Abstract
Background
Extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae poses serious challenges to clinicians because of its resistance to many classes of antibiotics.
Methods and Findings
The mechanism of synergistic activity of a combination of (−)-epigallocatechin-3-gallate (EGCG) and β-lactam antibiotics cefotaxime was studied on Extended-spectrum β-lactamase producing Escherichia coli (ESBL-EC), by visualizing the morphological alteration on the cell wall induced by the combination using atomic force microscopy (AFM). Cells at sub-MICs (sub-minimum inhibitory concentrations) of cefotaxime were initially filamentated but recovered to the normal shape later, whereas cells at sub-MICs of EGCG experienced temporal disturbance on the cell wall such as leakage and release of cellular debris and groove formation, but later recovered to the normal shape. In contrast, the combination of cefotaxime and EGCG at their respective sub-MICs induced permanent cellular damages as well as continuous elongation in cells and eventually killed them. Flow cytometry showed that intracellular oxidative stress levels in the cell treated with a combination of EGCG and cefotaxime at sub-MICs were higher than those in the cells treated with either cefotaxime or EGCG at sub-MICs.
Conclusions
These results suggest that the synergistic effect of EGCG between EGCG and cefotaxime against ESBL-EC is related to cooperative activity of exogenous and endogenous reactive oxygen species (ROS) generated by EGCG and cefotaxime, respectively.
Introduction
The reemergence of infectious diseases and the continuous development of antimicrobial drug resistance in pathogens have been causing an alarming deficit in effective antibacterial agents, leading to a growing threat to public healthcare worldwide. Extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae have become the most frequent nosocomial pathogens and have posed serious challenges to clinicians because of their resistance to many classes of antibiotics [1]. ESBLs are the enzymes produced by Gram-negative bacteria that mediate resistance to third-generation cephalosporins (such as cefotaxime and ceftriaxone) by hydrolysis of these antibiotics [2]. Plasmid-mediated ESBL enzymes are of special interest in the generation of ESBL variants [3]. Infections caused by Enterobacteriaceae producing ESBL often complicate the therapy and limit treatment options, often necessitating combination therapy [4]. Combinations of a β-lactam with either a β-lactamase inhibitor or a fluoroquinolone, or double β-lactam combinations are common, but these may not always prevent the emergence of resistance [4], [5]. Several reviews recently emphasized the urgent need for new therapeutic strategies [6], [7].
(−)-Epigallocatechin-3-gallate (EGCG), a main constituent of green tea polyphenol, has been reported to have great anti-infective potential [8], [9] and also aids other antibiotics against both antibiotic-resistant Gram-positive [10], [11] and Gram-negative bacteria [12], [13] at sub-minimum inhibitory concentrations (sub-MICs). Synergy between EGCG and β-lactam against β-lactamase producing Staphylococcus aureus can be easily explained by the fact that both antibacterial compounds attack the same site of the peptidoglycan layer in Gram-positive bacteria [11], [14]. Since EGCG is not able to bind the peptidoglycan layer of Gram-negative bacteria because of their outer membrane, it is suggested that the mechanism underlying the synergistic effect of EGCG with the antibiotics against Gram-negative bacteria is different from the mechanism underlying the synergistic effects against Gram-positive bacteria.
Atomic force microscopy (AFM) is a very useful tool for visualizing the morphology of bacterial surfaces in nanoscale, and has been used to study the antibacterial effects of antibiotics [15], [16], antimicrobial peptides [17], [18], and others [19], [20]. It has been most recently shown by AFM that EGCG has different modes of antibacterial action against Gram-negative and Gram-positive bacteria by direct binding to the peptidoglycan layer or through H2O2 production, respectively [19].
In this study, we used AFM to obtain high-resolution images of morphological changes in ESBL-Escherichia coli induced by a sole treatment of EGCG or cefotaxime at sub-MICs (sub-minimum inhibitory concentrations) or a co-treatment of EGCG and cefotaxime at their respective sub-MICs. To explain the cause of the morphological changes on the bacterial cell surface, oxidative stress response in ESBL-EC against the treatments were measured.
