Abstract
The mechanisms resulting in progressive immune dysfunction during the chronic phase of HIV infection are not fully understood. We have previously shown that arginase, an enzyme with potent immunosuppressive properties, is increased in HIV seropositive (HIV+) patients with low CD4+ T cell counts. Here we show that the cells expressing arginase in peripheral blood mononuclear cells of HIV+ patients are low-density granulocytes (LDGs) and that whereas these cells have a similar morphology to normal-density granulocyte, they are phenotypically different. Importantly, our results reveal that increased frequencies of LDGs correlate with disease severity in HIV+ patients.
Introduction
Since the initial report of AIDS in 1981, approximately 60 million people have become infected with HIV of which more than 30 million have died. Although depletion of CD4+ T cells explains much of the immune suppression in HIV infected individuals, the precise reasons for the onset of immunopathology during HIV infection are not yet fully understood [1], [2], [3], [4].
Arginase, an enzyme of the urea cycle, can also be expressed in cells of the immune system and has been shown to exert potent immunoregulatory functions: a reduction in the bioavailability of L-arginine by arginase results in impaired T cell responses, characterized by down-regulation of T cell proliferation, reduced expression of CD3ζ and cytokine production [5], [6], [7], [8]. To date, two mechanisms by which arginase depletes L-arginine have been described:
Transport of extracellular L-arginine into the cells by cationic amino acid transporter (CAT)2B to make it accessible for catabolization by arginase results in depletion of L-arginine in the microenvironment [9], [10];
Arginase can be released by neutrophils into the extracellular milieu, where it binds L-arginine and thereby reduces the level of free L-arginine available to T cells [11].
We have previously shown that arginase activity is higher in peripheral blood mononuclear cells (PBMCs) isolated from HIV-1 infected individuals with low CD4+ T cell counts and that this coincided with lower levels of L-arginine [12]. In the present study, we characterized the phenotype of arginase-expressing cells in HIV+ patients and tested the hypothesis that their frequency correlated with markers of disease severity.
Materials and Methods
Subjects and Samples
Thirtytwo HIV seropositive (HIV+) treatment-naïve individuals (mean age 42.1±12.2 years) were recruited from St Mary’s Hospital and 11 healthy volunteers (mean age 35±4.7 years) were recruited as control subjects. The study was approved by the National Research Ethics Service (05/Q0410/93) and all individuals gave written, informed consent before participation.
Twenty ml of peripheral blood was collected in EDTA tubes and PBMCs were isolated by density gradient centrifugation on Histopaque®-1077 (Sigma). Neutrophils were isolated from the erythrocyte fraction by dextran sulphate sedimentation [13].
Flow cytometry
The following antibodies were used: CD14FITC, CD15PE (BD Pharmingen), Arginase1Alexa Fluor® 647 (Hycult Biotechnology), CD11bPerCP-eFluro710, CD16eFluro450, CD33PE-Cy7 (eBioscience), CD13APC-Cy7 (Biolegend), CD66bFITC and CD63FITC (Beckman Coulter) (Tables 1 and 2). Analysis was performed on an FACS Canto II (BD Bioscience) and results were analyzed using FlowJo v8.7 (Tree Star, Ashland, OR).
Table 1. Phenotype Panel.
| Antigen | Fluorophore | Clone | Isotype | Produced by | Product code | Volume used (µL) | Quantity (µg) |
| CD15 | PE | H198 | mouse IgM | BD Pharmingen | 555402 | 20 | NP |
| CD11b | PerCP-eFluor710 | ICRF44 | mouse IgG1 | eBioscience | 46–0118 | 3 | 0.075 |
| CD13 | APC-Cy7 | WM15 | mouse IgG1 | Biolegend | 301710 | 7 | NP |
| CD16 | eFluor 450 | eBioCB16 | mouse IgG1 | eBioscience | 48–0168 | 3 | 0.15 |
| CD33 | PE-Cy7 | WM-53 | mouse IgG1 | eBioscience | 25–0338 | 3 | 0.15 |
| CD66b | FITC | 80H3 | mouse IgG1 | Beckman Coulter | IM05310 | 3 | NP |
LDGs and NDGs were isolated as described in materials and methods and the expression levels of phenotypic markers were determined by flow cytometry.
NP = Not provided by manufacturer.
