Abstract
Permeability-surface area products of the capillary wall, PSc, and the myocyte sarcolemma, PSpc, for D-glucose and 2-deoxy-D-glucose were estimated via the multiple indicator-dilution technique in isolated blood-perfused dog and Tyrode-perfused rabbit hearts. Aortic bolus injections contained 131I-albumin (intravascular reference), two of three glucoses: L-glucose (an extracellular reference solute), D-glucose, and 2-deoxy-D-glucose. Outflow dilution curves were sampled for 1–2.5 min without recirculation. The long duration sampling allowed accurate evaluation of PSpc by fitting the dilution curves with a multiregional axially distributed capillary-interstitial fluid-cell model accounting for the heterogeneity of regional flows (measured using microspheres and total heart sectioning). With average blood flow of 1.3 ml·g–1·min–1, in the dog hearts the PSc for D-glucose was 0.72 ± 0.17 ml·g–1·min–1 (mean ± SD; n = 11), and PSpc was 0.57 ± 0.15 ml·g–1·min–1. In the rabbit hearts with perfusate flow of 2.0 ml·g–1·min–1 (n = 6), PSc was 1.2 ± 0.1 and PSpc was 0.4 ± 0.1 ml·g–1·min–1. PSc for 2-deoxy-D-glucose was about 4% higher than for D-glucose and L-glucose in both preparations. Relative to L-glucose, there was no measurable transendothelial transport of either dextroglucose, indicating that transcapillary transport was by passive diffusion, presumably via the clefts between cells. The technique allows repeated measurements of D-glucose uptake at intervals of a few minutes; it may therefore be used to assess changes in transport rates occurring over intervals of several minutes.
Keywords: isolated heart preparation, coronary transport function, myocardial transsarcolemmal flux, L-glucose, dogs, rabbits
THERE HAS BEEN A substantial accumulation of data from isolated heart preparations and in vitro muscle preparations on the utilization of D-glucose by myocardium, as, for example, in the symposium edited by Opie (20). The data provided on glucose utilization have been estimated in these many studies from the arteriovenous differences or from the diminution in glucose concentration in the perfusate or in the bathing medium, and in earlier studies by estimating the glycogen formation. Lacking in these studies was knowledge of the unidirectional transsarcolemmal influx of glucose, a value that is independent of the net transsarcolemmal flux and that is needed to characterize the transport mechanism in normal or in compromised states. Crone (8) showed that the indicator-dilution technique was applicable, indeed invaluable, for the estimation of glucose permeability of the blood-brain barrier and in his studies showed that permeation was via a saturable facilitated transport mechanism. In the heart there are two membranes to consider, the capillary and the sarcolemmal membranes, making the analysis complex. The multiple-tracer indicator-dilution technique provides a suitable vehicle for measuring unidirectional transsarcolemmal uptake rates in laboratory and potentially for clinical situations. The analysis requires incorporating mathematical solutions for capillary-tissue units accounting for capillary and cell barriers to exchange, such as that of Goresky and his colleagues (29), as components of a multicapillary model accounting for flow heterogeneity.
The practical incentives to gain fundamental insight into the rates of transcapillary and transsarcolemmal transport of D-glucose have been much increased by the use of [18F]fluorodeoxyglucose; its relative regional deposition density in the heart can be estimated by positron emission tomographic reconstruction (24). With the appropriate analysis it should be possible to estimate regional transsarcolemmal uptake rates in the hearts of intact animals or humans, which would be a practical extension of the autoradiographic approach of Sokoloff et al. (33).
Our current studies were designed to provide estimates of cellular D-glucose uptake by comparison with a set of reference tracers of partially analogous characteristics. The three reference tracers were 1) 131I-albumin, which is a reference tracer for transport in the blood plasma; 2) L-glucose, which has the same molecular weight as D-glucose (mol wt = 180) and which this study shows to have the same capillary permeability-surface area product (PSc) as D-glucose; it serves as an extracellular reference tracer; its interstitial fluid (ISF) volume of distribution is the same as for sucrose (unpublished data) and presumably for D-glucose, and it does not participate in the facilitated transport available for most D-glucoses (15, 22); and 3) 2-deoxy-D-glucose (mol wt = 164), which is transported across cell membranes via the same carrier as D-glucose but which is not metabolized to CO2 and H20. On entering the cells, 2-deoxy-D-glucase, like D-glucose, is phosphorylated via hexokinase to form 2-deoxy-D-glucose-6-phosphate but, unlike glucose, cannot be transformed to glucose 1-phosphate form by phosphoglucomutase, nor to fructose 6-phosphate via the isomerase, and so cannot be metabolized through the glycolytic series. The 2-deoxy-D-glucose phosphate is neither soluble in the membrane, nor transported outward via a carrier, nor dephosphorylated very rapidly, since there is little or no glucose phosphatase in the heart, and it is therefore retained by the cells for a long time; these features have led to its use as an indicator of cellular glucose uptake and, by inference, metabolism. These factors, plus the fact that 2-deoxy-D-glucose will not necessarily be transported into the cell at the same rate as D-glucose, mean that a comparison of the kinetics of these two glucoses is useful.
Studies were undertaken mainly in blood-perfused dog hearts and in three Tyrode-perfused rabbit hearts. In these preparations we have acquired data on the transcapillary transport of glucose and of its analogues. The general principles of the experimentation and analyses are given by Bassingthwaighte and Goresky (3). The analysis is strengthened over previous efforts by virtue of the use of a new optimization technique by which the three dilution curves in a set are fitted simultaneously with the model solutions. This reduces the degrees of freedom in the parameter estimation and so provides more accurate estimates but could not be automated on our present computer system with limited memory, so it is left as an example of an improvement to be put into standard practice at a later time.
Experimental Methods
Indicator-Dilution Technique
Both dog and rabbit hearts were studied. Eighteen sets of dilution curves were obtained on isolated, Langendorff, nonworking, spontaneously beating hearts. The dog hearts were perfused with blood at 37.5°C taken from the femoral artery of an anesthetized, artificially ventilated support dog weighing about 30 kg, as described in detail previously (11). The support dogs were fasted overnight; blood glucose levels were not measured. The rabbit hearts were perfused with oxygenated Tyrode solution through an aortic cannula. The perfusate composition was (mM) Na 147.4, K 5.4, Ca 1.8, Mg 0.5, Cl–133.1, , , EDTA 0.01, and glucose 5 and was without albumin. The temperature of circulating solutions was maintained at 37.5-38°C, and 23 sets of dilution curves were obtained.
The perfusion rate was controlled with a roller pump on the inflow line. Perfusion pressure, perfusate temperature, coronary sinus pressure, heart rate, and heart weight were monitored and recorded continuously. Drainage from the coronary sinus, right atrium, and right ventricle was collected via a tapered cannula through the right ventricular free wall near the apex. The left ventricular Thebesian vein inflow plus aortic valve leakage were drained via a small cannula through the apex. Flows through the right and left ventricular cannulas were measured, and venous samples were collected for hematocrit and blood glucose determinations before injection of tracers and periodically thereafter. The arterial and perfusion pressures, sampling time, and heart rate were continuously recorded. At the end of each experiment, the wet weight of the myocardium devoid of fatty tissue was measured.