Materials and Methods
Bacterial Strain and Growth Condition
An ESBL-EC strain (BAA-198) [21] was obtained from ATCC. The strain was grown overnight with aeration in a round glass tube containing Mueller – Hinton Broth (MHB; not cation-adjusted; Becton Dickinson) at 37°C. The overnight culture was 100 times diluted with MHB to a final volumes of 5 ml and continued growing with aeration at 37°C till reaching stationary phase determined from optical density at 600 nm (OD600 = 4). Then those cultures with OD600 value of 4 were 100 times diluted with MHB and grown with various concentrations of EGCG (Sigma-Aldrich, St. Louis, MO), cefotaxime (Beta-Lactam antibiotics; Sigma-Aldrich) or their combinations either for MIC determination or time-kill studies. Bacterial growth was calculated by colony-forming unit (CFU) count. CFUs were measured by counting colonies after plating 20 µl of each culture on MHB plates and incubating the plates overnight.
Determination of MIC and Confirmation of the Synergistic Effect
MICs were determined by broth microdilution method [11]. Tubes containing various concentrations of EGCG or cefotaxime (or both) in MHB media were prepared. The total volume of media in each tube was 5 ml. The media were then inoculated with 50 µl of E. coli suspensions (OD600 = 4) and incubated at 37°C with aeration for 18 h. MIC is defined as the lowest concentration of an antimicrobial agent at which no visible growth will occur after overnight incubation.
The effects of combinations were confirmed by the checkerboard method [22]. Two-fold serial dilutions of cefotaxime were tested in combinations with serial dilutions of EGCG. The results were evaluated by a fractional inhibitory concentration (FIC) index. FIC was calculated as MIC of antibiotics alone or EGCG in combination divided by MIC of antibiotics or EGCG alone, and the FIC index was obtained by adding the FICs. FIC indices were interpreted as follows: ≤0.5, synergy; >0.5 to 1, addition; and >1, indifference.
Time-kill Studies
Time-related effects of EGCG, cefotaxime and their combinations were determined by measuring cultures’ CFUs. E. coli suspensions (OD600 = 4) were 100 times diluted with MHB media containing different concentrations of cefotaxime or EGCG (or both). The number of surviving bacteria was counted after 0, 1, 2, 4, 6, 8, 10, 12, 14, 16 and 18 h incubated at 37°C.
Scanning Electron Microscopy (SEM) Analysis
Bacterial suspensions were pre- and post-fixed in glutaraldehyde solution and then added to glass cover slips. The glass cover slips were dehydrated in a series of ethanol concentrations (30–95%) for 15 min followed by 100% ethanol for 20 min. Then the cells were coated with gold and imaged by scanning electron microscopy.
Preparation of AFM Samples
For AFM imaging in air, aliquots of bacterial suspension were added to glass cover slips and rinsed gently in deionized water. The glass cover slips were then dried in a covered Petri dish (50 mm × 50 mm) for several minutes and immediately moved to AFM for imaging.
AFM Operation
The AFM images were taken in air with a commercial AFM (Dimension 3100 with a Nanoscope III controller, Digital Instruments). All images were collected in tapping mode using silicon cantilevers (RTESP, Veeco Probes) with resonance frequencies of approximately 300 kHz and spring constants of approximately 50 Nm−1. Cantilevers were not reused to prevent cross-contamination. Height and amplitude images were simultaneously acquired at scan rates of 0.5−1 Hz with 512×512 resolutions. All visible images were given as amplitude images. To describe the topography of the bacterial surface quantitatively, section analyses and root-mean-square roughnesses (Rrms) were taken from the height images. Once AFM images of single cells were acquired, we selectively magnified areas (500 nm × 500 nm) of the cells except their both ends. Then, we randomly selected very small regions (500 nm2) from the magnified areas to reduce the effect of bacterial half cylindrical structure on Rrms calculation. Membrane roughness was an average of these values taken from at least 20 cells.
Oxidative Stress Detection using Flow Cytometry
Dihydrorhodamine 123 (DHR 123, Sigma), an oxidative stress probe, was used to measure intracellular oxidation levels in bacteria [23] after chemical treatment. EGCG, cefotaxime, or combinations thereof were added to the culture and incubated for either 4 h or 8 h. Then, cells were washed in phosphate-buffered saline (PBS) and incubated with 5 mg/L of DHR 123 for 1 h. Finally, the cells were washed, resuspended in PBS and transferred to flow cytometry tubes. The fluorescence intensity of the bacteria was measured by a FACSCalibur™ flow cytometer (BD), and the mean fluorescence channel (MFC) value of 20,000 cells was determined upon analysis of the live or dead cell population, which was defined by forward and side scatter using CellQuest Pro software (BD). Data were analyzed in FSC/FL-1 histograms.
