyclic adenosine 3′,5′-monophosphates and cytoplasmic Ca2+ transients. Endocrinology. 1994;134:596–602. doi: 10.1210/en.134.2.596. [DOI] [PubMed] [Google Scholar]
- 70.Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–92, discussion 592. doi: 10.1016/j.eururo.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 71.Bostwick DG, Qian J, Pacelli A, Zincke H, Blute M, Bergstralh EJ, et al. Neuroendocrine expression in node positive prostate cancer: correlation with systemic progression and patient survival. J Urol. 2002;168:1204–11. doi: 10.1016/S0022-5347(05)64626-5. [DOI] [PubMed] [Google Scholar]
- 72.Garmey EG, Sartor O, Halabi S, Vogelzang NJ. Second-line chemotherapy for advanced hormone-refractory prostate cancer. Clin Adv Hematol Oncol. 2008;6:118–22, 127-32. [PubMed] [Google Scholar]
- 73.Henriksson R, Bjermer L, Von Schoultz E, Grankvist K. The effect of estramustine on microtubuli is different from the direct action via oxygen radicals on DNA and cell membrane. Anticancer Res. 1990;10(2A):303–9. [PubMed] [Google Scholar]
- 74.Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, et al. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113:5927–37. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- 75.Falsaperla M, Morgia G, Tartarone A, Ardito R, Romano G. Support ellagic acid therapy in patients with hormone refractory prostate cancer (HRPC) on standard chemotherapy using vinorelbine and estramustine phosphate. Eur Urol. 2005;47:449–54, discussion 454-5. doi: 10.1016/j.eururo.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Ripoll EA, Rama BN, Webber MM. Vitamin E enhances the chemotherapeutic effects of adriamycin on human prostatic carcinoma cells in vitro. J Urol. 1986;136:529–31. doi: 10.1016/s0022-5347(17)44937-8. [DOI] [PubMed] [Google Scholar]
- 77.Brown M, Cohen J, Arun P, Chen Z, Van Waes C. NF-kappaB in carcinoma therapy and prevention. Expert Opin Ther Targets. 2008;12:1109–22. doi: 10.1517/14728222.12.9.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fenical W, Jensen PR, Palladino MA, Lam KS, Lloyd GK, Potts BC. Discovery and development of the anticancer agent salinosporamide A (NPI-0052) Bioorg Med Chem. 2009;17:2175–80. doi: 10.1016/j.bmc.2008.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lü L, Tang D, Wang L, Huang LQ, Jiang GS, Xiao XY, et al. Gambogic acid inhibits TNF-α-induced invasion of human prostate cancer PC3 cells in vitro through PI3K/Akt and NF-κB signaling pathways. Acta Pharmacol Sin. 2012;33:531–41. doi: 10.1038/aps.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deep G, Gangar SC, Agarwal C, Agarwal R. Role of E-cadherin in antimigratory and antiinvasive efficacy of silibinin in prostate cancer cells. Cancer Prev Res (Phila) 2011;4:1222–32. doi: 10.1158/1940-6207.CAPR-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El Touny LH, Banerjee PP. Akt GSK-3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression toward a poorly differentiated phenotype. Carcinogenesis. 2007;28:1710–7. doi: 10.1093/carcin/bgm103. [DOI] [PubMed] [Google Scholar]
- 82.Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawasaki BT, Hurt EM, Kalathur M, Duhagon MA, Milner JA, Kim YS, et al. Effects of the sesquiterpene lactone parthenolide on prostate tumor-initiating cells: An integrated molecular profiling approach. Prostate. 2009;69:827–37. doi: 10.1002/pros.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]