Injections
The timing of the injections into the arterial inflow, lasting 1.0–2.0 s for dog hearts and 0.5–1.0 s for rabbit hearts, was recorded, and the midpoint was used as zero time (t = 0). The injectate contained an intravascular reference substance, albumin, and two of the three test substances. The radioactive tracers were diluted with physiological saline or Tyrode solution; the volume of the injectate was 2.9 ml for dogs and 0.2–0.5 ml for rabbits.
Tracers
The following radiotracers were used: 1) 131I-RIHSA (radioiodinated human serum albumin as a reference intravascular tracer), 2) 2-deoxy-D-[1-3H]glucose, 3) D-[6-3H]glucose, 4) D-[1-14C]glucose, and 5) L-[1-14C]-glucose as an extracellular reference solute (all supplied by Amersham Radiochemical Center, London, UK). Three injection mixtures were prepared: 1) 2-deoxy-D-[1-3H]glucose + L-[1-14C]glucose + 131I-RIHSA, 2) D-[6-3H]glucose + L-[1-14C]glucose + 131I-RIHSA, and 3) 2-deoxy-D-[1-3H]glucose + D-[1-14C]glucose + 131I-RIHSA. The activities used were 40 μCi of 3H-labeled tracer, 20 μCi of 14C-labeled tracer, and 10 μCi of 131I-RIHSA for dog experiments, and 4, 2, and 1 μCi, respectively, for rabbit experiments. Radiochemical purity of these five tracers were tested by using paper chromatography; the results showed 98% purity for 131I-RIHSA and for L-glucose and 99% purity for the D-glucose and deoxyglucase.
Sampling
The blood and Tyrode samples were collected from the coronary sinus outflow (right ventricle) at intervals of 0.4–1.0 s for the first 30 samples and 2–4 s for the last 30 samples, depending on the flow rate used. The total collection period was 72–150 s. A volume of 0.1 ml of each outflow sample, each standard, and each background sample was pipetted into scintillation vials. For the blood samples, the proteins were precipitated with 0.1 ml of 70% perchloric acid and 0.2 ml of 30% hydrogen peroxide, and the vials were heated at 100° Celsius for 1 h. Ten milliliters of scintillation cocktail (Insta-Gel, Packard Instrument, Downers Grove, IL) were added for both the dog and rabbit samples.
Radioisotope activities
Counting was done in a liquid scintillation counter (Nuclear Chicago, Mark II, Searle) using three channels. Four sets of quench correction curves (background, 3H-, 14C-, and 131I-labels) of 35 data points each were made for both dog and rabbit experiments. The “best” least-squares polynomials were fitted to the quench-data points, and the polynomials were used to estimate the true counting rates of the 3H-, 14C-, and 131I-labels of each sample.
To test the accuracy of the sampling and counting methods, two D-glucoses (3H- and 14C-labeled) were injected simultaneously with 131I-RIHSA in one dog experiment. The dilution curves obtained for these D-glucoses should theoretically be identical. The analysis of samples showed an excellent agreement; the mean absolute difference between the 3H- and 14C-samples was 0.74%, and the correlation coefficient between the 54 sample points was 0.999992.
Calculation of dilution curves
The dilution curves contained relatively little statistical counting noise, since the peak counting rate was usually higher than 100,000 counts/10 min for each tracer. The reference curve was terminated at 0.1–0.01% of the peak and the glucose curves at 1.0–0.1% of the peak (no recirculation), so that counting errors ordinarily had standard deviations less than 3% and spillover corrections (using a Gaussian elimination technique to invert the matrix) added very little more, as demonstrated by the test with the two D-glucoses. Each dilution curve was normalized so that it is the “transport function” of the system from injection site to sampling site, h(t), which is the fraction of dose appearing in the venous outflow per second
| (1) |
where FB is the flow of blood or perfusate through the coronary bed (ml/s), q0 is the quantity of tracer injected (μCi), and C(t) is outflow concentration of the tracer in the effluent at time t (μCi/ml). Our notation is hR(t) for a reference intravascular tracer and hD(t) for any permeating or diffusible tracer. From these, one may calculate an apparent instantaneous extraction E(t) at each point: E(t) = 1 – hD(t)/hR(t). Emax is the maximum of E(t) after smoothing, as defined by Guller et al. (11) and occurs at about the time of the peak of hR(t).
Estimation of Flow Heterogeneity from Microsphere Deposition
Microsphere injections
In some, but not all, experiments, microspheres were injected into the aortic cannula to obtain an estimate of the heterogeneity of regional flows, as described by Yipintsoi et al. (36), using 9-μm diameter spheres labeled with 85Sr or 46Sc (3M, St. Paul, MN). Previous experiments on dogs (36) and baboons (14) show that these distributions change very little over many minutes in experiments with varying blood flows, so there is little need for injecting the microspheres and the diffusible tracers simultaneously. Two microsphere injections were made: 85Sr after the first or second injection of diffusible tracers and 46Sc after the third or fourth tracer injection. The volumes injected were 1 ml for dogs and 0.2 ml for rabbits, and the activities used were 1–3 μCi and 0.05–0.1 μCi, respectively. The total number of microspheres was 3–20 × 105 for dogs and 0.1–4 × 105 for rabbits, or 5,000–25,000 microspheres/g of heart.
Microsphere deposition densities
At the end of each dog experiment the heart was cooled on dry ice and sliced into 10 major sections, the atria, 4 right ventricular rings, and 5 left ventricular rings, and then into 297 finer sections, ordered as described by King et al. (14). The rabbit hearts were sliced similarly but into fewer pieces. The weight of the heart pieces ranged between 0.03 and 0.19 g. The radioactivity of each piece was determined by multichannel gamma counting, as described in detail by King and Bassingthwaighte (13). For each heart, the probability density function of regional microsphere activity was constructed; it was interpreted as equivalent to the probability density function of regional myocardial flows.1
The probability density function of microspheres (regional flows) per gram of tissue is given by a weighting function w(f), where the probability w is a function of the relative flow, f, and where f is defined as the local flow per unit mass of tissue divided by the measured mean flow per unit mass. In histogram notation, the relative flow in the ith class is fi; and the fraction of the organ having flows within the class centered at fi is wiΔfi. The sum of the wiΔfis is unity, to account for the whole heart. The tracer enters each of the i regions in proportion to flow, i.e., the ith region of relative mass wiΔfi receives the fraction fiwiΔfi of the tracer. The sum of the fiwiΔfi is unity, accounting for all of the flow.