Results
Synergistic Effect of Cefotaxime and EGCG Against ESBL-EC
MICs of cefotaxime and EGCG were determined to be 128 mg/L and 1500 mg/L, respectively. Any combinations of cefotaxime and EGCG at their sub-MICs showed synergistic effect against ESBL-EC (Table 1). Cefotaxime at 8 mg/L showed synergistic effect with the lowest concentration (50 mg/L) of EGCG, while EGCG at both 100 mg/L and 250 mg/L showed synergistic effect with the lowest concentration (4 mg/L) of cefotaxime.
Table 1. MIC and FIC indices of cefotaxime in combination with EGCG against ESBL-EC.
A, Cefotaxime alone; B, plus EGCG (50 mg/L); C, plus EGCG (100 mg/L); D, plus EGCG (250 mg/L). The MIC of EGCG alone was 1500 mg/L.
Fractional inhibitory concentration (FIC) was calculated as MIC of antibiotics alone or EGCG in com-bination divided by MIC of antibiotics or EGCG alone, and the FIC Index was obtained by adding theFICs.
FIC indices were interpreted as below: ≤0.5, synergy; >0.5 to 1, addition; and >1, indifference.
Time Dependent Effects of Cefotaxime and EGCG Against ESBL-EC
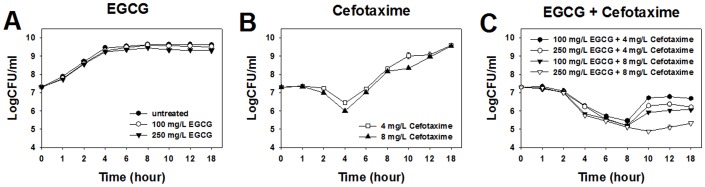
The bacterial growth was not affected by the treatment of EGCG at either 1/15 MIC (100 mg/L) or 1/6 MIC (250 mg/L) (Figure 1A), while it was inhibited up to 4 h after the treatment of cefotaxime at either 1/32 MIC (4 mg/L) or 1/16 MIC (8 mg/L) (Figure 1A and 1B). It was inhibited for more than 8 h at co-treatment of cefotaxime and EGCG at their respective sub-MICs (Figure 1C). Especially when co-treated with cefotaxime and EGCG at their respective 1/16 MIC and 1/6 MIC, bacterial growth was inhibited for up to 10 h (Figure 1C). These results indicate that the inhibitory effect of cefotaxime against ESBL-EC was extended by the addition of EGCG.
Figure 1. Time-kill curves of ESBL-EC treated with EGCG and cefotaxime at sub-MICs.
SEM Observation of Morphological Changes in ESBL-EC Induced by Cefotaxime and EGCG
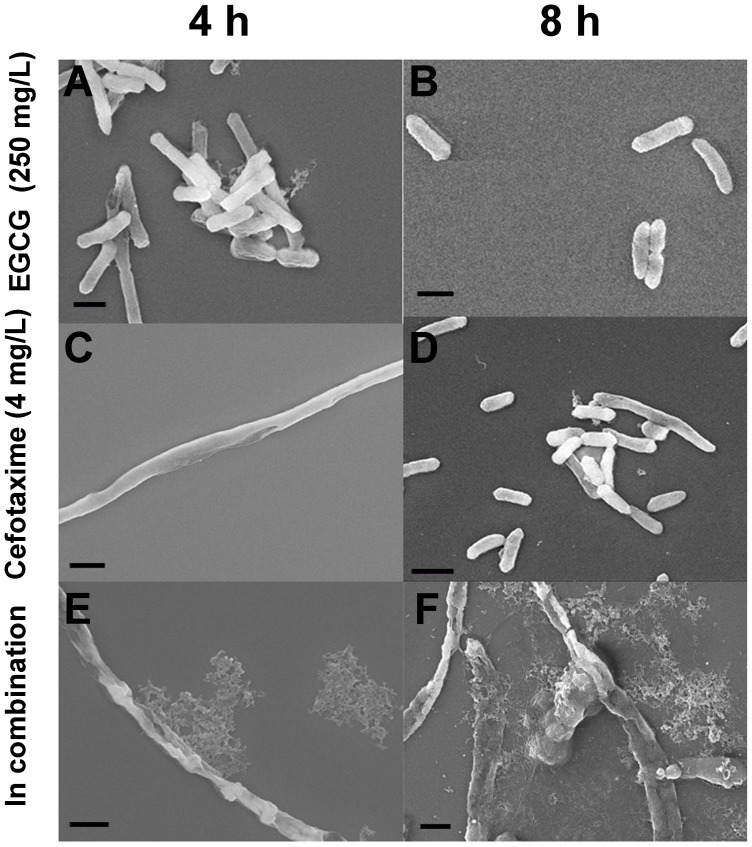
When treated with EGCG at 1/6 MIC (250 mg/L) for 4 h and 8 h, neither morphological changes nor significant leakages were observed in the cells (Figure 2A and 2B). When treated with cefotaxime at 1/32 MIC (4 mg/L), cells were elongated at 4 h (Figure 2C), but the normal cell shape was restored at 8 h (Figure 2D). When co-treated with cefotaxime at 1/32 MIC and EGCG at 1/6 MIC, cells were elongated and lost their cellular contents, leaving debris around them at 4 h (Figure 2E). However, they were not able to restore their normal cell shape at 8 h and became more severely damaged (Figure 2F).
