Abstract
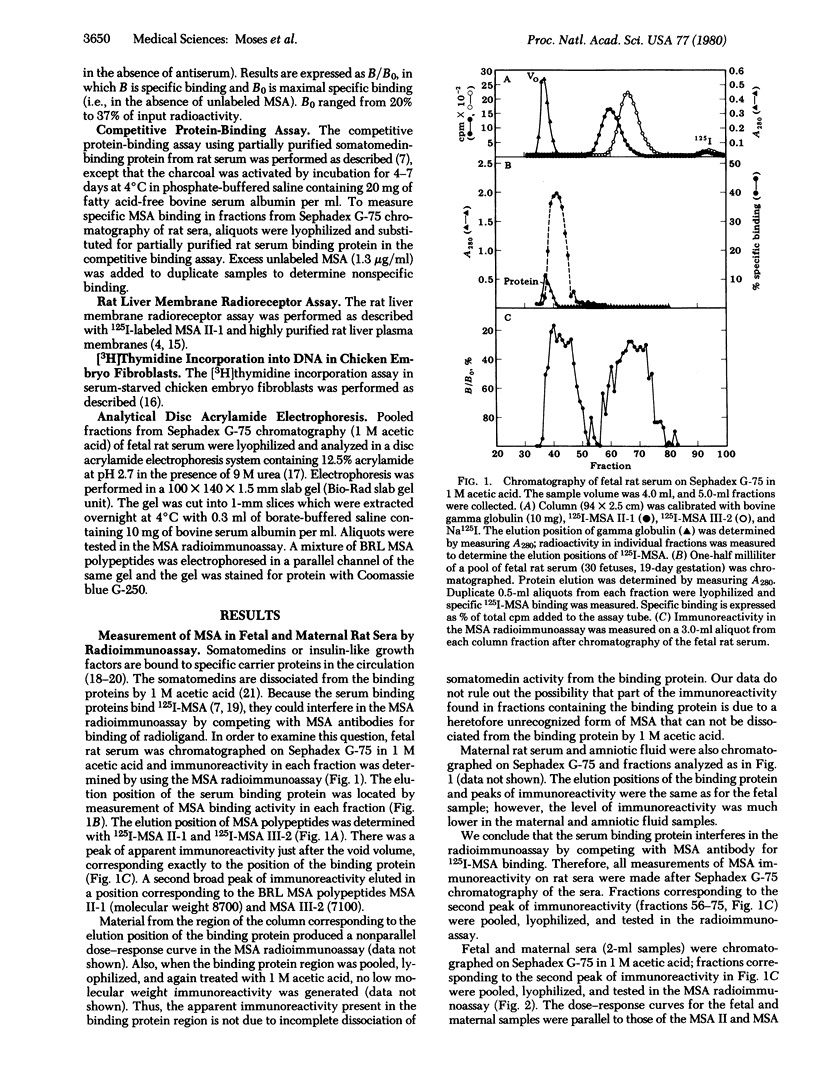
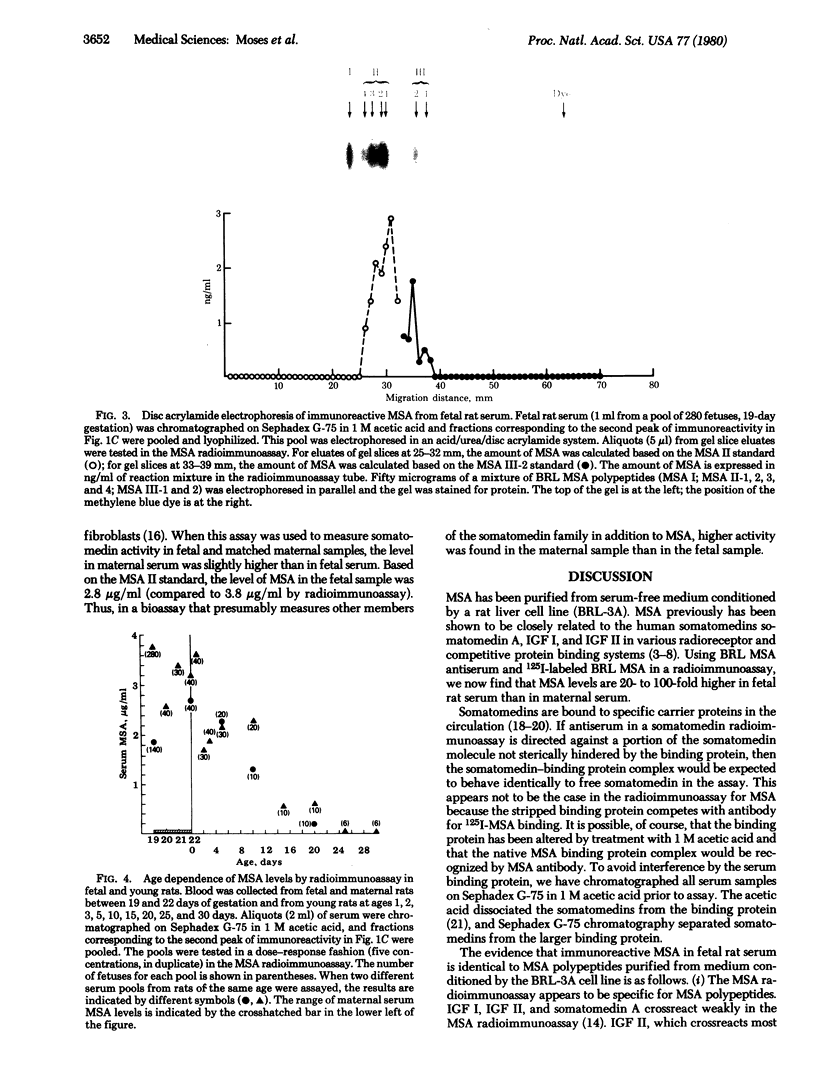
Multiplication-stimulating activity (MSA), purified from medium conditioned by the BRL-3A rat liver cell line, previously has been shown to be closely related to the human somatomedins or insulin-like growth factors. A radioimmunoassay was utilized to measure MSA levels in sera from fetal, maternal, and young rats. A serum somatomedin-binding protein was found to interfere in the radioimmunoassay by competing with antibody for binding 125I-labeled MSA. Therefore, prior to radioimmunoassay, sera were filtered on Sephadex G-75 in 1 M acetic acid to dissociate and separate somatomedin activity from the binding protein. Concentrations of MSA by radioimmunoassay were 20- to 100-fold higher in feta rat sera (1.8-4.4 micrograms/ml) than in maternal sera. MSA levels gradually decreased after birth, reaching maternal levels by day 25 of extrauterine life. MSA concentrations in feta rat sera also were found to be correspondingly high by a rat liver membrane radioreceptor assay and a competitive binding protein assay using rat serum somatomedin-binding protein. The findings of higher levels of MSA in fetal than in maternal rat sera and the gradual decline in MSA serum concentrations after birth are in direct contrast to total somatomedin activities measured by biosassay. Thus, MSA may function as a growth factor in the fetal rat whereas other somatomedins may play a role in stimulating growth during extrauterine life.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chochinov R. H., Mariz I. K., Daughaday W. H. Isolation of a somatomedin from plasma of rats bearing growth hormone secreting tumors. Endocrinology. 1977 Feb;100(2):549–556. doi: 10.1210/endo-100-2-549. [DOI] [PubMed] [Google Scholar]
- Cohen K. L., Short P. A., Nissley S. P. Growth hormone-dependent serum stimulation of DNA synthesis in chick embryo fibroblasts in culture. Endocrinology. 1975 Jan;96(1):193–198. doi: 10.1210/endo-96-1-193. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Raben M. S., Salmon W. D., Jr, van den Brande J. L., van Wyk J. J. Somatomedin: proposed designation for sulphation factor. Nature. 1972 Jan 14;235(5333):107–107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- Draznin B., Morris H. G., Burstein P. J., Schalch D. S. Serum growth hormone, somatomedin and its carrier protein in the rat: influence of age, sex, and pregnancy. Proc Soc Exp Biol Med. 1979 Oct;162(1):131–138. doi: 10.3181/00379727-162-40632. [DOI] [PubMed] [Google Scholar]