Methods of Analysis
For the analysis, the heart was described by an aggregate of capillary-tissue units in parallel. Each unit was a three-region (capillary, ISF, cell), two-barrier, axially distributed convection-diffusion model. A basic assumption is that diffusion in the radial direction (perpendicular to the capillary axis) is rapid compared with the transmembrane transport processes, which is justifiable because the intraregional radial distances are so short; intercapillary distances are less than 20 μm. The model is an extension of the one-barrier model of Bassingthwaighte (2) and is conceptually similar to the two-barrier model of Rose, Goresky, and Bach (29). Its virtues are the speed and stability of computation, providing solutions to fit to data extending over long durations such as many minutes or hours. Parameters of the model for fitting the dilution curves are as follows:
Fs, mean flow of solute-containing mother fluid (ml·g–1·min–1) = FB·(1 – Hct) (From data: FB is blood flow and Hct is hematocrit)
Heterogeneity of flows, given by the probability density function of microsphere deposition densities, wi and fi, dimensionless. The standard deviation of this density function divided by the mean is the relative dispersion of regional flows, RD. The local perfusate flow in the ith region is fiFs = Fsi. (For the multicapillary analysis the density function was represented by 5 or 7 regions, rather than the 25 classes into which the microsphere deposition densities had been partitioned)
PSc, capillary permeability-surface area product, ml·g–1·min–1
γISF, dimensionless ratio of interstitial volume of distribution, (ml/g), to the intracapillary volume of distribution, of the particular tracer
PSpc, permeability-surface area product of parenchyma1 cell (of sarcolemma of myocytes), ml·g–1·min–1
γpc, dimensionless ratio of the volume of tracer distribution within parenchymal cell, , to the intracapillary volume of distribution,
Gpc, clearance rate for intracellular sequestration or consumption, ml·g–1·min–1 [ is k5 in the notation of Rose, Goresky, and Bach (29)]
The differential equations for the system in each capillary-interstitial fluid-cell unit are
| (2) |
The subscripts are C for capillary, ISF for interstitium, and pc for parenchymal cell (myocyte). In addition to the parameters listed above, the other symbols are Cc, CISF, and Cpc for concentrations (counts/min per ml volume of distribution in each region) at position x at time t; x is distance along the capillary from the entrance at x = 0 to the exit at x = L. DC, DISF, and Dpc are axial diffusion coefficients; intracapillary diffusion has a small influence on the form of the dilution curves during the first 1–2 s following the appearance time; for glucose, with its low transport rate, diffusion has no discernible effect on the estimates of the other parameters, and the Ds were set to zero for this analysis. A fast numerical method (5), using the Lagrangian sliding fluid element approach of Bassingthwaighte (2) was used to obtain solutions to the equations, solved for each pathway with individual values for Fsi. Its accuracy, with axial diffusion, was evaluated by Lenhoff and Lightfoot (16) for a two-region solution and found to be within 1% of that of an analytic solution for the same model. The unit impulse response at the outflow from the ith region is hCi(t); note, however, that the model was used as a differential operator, thus avoiding convolution integration.
With PSC zero, the indicator remains intravascular and has a mean transit time . With PSC > 0, and PSpc = 0, the indicator remains extracellular, with . With PSC and PSpc, both >O and Gpc = 0, . The effect of Gpc > 0 is to shorten t̄ compared with the nonconsumed tracer with the same volume of distribution, because there is a greater fraction consumed in slow flow regions and at late times when the tracer is largely inside the parenchyma1 cell where the consumption occurs.
An aggregate of these capillary-tissue units plus large vessel units made up the whole heart model. The aggregate of parallel capillary-tissue units gives the overall transport function of the capillary exchange regions hC(t), using the flow weighting obtained from the microsphere distributions
| (3) |
For an individual tracer all units have the same parameters except for Fsi. The various diffusible tracers were allowed to have different values for PS and V in the initial analyses.
The capillary-tissue units were considered to be in series with large vessels which also disperse and delay the tracers as described by the large vessel transport functions hLV(t). The convolution of the set of capillary transport functions with a single large vessel transport function is appropriate when the large vessel dispersion is similar for all capillary-tissue regions. This gives the overall model transport function ĥ(t) as a convolution (denoted by the asterisk) of hLV and hC
| (4) |
Rose, Goresky, and Bach (29) used a heterogeneity model of a different form, having a set of dispersionless large vessels also in parallel and linking them via a 1:1 linear relationship to the capillaries. Their model has not been compared with ours but could give different parameter estimates.
Optimization of the fits of the model to the data
Two new approaches have been used in the analysis. First, the method of optimization of the parameter values to achieve a good fit of the model to the data is a new one based on the use of the sensitivity functions (17). Second, the degrees of freedom in the parameter evaluation are reduced strongly by fitting several components of a set of data (flows, dilution curves, and microsphere deposition densities) simultaneously with a multicomponent model.
The nature of sensitivity functions and their use was introduced by Levin, Kuikka, and Bassingthwaighte (17) but will be summarized here. Sensitivity functions, Sp(t), are defined as partial derivatives of the model solution with respect to each of the parameters of interest, p, as a function of time
| (5) |
where p is any of the parameters, PSC, γISF, PSpc, γpc, and Gpc. The caret denotes a model solution, as opposed to the observed dilution curve. The sensitivity functions for these parameters are shown in Fig. 1 for a representative model solution. Mathematical independence of the parameters is demonstrated by the fact that no sensitivity function is a scalar or a reciprocal of another.
Fig. 1.
Impulse responses and sensitivity functions (S) for a capillary interstitial fluid (ISF)-cell model solution with parameter values suitable for D-glucose in the heart. Notice that sensitivity functions are all different, indicating independence of the parameters in shaping model solutions. Note too that and Gpc have little influence at early times and more later, suggesting the importance of avoiding (or accounting for) reciruculation. Parameter values used to generate model solutions were 7-path model with relative dispersion of flows = 30%; Fs = 1.5 ml·g–1·min–1; PSC and PSpc = 0.75 and 0.5 ml·g–1·min–1; Gpc = 0.5 ml·g–1·min–1; γpc (which is ) = 10, and γISF (which is ) = 5. (Input function is a gamma variate function with a mean transit time of 3 s, a relative dispersion of 0.2, and a skewness of 1.0.) Scalar multiplier of 40 SPSpc and SGpc and of 100 for and indicate that sensitivity to PSC is higher than to any other parameter. Units of S, s-1/(units of parameter). See text for abbreviations.
For each free parameter there is a unique Sp(t) that differs from those of other parameters it describes the extent by which the model solution ĥ(t) would be increased by an increase in the parameter value,Thus for example, the influence of increasing PSC on ĥD(t) is to reduce its height at early times, as indicated by SPSC being negative. At a time late in the washout phase when hD(t) is higher than hR(t), an increase in PSC results in an increase in ĥD(t), so that SPSC is positive at late times. Although parameters are independent, there are interactions between them. Our optimization technique is based on the principle that their relative influences are different at each time t and that for each there is a period when its influence is maximal. The distance (with its negative or positive sign) between the model ĥD(t) and the data hD(t) around a particular time of maximal influence of one parameter gives information via the sensitivity functions on how much to change all of the free parameters.