Figure 2. SEM images of ESBL-EC treated with sub-MICs of EGCG, cefotaxime or their combinations.
Cells were: treated with 250 mg/L of EGCG for 4 h (A) and 8 h (B); treated with 4 mg/L of cefotaxime for 4 h (C) and 8 h (D); and treated with 250 mg/L of EGCG and 4 mg/L of cefotaxime in combination for 4 h (E) and 8 h (F). Scale bar: 1 µm.
AFM Observation of Morphological Changes in ESBL-EC Induced by Cefotaxime and EGCG
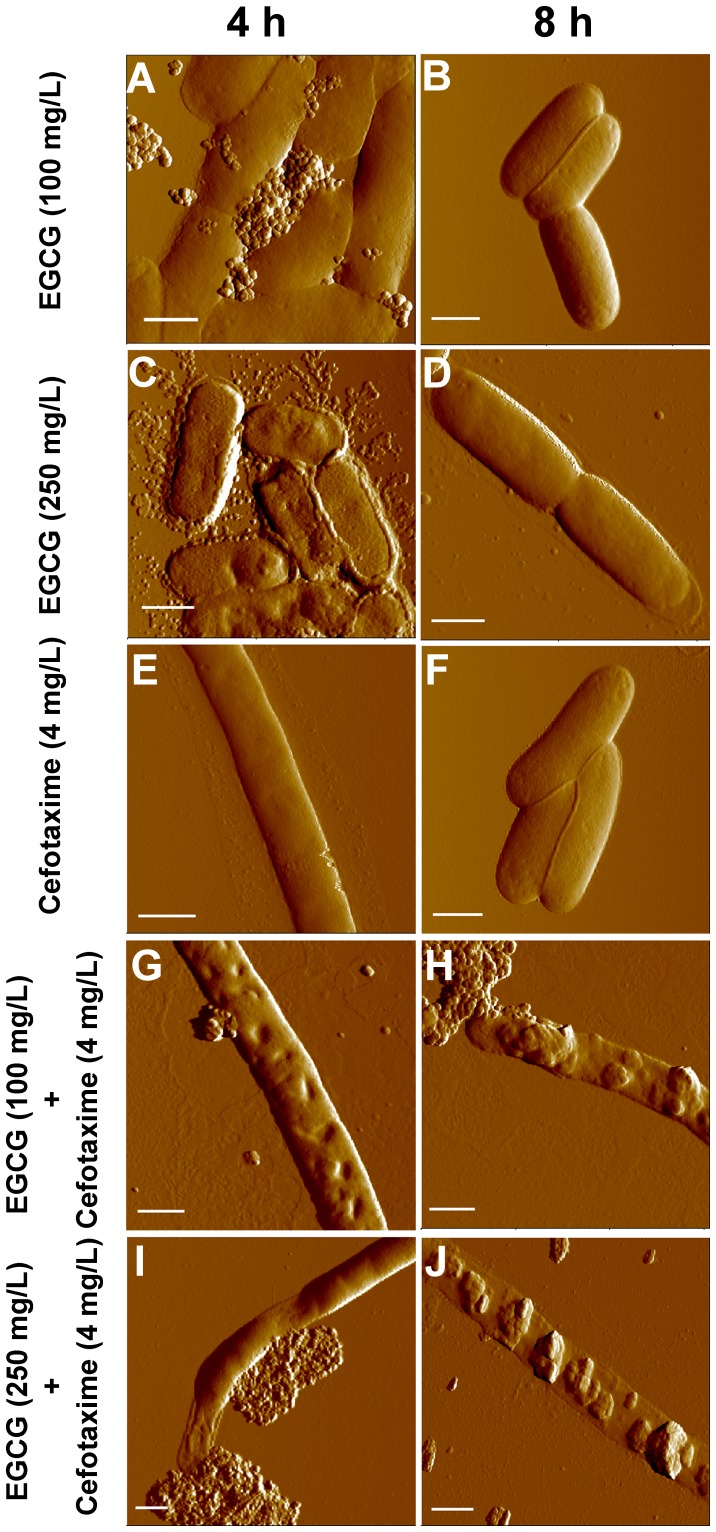
Untreated E. coli cells have very smooth surfaces (Rrms 1.3±0.1 nm, n = 20) (Figure S1). When treated with EGCG at 100 mg/L for 4 h, some leakages (Figure 3A) were observed in cells with slightly increased roughness (Rrms 6.8±1.8 nm, n = 20), possibly due to the leakages. Cells restored their smooth surface (Figure 3B) at 8 h and their surface displayed a low roughness (Rrms 1.5±0.6 nm, n = 20). When treated with EGCG at 250 mg/L for 4 h, large grooves were observed over the cell wall, leading to rough cell surfaces (Rrms 14.9±1.0 nm, n = 20) (Figure 3C). Occasionally, partial collapse of the cell wall was also observed and more debris was released from cell, indicating that cells were more damaged at 250 mg/L than at 100 mg/L. Like at 100 mg/L of EGCG, E. coli at 250 mg/L of EGCG also recovered from such damages at 8 h (Rrms 2.4±0.6 nm, n = 20) (Figure 3D). The treatment of cefotaxime at 4 mg/L for 4 h mostly induced elongation (Figure 3E). Interestingly, elongated cells were able to maintain relatively smooth surfaces (Rrms 3.6±1.0 nm, n = 20) on their cell walls (Figure 3E). With the same treatment, some cells were not elongated, but their surface was uneven as shown in supporting information (Figure S2B), and some other cells showed leakages with flattened cell bodies (Figure S2C). At 8 h, cells restored their native rod shape (Figure 3F) with smooth surfaces (Rrms 1.4±0.5 nm, n = 20).