- Dulak N. C., Temin H. M. A partially purified polypeptide fraction from rat liver cell conditioned medium with multiplication-stimulating activity for embryo fibroblasts. J Cell Physiol. 1973 Apr;81(2):153–160. doi: 10.1002/jcp.1040810204. [DOI] [PubMed] [Google Scholar]
- Dulak N. C., Temin H. M. Multiplication-stimulating activity for chicken embryo fibroblasts from rat liver cell conditioned medium: a family of small polypeptides. J Cell Physiol. 1973 Apr;81(2):161–170. doi: 10.1002/jcp.1040810205. [DOI] [PubMed] [Google Scholar]
- Hintz R. L., Liu F. Demonstration of specific plasma protein binding sites for somatomedin. J Clin Endocrinol Metab. 1977 Nov;45(5):988–995. doi: 10.1210/jcem-45-5-988. [DOI] [PubMed] [Google Scholar]
- Megyesi K., Kahn C. R., Roth J., Neville D. M., Jr, Nissley S. P., Humbel R. E., Froesch E. R. The NSILA-s receptor in liver plasma membranes. Characterization and comparison with the insulin receptor. J Biol Chem. 1975 Dec 10;250(23):8990–8996. [PubMed] [Google Scholar]
- Moses A. C., Nessley S. P., Cohen K. L., Rechler M. M. Specific binding of a somatomedin-like polypeptide in rat serum depends on growth hormone. Nature. 1976 Sep 9;263(5573):137–140. doi: 10.1038/263137a0. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Passamani J., White R. M. Further characterization of growth hormone-dependent somatomedin-binding proteins in rat serum and demonstration of somatomedin-binding proteins produced by rat liver cells in culture. Endocrinology. 1979 Feb;104(2):536–546. doi: 10.1210/endo-104-2-536. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Short P. A., Rechler M. M. Immunological cross-reactivity of multiplication-stimulating activity polypeptides. Eur J Biochem. 1980 Jan;103(2):401–408. doi: 10.1111/j.1432-1033.1980.tb04326.x. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Short P. A., Rechler M. M., Podskalny J. M. Purification and characterization of multiplication-stimulating activity. Insulin-like growth factors purified from rat-liver-cell-conditioned medium. Eur J Biochem. 1980 Jan;103(2):387–400. doi: 10.1111/j.1432-1033.1980.tb04325.x. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Fractionation of cell membrane protein by disc electrophoresis. Biochim Biophys Acta. 1967 Jan 18;133(1):168–170. doi: 10.1016/0005-2795(67)90051-7. [DOI] [PubMed] [Google Scholar]