Table 2. ARGINASE 1 Panel.
| Antigen | Fluorophore | Clone | Isotype | Produced by | Product code | Volume used (µL) | Quantity (µg) |
| CD15 | PE | H198 | mouse IgM | BD Pharmingen | 555402 | 20 | NP |
| CD13 | APC-Cy7 | WM15 | mouse IgG1 | Biolegend | 301710 | 7 | NP |
| CD16 | eFluor 450 | eBioCB16 | mouse IgG1 | eBioscience | 48–0168 | 3 | 0.15 |
| CD63 | FITC | CLBGran/12 | mouse IgG1 | Beckman Coulter | IM1165U | 14 | NP |
| Arginase 1 | Alexa Fluor® 647 | 6G3 | mouse IgG1 | Hycult biotech. | HM2162 | 2.75 | 0.275 |
LDGs and NDGs were isolated as described in materials and methods and the expression levels of phenotypic markers were determined by flow cytometry.
NP = Not provided by manufacturer.
Isolation of CD15+ cells
CD3+ and CD14+ cells were removed by positive selection, CD15+ cells were incubated with anti-CD15PE and selected using EasySep PE Positive Selection Kit (EasySep, Stem Cell Technologies, France). Purity of CD15+ cells was checked by flow cytometry (>95%). The remaining cells were transferred onto a microscope slide (Thermo Scientific, United Kingdom) using a cytospin centrifuge and stained with hematoxylin and eosin (H&E) (Reagena, Gamidor, United Kingdom).
Statistical Analyses
Data were evaluated for statistical differences using a Wilcoxon signed rank test, a two-tailed Mann-Whitney test and a Spearman’s rank test when appropriate (GraphPad Prism 5); differences were considered statistically significant at p<0.05. Unless otherwise specified, results are expressed as median±SEM.
Results
Phenotypic Characterization of Different Populations of Granulocytes
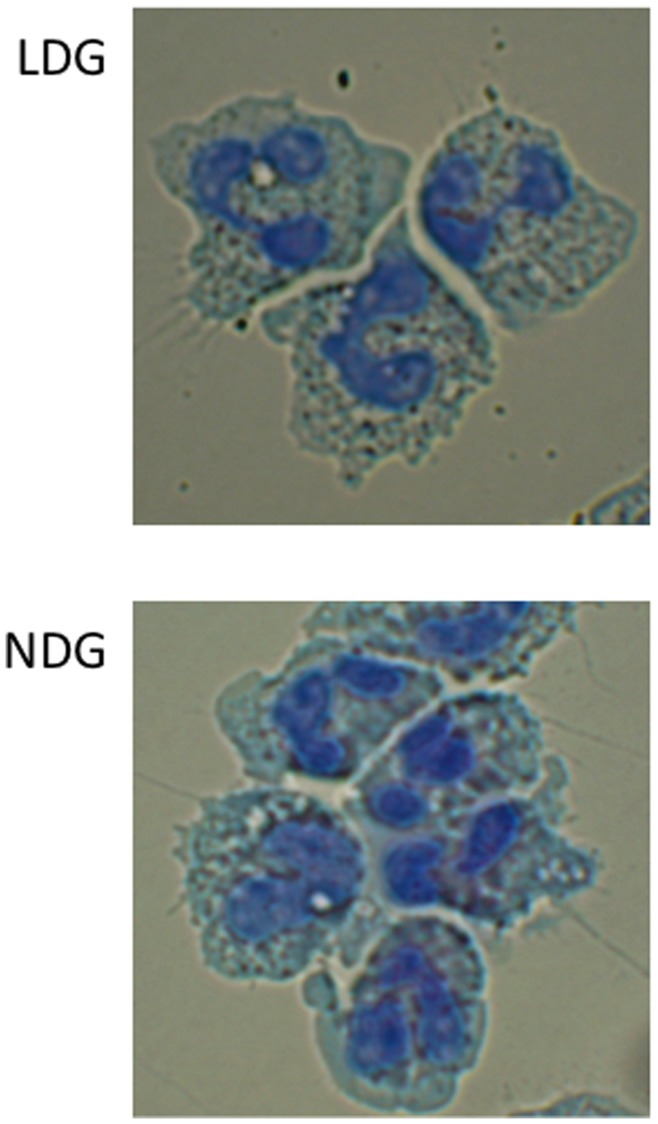