Figure 3. Topological images of ESBL-EC treated with sub-MICs of EGCG, cefotaxime or their combinations.
Cells were: treated with 100 mg/L of EGCG for 4 h (A) and 8 h (B); treated with 250 mg/L of EGCG for 4 h (C) and 8 h (D); treated with 4 mg/L of cefotaxime for 4 h (E) and 8 h (F); treated with 100 mg/L of EGCG and 4 mg/L of cefotaxime in combination for 4 h (G) and 8 h (H); and treated with 250 mg/L of EGCG and 4 mg/L of cefotaxime in combination for 4 h (I) and 8 h (J). Scale bar: 1 µm.
When co-treated with cefotaxime (4 mg/L) and EGCG (100 mg/L), cells became elongated with large grooves and partial leakages on their cell walls at 4 h (Figure 3G), increasing their surface roughness (Rrms 22.0±9.5 nm, n = 20). At 8 h, more severe leakages were observed all along the cell body, and the damaged cells lost their cellular contents, leaving flattened cell bodies and extremely rough cell surfaces (Rrms 43.2±12.6 nm, n = 20; Figure 3H). When treated with both cefotaxime (4 mg/L) and EGCG (250 mg/L), the leakage of vast cellular materials resulted in the partial flattening of cell bodies as well as increase in surface roughness (Rrms 40.0±7.3 nm, 21 n = 20) at 4 h (Figure 3I). At 8 h, the cell walls were totally collapsed, and severe cellular leakage caused flattening of the cells, leaving only empty envelopes (Figure 3J) and the highest roughness (Rrms 76.0±14.1 nm, n = 20). AFM images of whole cells for each co-treatment can be found in the Supporting Information section (Figure S3A – S3D).