For example, consider a hypothetical case where there are three free parameters, with the sensitivity functions for each. (For 5 parameters, the technique is identical, but discussing 3 is briefer.) The influences of parameters, PSC, γ, PSpc have their maxima at times t1, t2, and t3, i.e., the absolute values of the three sensitivity functions are relatively large at these times. But at each time there is some influence of all three parameters. The difference between the model functions ĥ(t1) and the observed h(t1), at a particular time t1, can be related to the values of the sensitivity functions at the same time t1 via an approximating linear equation containing three unknowns, the changes in parameter values, the Δp's, required to get a better fit at this time point. Similarly one would need other equations for other particular times. With two other times; t2 and t3, there are three equations with three unknowns
| (6) |
This can be written as an array of differences, D, where D1 = h(t1) – ĥ(t1), etc., and PSC is parameter 1, p1; γ is p2; PSpc, is p3; and the sensitivity function value for p1 at t1 is S11, etc., giving
| (7) |
This matrix was solved for the best estimates of ΔPSC, Δγ, and ΔPSpc by a standard matrix inversion technique, Gaussian elimination. The new parameter values are the previous values plus the changes, the Δp's.
In practice, time windows were used instead of single time points; these were short time spans around each maximum point of Si(t). D1, for example, represented an area between ĥ(t) and h(t) for several seconds around t1, divided by the duration of the period. By this mechanism the influence of particular data points is reduced, and the effect of noisy data is minimized. In addition, when the sensitivity function for one parameter is changing sign near time t1, the influence on this parameter is quite properly reduced toward zero.
With this optimization technique, one is not minimizing any global distance function such as a sum of squares or coefficient of variation but rather is matching model to data in sets of local regions that are defined by the sensitivity functions. The search ceases when the gradients no longer change or if the coefficient is less than 1%. The coefficient of variation (CV) that we report uses all the data points and therefore provides a global measure of the goodness of fit achieved but is not the function being minimized: it is CV = [Σ(ĥi – hi)2/(n – 1)]½/(Σĥi/n), where the index i denotes the value of h or ĥ at time ti, and n is the number of data points in the curve.
Reduction of degrees of freedom
Greatest accuracy in parameter estimation is achieved when the degrees of freedom are minimal, given that the model is physiologically appropriate. PSC for albumin is assumed to be zero, which is appropriate for the short times involved in these studies; the albumin curve, the measured mean flow, the distribution of regional flows (from microsphere data), and an assumed capillary volume, of 0.035 ml/g (6), thereby completely define the large vessel transport function, hLV(t), which is obtained by deconvolution in accordance with Eq. 4. This hLV(t) is used as the input function for the three h(t)s in each set; hR(t) should, and does automatically, give an exact fit to the observed hR(t) when the axial diffusion coefficient is set to zero. Recruitment with increasing flow, a physiological possibility, is not an issue since the PSs for the three glucoses were obtained in pairs without changing flow, nor is the assumption of a particular value for . Any other value from 0.005 to 0.5 would do as well, since the analysis determines PS/F, and F is measured experimentally.
For L-glucose only two parameters, PSC and γISF, were free. While there might otherwise be five free parameters for D-glucose and deoxyglucose, in practice these two were fixed when L-glucose was one of the three tracers injected: PSC was the same for D- as for L-glucose; PSC for deoxyglucose was (180/164)½ (=1.048, the expected ratio of free aqueous diffusion coefficients, proportional to the square root of the ratio of mol wts) times that for L-glucose (assuming permeation without significant steric hindrance); was the same for each pairing of the three glucoses. When D- and 2-deoxy-D-glucose were paired, both γISF and γpc were the same for both sugars, but PSC and PSpc were unconstrained. The results will show that the ratio of PSC for D- to deoxyglucose was not statistically different from 1.048, verifying the use of the assumption for L-glucose, as well as indicating the absence of substantial transcapillary escape via the plasmalemma of the endothelial cells.
RESULTS
Experimental Outflow Dilution Curves
The dog heart weights ranged from 102 to 230 g, and the rabbit hearts from 7.6 to 10.6 g. Perfusion pressures were 50–120 mmHg in dogs, 40–130 in rabbits. Heart rates were from 50 to 90 min-1 in dogs, 60–140 in rabbits. None of these appeared to influence the estimates of the parameters. The mean coronary flows and their relative dispersions (from the microsphere data) are given for the dog studies in Table 1 and for the rabbits in Table 2.