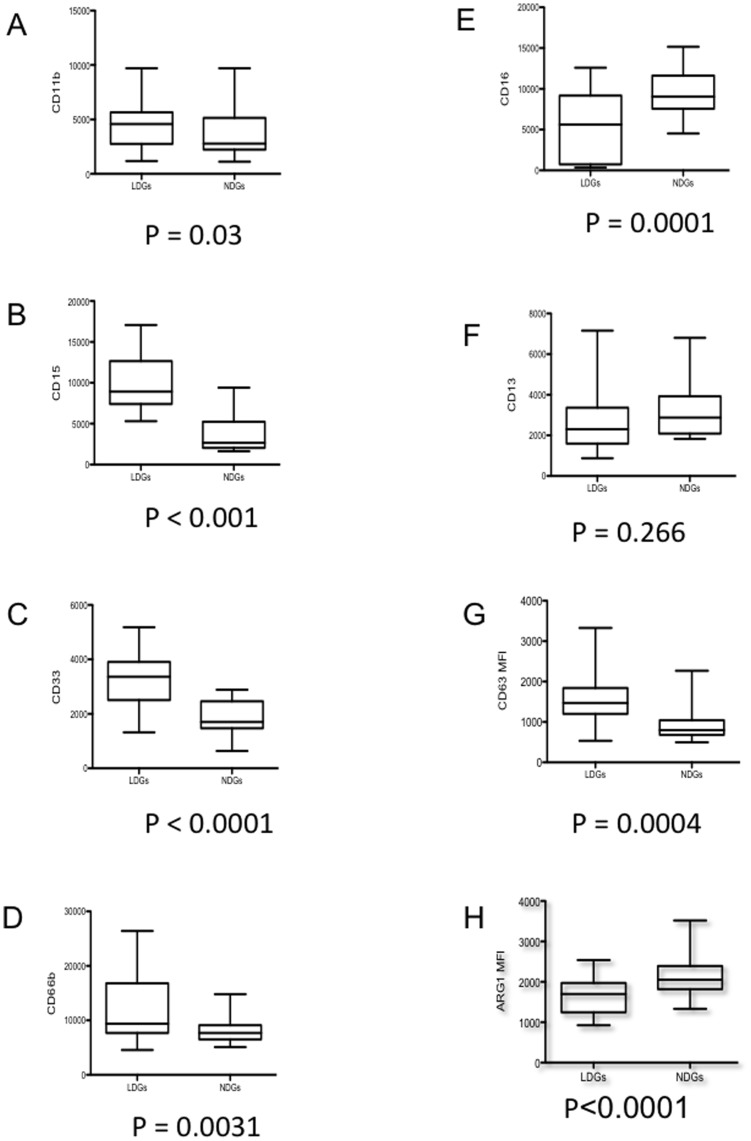
We have previously shown that arginase activity is significantly higher in PBMCs of HIV+ individuals with low CD4+ T cell counts. Furthermore, we showed that the cells expressing arginase in the PBMC fraction are granulocytes [12]. Typically granulocytes sediment with erythrocytes following density gradient centrifugation. Based on this difference in density we refer to granulocytes that co-purify in the PBMC fraction as low-density granulocytes (LDGs) and granulocytes which sediment with erythrocytes as normal-densitiy granulocytes (NDGs). To determine whether these two populations of granulocytes can be distinguished morphologically and phenotypically, we purified LDGs and NDGs and stained both populations with H&E. As shown in Figure 1, despite a difference in density, these two populations are morphologically similar: they are mature, segmented neutrophils. To answer whether they differ phenotypically, we assessed the expression levels of a panel of phenotypic markers of neutrophils. The mean fluorescence intensities (MFI) of CD11b, CD15, CD33 and CD66b were significantly greater (Figures 2 A–D) on LDGs than on NDGs. CD16 MFI was significantly reduced on LDGs (Figure 2 E). No significant difference was observed in the MFI of CD13 (Figure 2 F).
Figure 1. Morphology of LDGs and NDGs.
LDGs and NDGs were isolated as described in materials and methods and their morphology was compared after H&E staining. Data show the results of one representative experiment out of five independent experiments.
Figure 2. Phenotypic analysis of LDGs and NDGs.