Oxidative Stress Level in ESBL-EC Treated with Cefotaxime and EGCG
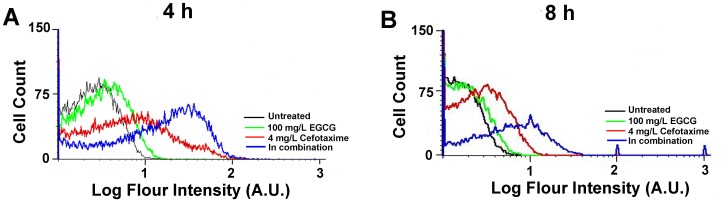
DHR was used as an indicator of intracellular H2O2 [23]–[26]. At both 4 h and 8 h, bacterial cells co-treated with EGCG and cefotaxime showed higher oxidative stress response than those treated with either EGCG or cefotaxime (Figure 4A and 4B). The cells with any of the treatments for 4 h showed higher oxidative stress response than those at 8 h (Figure 4A and 4B), indicating that antibacterial effects of all the treatment decrease over time.
Figure 4. Oxidative stress response in ESBL-EC treated with sub-MICs of EGCG, cefotaxime or their combinations.
Cells were either treated for 4 h (A) or 8 h (B).
Discussion
The sole treatment of either cefotaxime or EGCG at sub-MICs temporarily inhibits ESBL-EC. The cefotaxime induced filamentation in the cells. Filamentation is a typical feature of the morphological change induced by β-lactam antibiotics against Gram-negative bacteria [15], [27]. It was reported that β-lactam antibiotics induced SOS response in E. coli. During the SOS response, bacteria stop dividing and become filamentated. As a result, filamentated bacteria were able to survive under the selective pressure of β-lactam antibiotics [2], [27]. Similarly, filamenated cells were observed at 4 h after cefotaxime treatment, as shown in Figure 2C and 3E. Neither damages nor leakage were observed in the filamentated cells (Figure 3E and S2A), indicating that the cell walls maintained integrity during filamentation. ESBL-EC can obtain resistance to cefotaxime at 8 h. The strain produces β-lactamase to hydrolyze β-lactam antibiotics [2]. This might cause the strain to recover normal cell shape (Figure 2D and 3F) as well as normal growth rate (Figure 1B). Although EGCG at sub-MICS induced cellular damages in ESBL-EC (Figure 3A and 3C), their growth was not significantly inhibited by the treatments (Figure 1A). Furthermore, the strain recovered normal shape at 8 h (Figure 3B and 3D), suggesting that EGCG can cause only a temporal disturbance on the cell wall of ESBL-EC. EGCG is known to generate reactive oxygen species (ROS) by auto-oxidation [28], [29]. The production rate of H2O2 by EGCG increases greatly in the first hour and decreases thereafter [28].
ESBL-EC was not able to withstand oxidative stress exerted by co-treatment of EGCG and cefotaxime. Similarly, its growth was more severely inhibited by co-treatment of H2O2 and cefotaxime, compared to in the sole treatment of either H2O2 or cefotaxime (Figure S4C). During the SOS response against oxidative stress, cells become filamentated until they remove oxidative stress agents such as H2O2 and antibiotics. Since ESBL-EC cells were kept filamentated with severe damages even at 8 h (Figure 3H and 3J) by the co-treatment, it is suggested that ESBL-EC cells are not able to hydrolyze cefotaxime in the presence of EGCG. This can lead to a hypothesis that β-lactamase is damaged by excessive oxidative stress induced by the co-treatment. As shown in Figure 4, in fact, the cells upon the co-treatment experienced a higher oxidative stress than those upon the sole treatment of either EGCG or cefotaxime. Gram-negative bacteria experience oxidative stress due to H2O2 produced by EGCG [19], [28]. The inhibitory effect of β-lactam antibiotics is also known to be related to endogenous hydroxyl radical (OH•) damage, which initiates SOS response [30]. The cell wall of Gram-negative bacteria is not likely to be directly attacked by EGCG due to the presence of the outer membrane and lipopolysaccharide. Herein, we propose a mechanism for the synergistic effect between cefotaxime and EGCG as a converging attack of exogenous and endogenous oxidative stress generated by EGCG and cefotaxime, respectively. Oxidative stress not only initiates membrane degradation, but also causes cell wall collapse and significantly disrupts cellular proteins [24]. Our AFM images and FACS data suggest that the synergistic effect between EGCG and cefotaxime thus may be explained as the synergy between exogenous and endogenous ROS, which are lethal to ESBL-EC. Similarly,
Synergistic effect between cefotaxime and EGCG was only observed when EGCG was used at 100 mg/L, which is considerably above physiological levels. Therefore, combined use of EGCG with cefotaxime could be useful only for topical application to the skin infected with ESBL-EC.