TABLE 1.
Capillary and sarcolemmal permeabilities in isolated blood-perfused dog hearts
| Expt No. | Fs, ml·g–1·min–1 | RD, μm |
PSC, ml·g–1·min–1 |
γ ISF |
PSpc, ml·g–1·min–1 |
γ pc | CV |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D-Glc | Deoxy-Glc | D-Glc | Deoxy-Glc | L-Glc | D-Glc | Deoxy-Glc | |||||
| 30127-1 | 0.53 | 0.37 | 0.49 | 7.8 | 0.3* | 12.5* | 0.22 | 0.19 | |||
| 30127-2 | 0.51 | 0.37 | 0.51 | 6.0 | 0.6* | 12.5* | 0.36 | 0.20 | |||
| 30127-3 | 0.49 | 0.54 | 0.45 | 5.1 | 0.3* | 12.5* | 0.27 | 0.25 | |||
| 30127-4 | 0.41 | 0.54 | 0.39 | 4.0 | 0.6* | 12.5* | 0.36 | 0.39 | |||
| 3028-1 | 1.48 | 0.28 | 0.76 | 4.2 | 0.2 | 9.8 | 0.11 | 0.04 | |||
| 3028-2 | 1.48 | 0.28 | 0.75 | 4.7 | 0.5 | 12.8 | 0.18 | 0.10 | |||
| 3028-3 | 1.47 | 0.28 | 0.71 | 0.73 | 7.0 | 0.8 | 0.2 | 12.6 | 0.23 | 0.06 | |
| 3028-4 | 1.52 | 0.3 | 0.62 | 3.7 | 0.1 | 12.9 | 0.13 | 0.12 | |||
| 3028-5 | 1.62 | 0.3 | 0.60 | 4.0 | 0.4 | 12.9 | 0.12 | 0.13 | |||
| 3028-6 | 1.64 | 0.3 | 0.65 | 0.68 | 5.0 | 0.3 | 0.2 | 14.0 | 0.22 | 0.27 | |
| 22028-1 | 1.72 | 0.30 | 0.81 | 5.2 | 0.3 | 12.5* | 0.12 | 0.10 | |||
| 22028-3 | 2.36 | 0.30 | 0.81 | 0.86 | 6.1 | 0.8 | 0.6 | 12.0 | 0.12 | 0.22 | |
| 4048-1 | 1.37 | 0.56 | 0.91 | 3.9 | 0.1 | 12.9 | 0.14 | 0.06 | |||
| 4048-2 | 1.28 | 0.56 | 0.82 | 4.4 | 0.5 | 13.0 | 0.11 | 0.20 | |||
| 4048-3 | 1.07 | 0.56 | 0.96 | 0.97 | 6.6 | 0.6 | 0.4 | 14.8 | 0.073 | 0.05 | |
| 4048-4 | 1.66 | 0.49 | 1.30 | 7.4 | 0.4 | 12.5* | 0.15 | 0.06 | |||
| 4048-5 | 1.61 | 0.49 | 0.74 | 5.4 | 0.5 | 12.9 | 0.13 | 0.24 | |||
| 4048-6 | 1.36 | 0.49 | 0.97 | 1.00 | 6.5 | 0.7 | 0.5 | 13.3 | 0.19 | 0.08 | |
| Mean | 1.31 | 0.41 | 0.72 | 0.80 | 5.4 | 0.57 | 0.30 | 12.7 | 0.18 | 0.19 | 0.12 |
| ± SD | ±0.51 | ±0.11 | ±0.17 | ±0.22 | ±1.25 | ±0.15 | ±0.15 | ±0.94 | ±0.087 | ±0.083 | ±0.081 |
| n | 18 | 18 | 11 | 12 | 18 | 11 | 12 | 18 | 13 | 11 | 12 |
Fs, flow of solute-containing mother fluid; RD, relative dispersion; PSC and PSpc, permeability-surface area product of capillaries and parenchymal cells, respectively; γISF and γpc, dimensionless ratios of interstitial volume of distribution and of volume of tracer distribution; CV, coefficient of variation. Experiment numbers have animal number first, then run number. In individual runs for L-glucose, PSC and were estimated jointly with a dextroglucose. was also estimated jointly for the dextroglucose runs. Values of PSC and γISF for L-glucose were obtained for each experiment, except where both D- and 2-deoxy-D-glucose were paired. Values for γISF are those reported here. Values for PSC were identical to those of D-glucose or were 1.048 times that of 2-deoxy-D-glucose; average PSC (L-Glc) was 0.69 ± 0.22 (n = 13). Estimates of infinite intracellular consumption averaged roughly 0.2 ml·g–1·min–1 but were too imprecise to merit individual reporting.
Data not sufficiently accurate to permit estimation.
TABLE 2.
Capillary and sarcolemmal permeabilities in isolated Tyrode-perfused rabbit hearts
| Expt No. | Fs, ml·g–1·min–1 | RD, μm |
PSC, ml·g–1·min–1 |
γ ISF |
PSpc, ml·g–1·min–1 |
γ pc | CV |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D-Glc | Deoxy-Glc | D-Glc | Deoxy-Glc | L-G1c | D-G1c | Deoxy-Glc | |||||
| 13078-1 | 1.7 | 0.30 | 1.1 | 7.9 | 0.4 | 9.0 | 0.098 | 0.092 | |||
| 13078-2 | 1.7 | 0.30 | 1.3 | 1.40 | 9.5 | 0.4 | 0.5 | 7.5 | 0.039 | 0.055 | |
| 13078-3 | 1.7 | 0.44 | 1.3 | 9.0 | 0.42 | 9.0 | 0.079 | 0.061 | |||
| 13078-4 | 1.7 | 0.44 | 1.2 | 1.25 | 10.0 | 0.5 | 0.42 | 10.0 | 0.056 | 0.039 | |
| 12068-1 | 2.8 | 0.3 | 1.0 | 8.5 | 0.6 | 13.0 | 0.19 | ||||
| 12068-2 | 2.4 | 0.3 | 1.0 | 1.04 | 9.0 | 0.3 | 0.16 | 12.0 | 0.18 | 0.17 | |
| Mean ± SD | 2.0 | 1.15 | 1.23 | 9.0 | 0.45 | 0.36 | 10.1 | 0.09 | 0.10 | 0.08 | |
| n | ±0.5 | ±0.14 | ±0.18 | ±0.7 | ±0.1 | ±0.18 | ±2.1 | ±0.06 | ±0.07 | ||
| 23 | 23 | 3 | 23 | 23 | 3 | 23 | 2 | 23 | 3 | ||
Abbreviations as in Table 1.
Experimental outflow dilution curves are shown in Fig. 2 and given in numerical form in Table 3. The paired D-and L-glucose dilution curves were essentially identical during the upslope and peak of the curves. From this we conclude that the transport rates for D- and L-glucose across the capillary wall are identical. Because L-glucose does not enter cells (except very slowly), we conclude further that endothelial luminal surface uptake of D-glucose is negligible and infer that both D- and L-glucose permeate via passive diffusion through aqueous channels, presumably the clefts between endothelial cells.
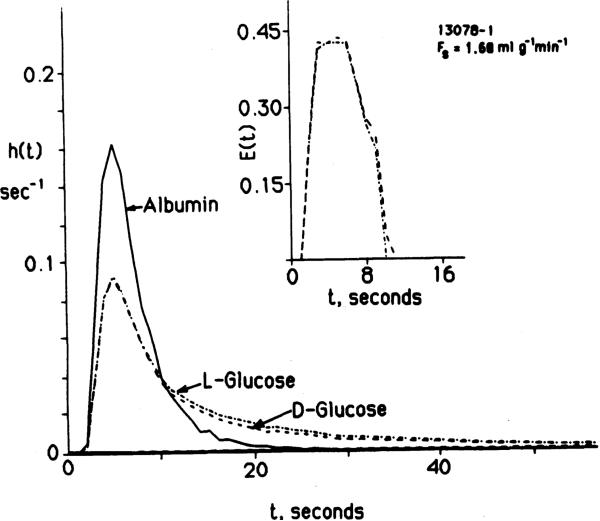
Fig. 2.
Coronary sinus outflow dilution curves for D- and L-glucose in a rabbit heart after injection of tracers into the aortic cannula at t = 0. Main peaks of impulse responses for D- and L-glucose are nearly identical. Extraction curves, E(t) in insert, provide a more critical test of their similarity and show the same result. Tail of D-glucose curve is lower than that of L-glucose, in accord with uptake by myocardial cells. (Expt no. 13078-l. Data given in numerical form in Table 3.)
TABLE 3.