LDGs and NDGs were isolated as described in materials and methods (n = 22) and the expression levels of CD11b (A), CD15 (B), CD33 (C), CD66b (D), CD16 (E), CD13 (F), CD63 (G) and arginase 1 (H) were determined by flow cytometry. Isotype controls: <1%. Statistical significance was determined by a two-tailed Mann-Whitney test. Box = interquartile range and median; whiskers = range.
To establish whether this phenotype differs from that of healthy individuals, we measured the expression levels of the same panel of cell surface markers on LDGs and NDGs from healthy individuals. As shown in table 3, there was also an increase in the MFI of CD15, CD33, CD63 and CD66b, and a decrease in the MFI of CD16 and arginase 1. There were no significant differences in the MFI of CD11b. However, there was a significant decrease in CD13 MFI on LDGs as compared to NDGs (Table 3).
Table 3. MFI of phenotypic markers of LDGs and NDGs.
| Controls | Controls | HIV+ patients | ||
| Phenotypic markers | LDGs (MFI) | NDGs (MFI) | % change | % change |
| CD11b | 3267±587 | 4334±183 | −6.9±10.4 | 29.0±9.2% |
| CD13 | 305±200 | 2196±167 | −65.4±11.0 | −7.5±10.0% |
| CD15 | 5313±270 | 2267±201 | 106.1±12.2 | 239.4±46.6% |
| CD16 | 384±81 | 15678±1197 | −97.2±0.4 | −46.4±8.4% |
| CD33 | 1385±327 | 506±95 | 176.4±19.1 | 82.8±12.1% |
| CD66b | 11954±929 | 8393±326 | 58.0±9.7 | 61.2±17.3 |
| CD63 | 2077±129 | 987±68 | 111.8±18.7 | 84.7±19.3 |
| Arginase 1 | 11899±592 | 16463±884 | −29.5±5.1 | −22.8±4.5 |
LDGs and NDGs were isolated as described in materials and methods and the expression levels of phenotypic markers were determined by flow cytometry (median±SEM). The percentage increase or decrease in MFI was calculated for controls (n = 11) and HIV+ patients (figures 2A–G, n = 22).
The blood from the controls and HIV+ patient with high or low CD4+ T cell counts was processed immediately and in the exact same way, therefore excluding that any differences observed were due to the handling procedure.
These results show that NDGs and LDGs are morphologically mature neutrophils, but that they differ in the expression level of markers of polymorphonuclear cells.
LDGs Express Higher Levels of CD63 and Lower Levels of Arginase
In neutrophils, arginase is localized in azurophilic granules [13], which express CD63; following activation, neutrophils degranulate and the release of arginase-containing azurophilic granules results in the incorporation of CD63 into the cell surface membrane of neutrophils [14], [15], [16], [17]. The arginase released by activated neutrophils has been shown to induce a profound suppression of T cell proliferation and cytokine synthesis [18]. To test whether LDGs have released their azurophilic granules, we measured the MFI of CD63 on LDGs and the MFI of arginase 1 in LDGs. As shown in Figures 2 G–H, CD63 MFI was significantly higher (p<0.0001) and arginase MFI was significantly lower (p = 0.0004) in LDGs as compared to NDGs. These results suggest that LDGs have degranulated and released their arginase.