Supporting Information
Topological images of ESBL-EC without any antibacterial treatment.
(DOCX)
Topological images of elongated ESBL-EC and cells failed in filamentation. Cells were: elongated (A); ghost cell (B) and severely leaked cell (C) after treatment of cefotaxime at 4 mg/L for 4 h.
(DOCX)
Topological images of ESBL-EC co-treated with sub-MICs of EGCG and cefotaxime. Cells were: treated with 100 mg/L of EGCG and 4 mg/L of cefotaxime in combination for 4 h (A) and 8 h (B) and treated with 250 mg/L of EGCG and 4 mg/L of cefotaxime in combination for 4 h (C) and 8 h (D). Scale bar: 10 µm.
(DOCX)
Time-kill curves of ESBL-EC treated with H2O2 and cefotaxime at sub-MICs.
(DOCX)
Funding Statement
This work was equally supported by grants (SRC Program: 2012-0000647, #20110021114, #2012-0001138) from Korean National Research Foundation as well as a grant (Code #PJ007492) from Korean Rural Development Administration. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Al-Muharrmi Z, Rafay A, Balkhair A, Jabri AA (2008) Antibiotic combination as empirical therapy for extended spectrum Beta-lactamase. Oman Med J 23: 78–81. [PMC free article] [PubMed] [Google Scholar]
- 2. Bradford PA (2001) Extended-spectrum beta-lactamases in the 21 st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14: 933–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rice L (2001) Evolution and clinical importance of extended-spectrum beta-lactamases. Chest 119: 391S–396S. [DOI] [PubMed] [Google Scholar]
- 4. Klibanov OM, Raasch RH, Rublein JC (2004) Single versus combined antibiotic therapy for gram-negative infections. Ann Pharmacother 38: 332–337. [DOI] [PubMed] [Google Scholar]
- 5. Sader HS, Huynh HK, Jones RN (2003) Contemporary in vitro synergy rates for aztreonam combined with newer fluoroquinolones and beta-lactams tested against gram-negative bacilli. Diagn Microbiol Infect Dis 47: 547–550. [DOI] [PubMed] [Google Scholar]
- 6. Bassetti M, Righi E, Viscoli C (2008) Pseudomonas aeruginosa serious infections: mono or combination antimicrobial therapy? Curr Med Chem 15: 517–522. [DOI] [PubMed] [Google Scholar]
- 7. Nicasio AM, Kuti JL, Nicolau DP (2008) The current state of multidrug-resistant gram-negative bacilli in North America. Pharmacotherapy 28: 235–249. [DOI] [PubMed] [Google Scholar]
- 8. Cho YS, Schiller NL, Oh KH (2008) Antibacterial effects of green tea polyphenols on clinical isolates of methicillin-resistant Staphylococcus aureus . Curr Microbiol 57: 542–546. [DOI] [PubMed] [Google Scholar]
- 9. Shimamura T, Zhao WH, Hu ZQ (2007) Mechanism of Action and Potential for Use of Tea Catechin as an Anti-infective Agent. Anti-Infect Agents Med Chem 6: 57–62. [Google Scholar]
- 10. Hu ZQ, Zhao WH, Yoda Y, Asano N, Hara Y, et al. (2002) Additive, indifferent and antagonistic effects in combinations of epigallogactechin gallate with 12 non-β-lactam antibiotics against methicillin-resistant Staphylococcus aureus . J Antimicrob Chemother 50: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 11. Zhao WH, Asano N, Hu ZQ, Shimamura T (2003) Restoration of antibacterial activity of β-lactams by epigallocatechin gallate against β-lactamase-producing species depending on location of β-lactamase. J Pharm Pharmacol 55: 735–740. [DOI] [PubMed] [Google Scholar]
- 12. Cho YS, Oh JJ, Oh KH (2011) Synergistic anti bacterial and proteomic effects of epigallocatechin gallate on clinical isolates ofimipenem-resistant Klebsiella pneumoniae . Phytomedicine 18: 941–946. [DOI] [PubMed] [Google Scholar]
- 13. Osterburg A, Gardner J, Hyon SH, Neely A, Babcock G (2009) Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green teapolyphenol (−)-epigallocatechin-3-gallate (EGCG). Clin Microbiol Infect 15: 341–346. [DOI] [PubMed] [Google Scholar]