- Nissley S. P., Rechler M. M. Multiplication-stimulating activity (MSA): a somatomedin-like polypeptide from cultured rat liver cells. Natl Cancer Inst Monogr. 1978 May;(48):167–177. [PubMed] [Google Scholar]
- Rechler M. M., Eisen H. J., Higa O. Z., Nissley P., Moses A. C., Schilling E. E., Fennoy I., Bruni C. B., Phillips L. S., Baird K. L. Characterization of a somatomedin (insulin-like growth factor) synthesized by fetal rat liver organ cultures. J Biol Chem. 1979 Aug 25;254(16):7942–7950. [PubMed] [Google Scholar]
- Rechler M. M., Fryklund L., Nissley S., Hall K., Podskalny J. M., Skottner A., Moses A. C. Purified human somatomedin A and rat multiplication stimulating activity. Mitogens for cultured fibroblasts that cross-react with the same growth peptide receptors. Eur J Biochem. 1978 Jan 2;82(1):5–12. doi: 10.1111/j.1432-1033.1978.tb11991.x. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Podskalny J. M., Nissley S. P. Characterization of the binding of multiplication-stimulating activity to a receptor for growth polypeptides in chick embryo fibroblasts. J Biol Chem. 1977 Jun 10;252(11):3898–3910. [PubMed] [Google Scholar]
- Schlumpf U., Heimann R., Zapf J., Froesch E. R. Non-suppressible insulin-like activity and sulphaton activity in serum extracts of normal subjects, acromegalics and pituitary dwarfs. Acta Endocrinol (Copenh) 1976 Jan;81(1):28–42. doi: 10.1530/acta.0.0810028. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Temin H. M. Purified multiplication-stimulating activity from rat liver cell conditioned medium: comparison of biological activities with calf serum, insulin, and somatomedin. J Cell Physiol. 1974 Oct;84(2):181–192. doi: 10.1002/jcp.1040840204. [DOI] [PubMed] [Google Scholar]
- Stuart M. C., Lazarus L., Moore S. S., Smythe G. A. Somatomedin production in the neonatal rat. Horm Metab Res. 1976 Nov;8(6):442–445. doi: 10.1055/s-0028-1093610. [DOI] [PubMed] [Google Scholar]
- Zapf J., Waldvogel M., Froesch E. R. Binding of nonsuppressible insulinlike activity to human serum. Evidence for a carrier protein. Arch Biochem Biophys. 1975 Jun;168(2):638–645. doi: 10.1016/0003-9861(75)90296-9. [DOI] [PubMed] [Google Scholar]