The Frequency of LDGs Correlates with Markers of Disease Severity
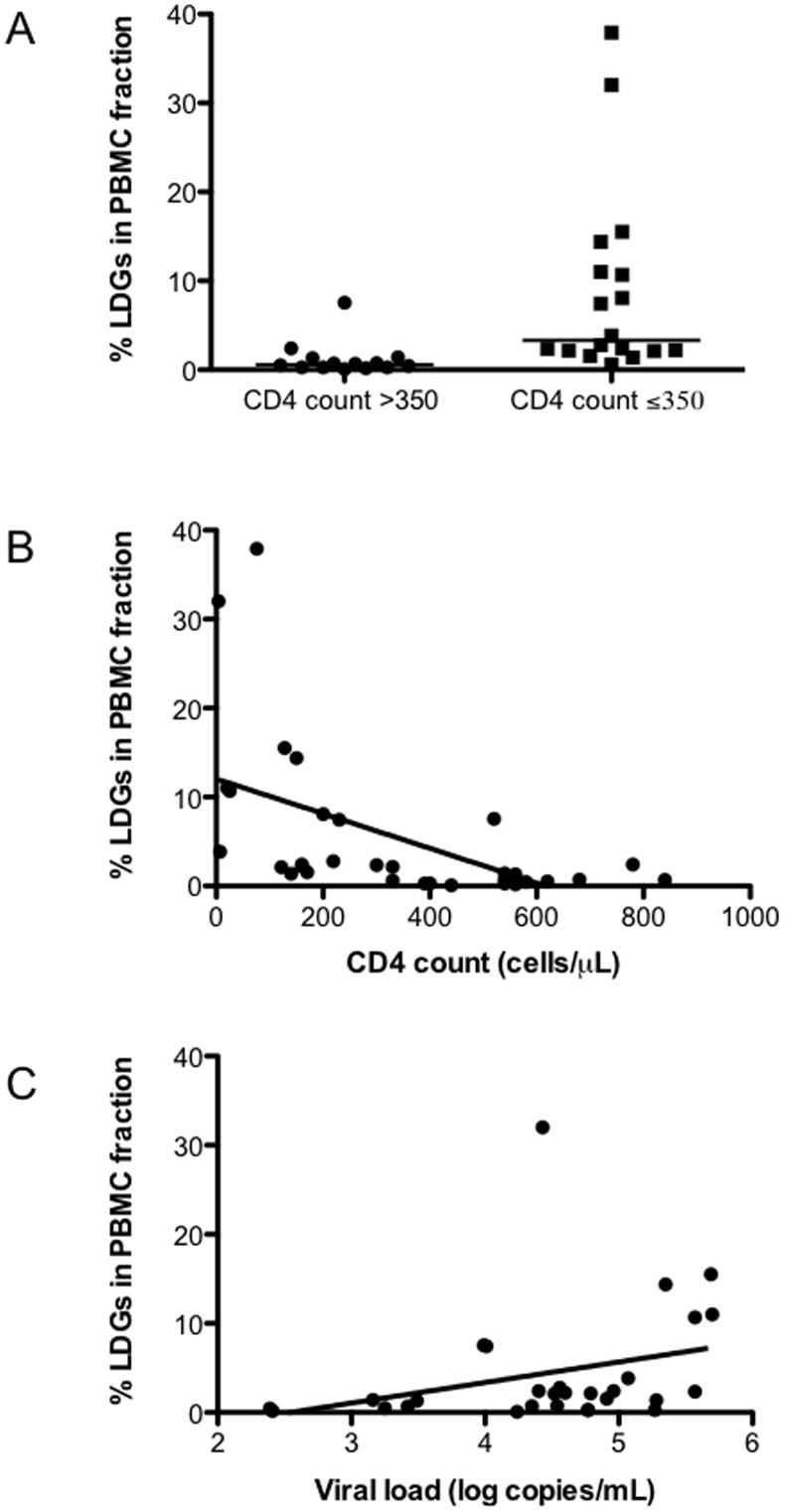
Markers of disease severity in HIV-1 infection include CD4+ T cell count and viral load. Consequently we assessed whether the frequency of LDGs in healthy controls and HIV patients correlated with markers of disease severity. First, we compared the frequency of LDGs in healthy controls and HIV patients. The frequency of LDG was significantly lower in healthy controls as compared to HIV+ patients with CD4+ T cell counts >350 cells/µl (0.24±0.3 vs 0.60±0.52, p = 0.0160) and HIV+ patients with CD4+ T cell counts <350 cells/µl (0.24±0.3 vs 3.33±2.51, p<0.0001) (data not illustrated). Next we determined whether the frequencies of LDGs varied between individuals with low or high CD4+ T cell counts. As shown in Figure 3A, the percentage of LDGs in the PBMCs of HIV+ individuals with CD4+ T cell counts <350 cells/µl was significantly higher (3.33±2.51 vs 0.60±0.52, p = 0.0001). Similar results were obtained with the absolute counts of LDGs per ml of blood (6.17±6.78×104 vs 1.94±1.41×104, p = 0.0037). To assess the potential clinical significance of this difference, we tested for a correlation between the percentages of LDGs in the PBMCs against absolute CD4+ T cell count and HIV-1 viral load. Results in Figure 3B show a strong inverse correlation between CD4+ T cell counts and percentage of LDGs (r = −0.6908, p<0.0001). There was also a significant correlation between HIV-1 viral load and percentage of LDGs (Figure 3C, r = 0.4661, p = 0.0108). These results show that in HIV+ patients, the frequency of LDGs present in PBMCs correlated with markers of disease severity.