- 14. Zhao WH, Hu ZQ, Okubo S, Hara Y, Shimamura T (2001) Mechanism of Synergy between Epigallocatechin Gallate and β-lactams against Methicillin-Resistant Staphylococcus aureus . Antimicrob Agents Chemother 45: 1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Braga PC, Ricci D (1998) Atomic Force Microscopy: Application to Investigation of Escherichia coli Morphology before and after Exposure to Cefodizime. Antimicrob Agents Chemother 42: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Y, Wang K, Tan W, He X, Jin R, et al. (2006) Atomic Force Microscopy Study of Different Effects of Natural and Semisynthetic β-lactam on the Cell Envelope of Escherichia coli . Anal Chem 78: 7341–7345. [DOI] [PubMed] [Google Scholar]
- 17. Li A, Lee PY, Ho B, Ding JL, Lim CT (2007) Atomic force microscopy study of the antibacterial action of Sushi peptides on Gram-negative bacteria. Biochim Biophys Acta 1768: 411–418. [DOI] [PubMed] [Google Scholar]
- 18. Meincken M, Holroyd DL, Rautenbach M (2005) Atomic Force Microscopy Study of the effect of Antimicrobial Peptides on the Cell Envelope of Escherichia coli . Antimicrob Agents Chemother 49: 4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui Y, Oh YJ, Lim J, Youn M, Lee I, et al. (2012) AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3 gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol 29: 80–87. [DOI] [PubMed] [Google Scholar]
- 20. Sahu K, Bansal H, Mukherjee C, Sharma M, Kumar P (2009) Atomic force microscopic study on morphological alterations induced by photodynamic action of Toluidine Blue O in Staphylococcus aureus and Escherichia coli . J Photochem Photobiol B 96: 9–16. [DOI] [PubMed] [Google Scholar]
- 21. Jacoby GA, Sutton L (1991) Properties of plasmids responsible for production of extended spectrum beta-lactamases. Antimicrob Agents Chemother 35: 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norden CW, Wentzel H, Keleti E (1979) Comparison of techniques for measurement of in vitro antibiotic synergism. J Infect Dis 140: 629–633. [DOI] [PubMed] [Google Scholar]
- 23. Yeom J, Imlay JA, Park W (2010) Iron homeostasis affects antibiotic-mediated cell death in Pseudomonas species. J Biol Chem 285: 22689–22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cabiscol E, Tamarit J, Ros J (2000) Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3: 3–8. [PubMed] [Google Scholar]
- 25. Henderson LM, Chappell JB (1993) Dihydrorhodamine 123: a fluorescent probe for superoxide generation? Eur J Biochem 217: 973–980. [DOI] [PubMed] [Google Scholar]
- 26. Semenza GL (1999) Perspectives on Oxygen Sensing. Cell 98: 281–284. [DOI] [PubMed] [Google Scholar]
- 27. Justice SS, Hunstand DA, Cegelski L, Hultgren SJ (2008) Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol 6: 162–168. [DOI] [PubMed] [Google Scholar]
- 28. Arakawa H, Maeda M, Okubo S, Shimamura T (2004) Role of Hydrogen Peroxide in Bactericidal Action of Catechin. Biol Pharm Bull 27: 277–281. [DOI] [PubMed] [Google Scholar]
- 29. Hou Z, Sang S, You H, Lee MJ, Hong J, et al. (2005) Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res 65: 8049–8056. [DOI] [PubMed] [Google Scholar]
- 30. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ (2007) A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130: 797–810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Topological images of ESBL-EC without any antibacterial treatment.
(DOCX)
Topological images of elongated ESBL-EC and cells failed in filamentation. Cells were: elongated (A); ghost cell (B) and severely leaked cell (C) after treatment of cefotaxime at 4 mg/L for 4 h.
(DOCX)
Topological images of ESBL-EC co-treated with sub-MICs of EGCG and cefotaxime. Cells were: treated with 100 mg/L of EGCG and 4 mg/L of cefotaxime in combination for 4 h (A) and 8 h (B) and treated with 250 mg/L of EGCG and 4 mg/L of cefotaxime in combination for 4 h (C) and 8 h (D). Scale bar: 10 µm.
(DOCX)
Time-kill curves of ESBL-EC treated with H2O2 and cefotaxime at sub-MICs.
(DOCX)