Example data set (13078-1) and model fit
| Time, s | Albumin |
[14C]L-glucose |
[3H]D-glucose |
|||
|---|---|---|---|---|---|---|
| ĥ R | ĥ R | h D1 | ĥ D1 | h D2 | ĥ D2 | |
| 1 | 10 | 20 | 10 | 155 | 10 | 155 |
| 2 | 5,129 | 5,754 | 3,802 | 5,888 | 3,802 | 5,888 |
| 3 | 75,857 | 81,283 | 42,658 | 40,738 | 41,687 | 40,738 |
| 4 | 147,910 | 151,355 | 81,283 | 79,432 | 83,176 | 79,432 |
| 5 | 165,958 | 165,958 | 91,201 | 93,325 | 91,201 | 93,325 |
| 6 | 151,355 | 147,910 | 83,176 | 85,113 | 83,176 | 85,113 |
| 7 | 112,201 | 109,647 | 69,183 | 69,183 | 69,183 | 69,183 |
| 8 | 79,432 | 77,624 | 56,234 | 54,954 | 56,234 | 54,954 |
| 9 | 63,095 | 61,659 | 46,773 | 45,708 | 45,708 | 44,668 |
| 10 | 39,810 | 38,904 | 38,904 | 36,308 | 37,153 | 34,673 |
| 11 | 30,903 | 30,903 | 33,113 | 30,903 | 30,903 | 29,512 |
| 12 | 23,442 | 23,442 | 30,199 | 27,542 | 27,542 | 25,704 |
| 13 | 17,783 | 17,378 | 26,302 | 23,988 | 23,988 | 22,387 |
| 14 | 14,791 | 14,454 | 23,442 | 21,877 | 21,877 | 19,952 |
| 15 | 11,481 | 11,481 | 21,379 | 20,417 | 19,498 | 17,783 |
| 16 | 9,332 | 9,120 | 19,054 | 18,621 | 16,982 | 15,849 |
| 17 | 6,918 | 6,761 | 17,783 | 16,982 | 15,488 | 14,454 |
| 18 | 5,370 | 5,370 | 16,596 | 15,849 | 14,125 | 12,882 |
| 19 | 3,715 | 3,548 | 15,488 | 14,454 | 12,882 | 11,749 |
| 20 | 3,020 | 3,020 | 14,454 | 13,489 | 12,023 | 10,715 |
| 21 | 2,570 | 2,512 | 13,489 | 12,882 | 11,220 | 10,000 |
| 22 | 2,089 | 2,042 | 12,589 | 12,023 | 10,471 | 9,332 |
| 23 | 1,820 | 1,778 | 11,749 | 11,481 | 9,772 | 8,710 |
| 24 | 1,549 | 1,549 | 10,471 | 10,965 | 8,912 | 8,128 |
| 25 | 1,445 | 1,445 | 10,471 | 10,471 | 8,710 | 7,762 |
| 26 | 1,380 | 1,380 | 9,550 | 10,000 | 8,128 | 7,413 |
| 27 | 1,288 | 1,288 | 8,511 | 9,550 | 7,244 | 6,918 |
| 28 | 871 | 851 | 7,762 | 8,912 | 6,456 | 6,456 |
| 32 | 813 | 813 | 6,761 | 7,586 | 5,754 | 5,370 |
| 36 | 741 | 724 | 6,026 | 6,309 | 5,129 | 4,467 |
| 40 | 646 | 646 | 5,012 | 5,370 | 4,365 | 3,890 |
| 44 | 575 | 575 | 4,074 | 4,467 | 3,715 | 3,311 |
| 48 | 468 | 468 | 3,388 | 3,715 | 3,236 | 2,951 |
| 52 | 251 | 245 | 2,884 | 3,020 | 2,884 | 2,512 |
| 56 | 219 | 219 | 2,455 | 2,512 | 2,884 | 2,239 |
| 60 | 200 | 200 | 2,089 | 2,089 | 2,291 | 2,042 |
| 64 | 182 | 182 | 1,778 | 1,738 | 2,138 | 1,862 |
| 68 | 155 | 155 | 1,585 | 1,445 | 1,905 | 1,698 |
| 72 | 126 | 126 | 1,349 | 1,202 | 1,738 | 1,585 |
| 76 | 100 | 98 | 1,175 | 1,000 | 1,622 | 1,479 |
| 80 | 135 | 138 | 1,000 | 851 | 1,445 | 1,380 |
| 84 | 78 | 76 | 912 | 692 | 1,413 | 1,288 |
| 88 | 45 | 45 | 776 | 562 | 1,288 | 1,175 |
| 92 | 59 | 59 | 692 | 479 | 1,202 | 1,122 |
| 96 | 145 | 145 | 617 | 457 | 1,096 | 1,096 |
| 100 | 85 | 85 | 550 | 355 | 1,000 | 1,023 |
| 104 | 52 | 25 | 501 | 219 | 933 | 871 |
| 108 | 24 | 10 | 447 | 78 | 832 | 741 |
| 112 | 71 | 398 | 10 | 794 | 603 | |
| 116 | 43 | 380 | 776 | 457 | ||
| 120 | 24 | 324 | 692 | 316 | ||
| 124 | 66 | 295 | 676 | 182 | ||
| 128 | 36 | 251 | 603 | 42 | ||
| 132 | 33 | 234 | 562 | 10 | ||
| 136 | 40 | 229 | 562 | |||
| 140 | 29 | 209 | 537 | |||
| 144 | 29 | 186 | 468 | |||
Fs = 1.68 ml·g–1·min–1. Model parameters: PSc for D- and L-glucose = 1.13 ml g–1·min–1; γISF for D- and L-glucose = 7.94 ml/g; PSpc for D-glucose = 0.46 ml·g–1·min–1. (zero for L-glucose); γpc = 9.0 ml/g; Gpc = 0.03 ml·g–1·min–1. See also Figs. 2 and 3. The transport functions, h(t), fraction per second are multiplied by 106. Other abbreviations as in Table 1.
The curves differed during the washout phase. The difference was small, even though no fatty acid was available in the perfusate in the rabbit experiments. In the later phase of washout both the D- and 2-deoxy-D-glucose curves often, but not always, become closer to and then cross above the L-glucose curve paired with it. This pattern is consistent with the reflux of tracer from the cells through the ISF to the effluent perfusate.
Influences of Parameter Values on Model Solutions
From the similarity of the D- and L-glucose curves in Fig. 2 one can see that capillary PSC and the initial back diffusion from ISF must be the same for D- and L-glucose. These effects are shown by the sensitivity functions in Fig. 1 to occur early, while cellular effects occur later.
The influences of PSpc and Gpc, on the dilution curve, shown in Fig. 3, are all on the tails of the curves and not on the unslope and peaks. Figure 3A shows the effects of increasing rates of permeation into the myocytes, in an artificial situation in which there is no reflux from the cell. The absence of cellular efflux is equivalent to having an infinite intracellular consumption, Gpc, or an infinite intracellular volume of distribution, . With intermediate values of PSpc from 0.25 to 8 ml·g–1·min–1, the height of the peak of hD(t) is little affected, but the height of the tail beyond t = 20 s is greatly reduced by cellular influx in the absence of efflux. With both and PSpc very high, the ISF concentration is held at zero and there is no return flux from cell or ISF to the capillary. Then in the ith pathway the transport function for the permeating tracer, hDi(t), has a shape identical to that of the intravascular reference, hRi(t), but scaled down according to the local capillary permeability and local flow
| (8) |
This condition of no back diffusion from ISF to capillary is the hypothetical basis of the classic Crone-Renkin expression for estimating capillary permeability, although these authors considered only one capillary. The composite outflow dilution curve hD(t) is the weighted sum of the individual hDi(t)s, as in Eq. 3; its shape is different from that of the hR(t) because the fraction of tracer taken up by the cells is greater in low-flow pathways than high-flow pathways, in accordance with the early, rising value of the instantaneous extraction E(t) in Fig. 1.