Figure 3. Frequency of LDGs in PBMCs of HIV patients.
PBMCs from HIV+ patients with CD4+ T cell counts >350 (n = 14) or <350 cells/µL (n = 18) were isolated by Ficoll gradient and the frequency of CD15+ arginase+ cells was determined by flow cytometry, statistical significance was determined by a two-tailed Mann-Whitney test (A). Correlation between %LDGs and CD4+ T cell counts (B) or viral load (C), statistical significance was determined by a Spearman’s rank test. Isotype controls: <1%.
Discussion
Arginase-induced L-arginine catabolism is a well-established mechanism of immune suppression [5], [6], [7], [8]. We have recently shown that arginase activity is abnormally high in PBMCs of untreated HIV+ individuals with low CD4+ T cell counts and that this coincides with lower levels of L-arginine [12]. Here we consolidate and extend our previous results and show that the frequency of arginase-expressing cells is closely associated with the two most widely accepted markers of disease severity: CD4+ T cell counts and plasma HIV-1 viral loads.
Following density gradient centrifugation, LDGs co-purify in the PBMC fraction, whereas NDGs sediment with erythrocytes. This difference could result from i) different degrees of maturation and ii) different activation and degranulation states:
During neutrophil maturation there is a change in expression of surface antigens [19]. In untreated HIV+ individuals, LDGs display high expression levels of CD33, a marker of immature neutrophils, and lower levels of CD16, a marker found on mature neutrophils, suggesting that these cells are immature [19]. A study by Brandau et al. identified immature granulocytes from individuals with cancer that co-purify in the PBMC fraction [20]. However, our results show that LDGs are morphologically similar to NDGs, as they are segmented neutrophils. We cannot exclude the possibility that the population of LDGs isolated from HIV+ individuals represents a heterogeneous population of neutrophils that does not exclusively comprise mature activated cells; indeed, Rodriguez et al showed that in renal cell carcinoma, <10% of the myeloid derived suppressor cells (MDSCs) were immature granulocytes and that ∼90% were mature segmented granulocytes [21].
The degree of activation of neutrophils and degranulation depends on the strength of the activating signal: the order of granule release follows a strict hierarchy requiring increasing activation: 1) secretory granules; 2) gelatinous (tertiary) granules; 3) specific (secondary) granules and 4) azurophilic (primary) granules. From the panel of markers used in the present study, the following markers were significantly increased on LDGs: CD11b, present in the membrane of secretory vesicles, gelatinase granules and specific granules; CD63, found in the membrane of azurophilic granules; and CD66b, detected in the membrane of specific granules. Further, the intensity of arginase, which is present in azurophilic granules [13], was lower in LDGs as compared to NDGs. Our results suggest that LDGs are activated neutrophils that have degranulated because they have increased cell surface expression of CD66b, CD63 and CD11b and decreased intracellular arginase 1 expression.
The presence of CD15+ granulocytes in the PBMC fraction has been demonstrated in a number of different conditions including cancer, pregnancy, trauma and SLE, each of which is frequently accompanied by a degree of immune suppression. We have shown that arginase-induced L-arginine depletion and the subsequent T cell inhibition is a mechanism of immune suppression that is restricted to the site of pathology [22]. Therefore, it is possible that the increased frequencies of LDGs we observed in the peripheral blood of HIV+ individuals are only a weak reflection of the events occurring at the principal sites of HIV infection, i.e. in the solid lymphoid tissue. Indeed, a recent study showed in a model of prostate-specific inflammation, that MDSCs isolated from the site of inflammation were more immunosuppressive than those isolated from the periphery [23].
The impact of arginase-induced L-arginine depletion on T cell effector functions has been well established [5], [6], [7], [8]. In HIV+ individuals with low CD4+ T cell counts, we and others have already reported a pronounced downregulation of CD3ζ, which is one of the hallmarks of T cell suppression induced by L-arginine depletion [12]. We propose that the following mechanism contributes to poor T cell function in these individuals: LDGs release arginase, which reduces the concentration of free L-arginine in the microenvironment [12], thereby preventing efficient T cell responses. Indeed, we have previously shown that PMN release their arginase and that this inhibits T cell proliferation in an arginase-dependent manner [18].
More work is needed to understand the causes of the observed neutrophil activation and the subsequent release of arginase, as it is not yet not possible to answer whether it is the virus or the immune system or a combination of both that accounts for the increased frequency of activated low-density granulocytes in HIV+ patients. A better understanding of the complex mechanisms leading to progressively impaired immune functions might prove helpful in improving the existing treatment not only for HIV+ individuals, but also for other chronic infectious diseases such as tuberculosis and leishmaniasis, in which a degree of immune suppression may be caused by abnormal arginase activity.
Acknowledgments
We thank Drs E. Riley, M. Simon, C. Bangham and S. Herath for helpful discussions and critical reading of the manuscript.
Funding Statement
This work was supported by a grant from The Wellcome Trust (07664/Z/05/Z, PK). TC was a recipient of an Imperial College London MB/PhD fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shearer GM, Clerici M (1991) Early T-helper cell defects in HIV infection. AIDS 5: 245–253. [DOI] [PubMed] [Google Scholar]
- 2. Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, et al. (1989) Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest 84: 1892–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy JA (2007) HIV and the pathogenesis of AIDS; press A, editor.
- 4. Appay V, Sauce D (2008) Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol 214: 231–241. [DOI] [PubMed] [Google Scholar]
- 5. Bronte V, Zanovello P (2005) Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 5: 641–654. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez PC, Ochoa AC (2008) Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev 222: 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Munder M (2009) Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol 158: 638–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Youn JI, Gabrilovich DI (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40: 2969–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kropf P, Fuentes JM, Fahnrich E, Arpa L, Herath S, et al. (2005) Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. Faseb J 19: 1000–1002. [DOI] [PubMed] [Google Scholar]
- 10. Ochoa AC, Zea AH, Hernandez C, Rodriguez PC (2007) Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 13: 721s–726s. [DOI] [PubMed] [Google Scholar]
- 11. Muller I, Munder M, Kropf P, Hansch GM (2009) Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol 30: 522–530. [DOI] [PubMed] [Google Scholar]
- 12. Cloke T, Garvery L, Choi BS, Abebe T, Hailu A, et al. (2010) Increased arginase activity correlates with disease severity in HIV seropositive patients. Journal of Infectious Diseases 202: 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, et al. (2005) Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood 105: 2549–2556. [DOI] [PubMed] [Google Scholar]
- 14. Hakkert BC, Kuijpers TW, Leeuwenberg JF, van Mourik JA, Roos D (1991) Neutrophil and monocyte adherence to and migration across monolayers of cytokine-activated endothelial cells: the contribution of CD18, ELAM-1, and VLA-4. Blood 78: 2721–2726. [PubMed] [Google Scholar]
- 15. Kuijpers TW, Hakkert BC, Hoogerwerf M, Leeuwenberg JF, Roos D (1991) Role of endothelial leukocyte adhesion molecule-1 and platelet-activating factor in neutrophil adherence to IL-1-prestimulated endothelial cells. Endothelial leukocyte adhesion molecule-1-mediated CD18 activation. J Immunol 147: 1369–1376. [PubMed] [Google Scholar]
- 16. Kuijpers TW, Tool AT, van der Schoot CE, Ginsel LA, Onderwater JJ, et al. (1991) Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood 78: 1105–1111. [PubMed] [Google Scholar]
- 17. Martin-Martin B, Nabokina SM, Blasi J, Lazo PA, Mollinedo F (2000) Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood 96: 2574–2583. [PubMed] [Google Scholar]
- 18. Munder M, Schneider H, Luckner C, Giese T, Langhans CD, et al. (2006) Suppression of T cell functions by human granulocyte arginase. Blood 108: 1627–1634. [DOI] [PubMed] [Google Scholar]
- 19. Elghetany MT (2002) Surface antigen changes during normal neutrophilic development: a critical review. Blood Cells Mol Dis 28: 260–274. [DOI] [PubMed] [Google Scholar]
- 20. Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, et al. (2011) Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol 89: 311–317. [DOI] [PubMed] [Google Scholar]
- 21. Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, et al. (2009) Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 69: 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Modolell M, Choi B-S, Ryan RO, Hancock M, Titus RG, et al. (2009) Local suppression of T cell responses by arginase-induced L-arginine depletion in nonhealing leishmaniasis. PLoS Neglected Tropical Diseases 14: e480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haverkamp JM, Crist SA, Elzey BD, Cimen C, Ratliff TL (2011) In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur J Immunol 41: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]