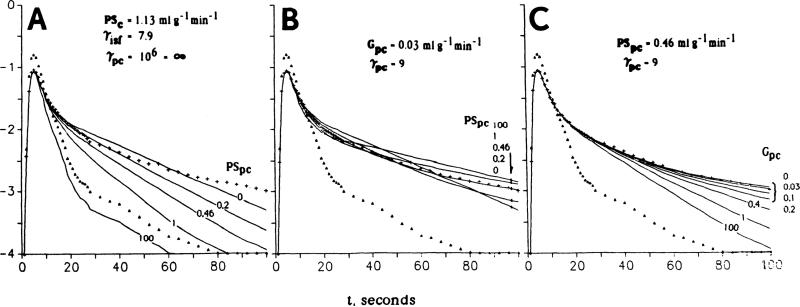
Fig. 3.
Multicapillary solutions for a capillary interstitial fluid (ISF)-cell-reaction model of D-glucose outflow dilution curves from same Krebs-Ringer perfused rabbit heart as in Fig. 2 (no. 13078-l). A: solutions for a reduced model for capillary permeation and cellular uptake without return flux from cell but with return flux from ISF to capillary. Heterogeneity of flows was approximated using 7 parallel pathways with a Gaussian distribution of flows with relative dispersion 30%. Five solutions are drawn for different values of the rate of cellular influx (PSpc), all other parameters being constant. Fs = 1.68 ml·g–1·min–1; PSc = 1.13 ml·g–1·min–1; γpc = 7.9; γpc = infinity, to prevent reflux from the cell. B: effects of cellular permeation, now with return flux from cell to ISF. Intracellular consumption, Gpc = 0.03 ml·g–1·min–1; other parameters same as for A. Best fit is obtained with PSpc = 0.42 ml·g–1·min–1 and γpc = 9. C: effect of Gpc on shapes of tails of outflow dilution curves. Increasing rates of consumption reduce reflux from cell into outflow, lowering tails of curves. Best fit values were PSc = 1.13 ml·g–1·min–1, γISF = 7.9, PSpc = 0.46 ml·g–1·min–1, γpc = 9, and Gpc = 0.03 ml·g–1·min–1. When PSpc, is higher, sensitivity to Gpc is higher. (Numerical values of data points and of model solutions are listed in Table 3.)
Allowing bidirectional flux across the cell membrane (Fig. 3B) gives an improved fit to the D-glucose curve. The curve for PSpc = 0 is the best fit to the simultaneously obtained L-glucose curve. Initially the curves for PSpc = 0 and ≠0 are the same, but they separate on the downslope. Later, return flux from the cell results in the model D-glucose curve crossing above L-glucose (that for PSpc = 0), a marked difference from the behavior in Fig. 3A, where the separation increases.
Figure 3C shows that consumption or sequestration within the cell reduces the return flux from the cell and the mean transit time of the outflow curve. During the 1st min the effect on ĥD(t) of increasing Gpc is the same as enlarging . Later, the effects differ in that an enlarged gives reflux later, whereas chemical reaction gives rise to metabolite species. Within the 2 min of data collection in these experiments, the difference between reversible binding and reaction cannot be ascertained accurately. Constraining the values of to the neighborhood of the known intracellular water space reduces the variability of estimates of Gpc.
The effect of intracellular consumption on the amount and time course of untransformed tracer-labeled substrate emerging in the outflow dilution curve is greater when the rate'of entry into the cell is higher; when PSC and PSpc are higher, sensitivity to Gpc is higher. The effect of Gpc is always to reduce return flux of untransformed tracer into the outflow. At high values of Gpc all intracellular tracer is consumed, and the curves are the same as for zero reflux (Fig. 3A). Thus the key to the unraveling of the relative effects of the various parameters on the model solutions to be fitted to the data is that each parameter influences different parts of the outflow dilution curves in different ways so that each parameter has a unique influence on the curve, independent of all of the other parameters. That this should occur is indicated by differences between the sensitivity functions shown in Fig. 1.
Parameter Values Estimated from Dilution Curves
The data for the blood-perfused dog hearts and for the Tyrode-perfused rabbit hearts fall into two separate ranges of perfusate flow. The higher viscosity and lower “plasmacrit” in the dog studies result in lower flows than in the rabbit experiments. Even so, the flows in our dog studies are still on the average higher than normal flows in dog hearts at rest. High flows are desirable only from the point of view of obtaining accurate estimates of PSC and γISF. Lower flows and even longer total sampling durations would have helped in estimating γpc and Gpc.
In Tables 1 and 2 are listed the parameter values resulting from the fitting of the models to the experimental curves from dog and rabbit hearts. An example of solutions fitted to a D-glucose curve in a rabbit heart is shown in Fig. 3. Model solutions for D- and 2-deoxy-D-glucose are shown from a dog heart in Fig. 4. In the optimization γISF was forced to have the same value for each of the pair of permeant tracers. This takes advantage of the physiological expectation that D-, L-, and 2-deoxy-D-glucose should have the same volume of distribution in the ISF and also reduces the number of free parameters and gives a more reliable estimate of γISF (which is ). Each of the two glucose models in a run has the same flow heterogeneity, the same vascular volumes, and the same interstitial volumes .
Fig. 4.
Outflow dilution curves for D-glucose and deoxyglucose and albumin (dog expt 4048-6) fitted with the model. Deoxyglucose curve is shifted downward by half a logarithmic decade (ordinate values divided by 10½) to display it separately from D-glucose curve. Parameter estimates logarithmic for D- and deoxyglucose were PSc, 0.97 and 1.0; PSpc = 0.7 and 0.5; Gpc, = 0.01 and 0.05 ml·g–1·min–1; γISF = 6.5 and γpc = 13.3 ml/g for both. Coefficients of variation were 0.19 and 0.09.
PSpc, for L-glucose was fixed at zero, in accord with its role as an extracellular reference tracer. Although this is the expected value, since L-glucose is reported not to enter cells (9, 19, 21), a preliminary set of analyses was done on the L-glucose curves with PSpc, free to vary. The result was that the estimates of PSpc for L-glucose were very low, usually less than 0.05 ml·g–1·min–1, and the confidence intervals calculated from the optimization almost always included zero. Therefore, there was no clear evidence for a nonzero value of PSpc, for L-glucose. In studies on awake rabbits in our laboratory we estimated the intracellular concentration of tracer-labeled L-glucose ( using sucrose as reference) was less than 3% of the plasma concentration 30 min after intravenous injection. Therefore, in the subsequent analyses, PSpc for L-glucose was fixed at zero, reducing the degrees of freedom in fitting the pairs of glucose curves with the dual model.
A further logical restriction was to assume that the values for PSC were proportional to their free diffusion coefficients over the 5% range involved here, namely to set PSC for D-glucose equal to that of L-glucose and to fit PSC for deoxyglucose at 1.048 times that for L-glucose. When these constraints were set aside in the interest of seeing what results would emerge from the unbiased estimates of PSC, the goodness of fit of the models to the data was only negligibly improved, indicating that the constraining was not only useful in reducing the degrees of freedom, but that there was no evidence that the basic assumption might be wrong.
Estimates of PSC
The values of PSC for blood-perfused dog hearts were lower than for the Tyrode-perfused rabbit hearts. The ratios of deoxyglucose to D-glucose were 1.042 ± 0.02 (n = 8 in 5 dogs and 3 rabbits), close to the expected ratio, 1.048. Thus the PSCs for these glucoses are apparently in proportion to their free diffusion coefficients in water. For D-glucose the mean value of PSC was 0.72 ± 0.17 (n = 11) for dog myocardial capillaries and 1.15 ± 0.14 (n = 6) for rabbit myocardial capillaries when Tyrode perfused. For 2-deoxy-D-glucose, PSC in dog hearts averaged 0.80 ± 0.22 (n = 12) ml·g–1·min–1 and in three rabbit hearts 1.2 ± 0.2 (n = 3). Values for L-glucose are not reported in Tables 1 or 2: when paired with D-glucose, PSC (L-glucose) and PSC (D-glucose) were defined as equal and the best value determined for the pair; when 2-deoxy-D-glucose and L-glucose were paired, PSC (deoxyglucose)/PSC(L-glucose) was fixed at 1.048. In dog hearts the mean PSC (L-glucose) was 0.69 ± 0.22 (n = 13) ml·g–1·min–1 and in rabbit hearts 1.2 ± 0.1 (n = 3), the higher value in rabbits probably being attributable to the lack of albumin in the perfusate.
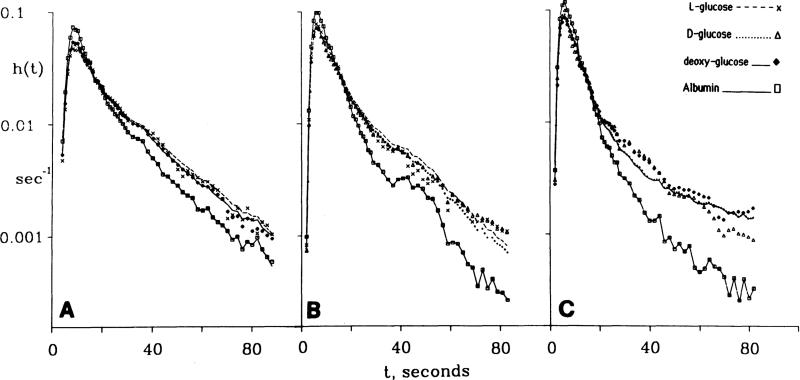
A test of adequacy of the conclusion that the PSCs were in proportion to free diffusion coefficients is given by Fig. 5 in which their ratios were fixed. Three sets of dilution curves from one dog study, nine h(t)s, obtained in sequence without changing the flow were fitted with four free parameters only: one degree of freedom for PSC, one for , and two for PSpc. The value for was fixed at γpc = 12.5 , about an average value from the analyses summarized in Tables 1 and 2. Gpc for D-glucose and 2-deoxy-D-glucose was fixed from an approximate calculation from a steady-state extraction of 5% to be 0.2 ml·g–1·min–1 (see discussion following Eq. 12). The results in Fig. 5, A–C, show that in spite of the reduction of degrees of freedom to 4 instead of 24 (2 for each L-glucose curve, 5 for each of the 4 dextroglucose curves), the fitting of the model solutions to the data are quite good, particularly in the first 20–30 s in which PSpc, and Gpc have less influence; the overall coefficient of variation for the 6 glucoses was 0.11. The conclusion is that the constraining is useful and acceptable in obtaining good estimates of PSC; such approaches could therefore have been used on other triple sets of data, but this mode of analysis is left for future studies when it can be automated. Logical constraining of PSC and (or γISF) should probably be used more generally, since the result will be more accurate estimates of the parameters which should be left free, PSpc and Gpc.
Fig. 5.
Constrained fitting of a capillary interestitial-fluid (ISF)-cell-reaction model to 9 coronary outflow dilution curves for albumin, D-, L-, and 2-deoxy-D-glucose. (Expts 30284, 2, and 3.) Symbols are data points; lines give model solutions fitted to data under strong constraints. Regional flows were from microsphere deposition densities. The only free parameters were 1 value each for PSc and and 2 for PSpc and for D- and 2-deoxy-D-glucose (see text). Since PSC affects most strongly the first 20–30 s of the curves, result is excellent fitting of parts of curve sensitive to PSc and poorer fitting tails at 30–80 s where , PSpc, and have influences.
Estimates of
Values for γISF, or , ranged from 3.7 to 7.8 with a mean of 5.4 ± 1.2 (n = 18) in dogs (Table 1) and from 7.9 to 10.0 with a mean of 9.0 ± 0.7 (n = 6) in the Tyrodeperfused rabbit hearts (Table 2), which are ordinarily moderately edematous. Using = 0.035 ml/g, in dogs = 0.0035 × 5.4 ≈ 0.19 ml/g and in rabbits ≈ 0.30 ml/g.
Estimates of PSpc
Values for PSpc for d- and 2-deoxy-D-glucose are given in Tables 1 and 2. The estimates are apparently independent of the flow, over a range of mean myocardial plasma flow from 0.41 to over 2.0 ml·g–1·min–1 in dogs and from 1.7 to 2.8 in rabbits. Since intercapillary diffusion distances are small enough, 17–22 μm, so that diffusional equilibration times locally over regions with these diameters are small, 10–60 ms, the rate of equilibration within the ISF is rapid compared with transmembrane transport rate constants, such as . This means that all of the parenchymal cell surface is quickly exposed to a more or less uniform extracellular concentration (CISF) even if only every other capillary is open. Thus constancy of PSpc in the presence of some flow effect to recruit capillaries implies that recruitment occurs within neighboring groups of capillaries, as was observed by Rose et al. (29).
Estimates of
Estimates of or γpc can be made from the outflow dilution curves when there is reflux of tracer from cells to outflow. L-glucose is not useful in this regard since it does not enter cells. D-glucose and 2-deoxy-D-glucose are phosphorylated via hexokinase, and only the unphosphorylated form can exit from the cell, so estimates of γpc are less accurate than if Gpc were zero. (Following this argument, one might choose 3-O-methylglucose, which is presumably not reacted with inside the cell, as a good indicator for measuring γpc, but this was not a primary goal here). Presumably the phosphorylated forms are too polar to traverse the membrane. In the absence of a significant phosphatase reaction in the cell (hydroylzing the phosphate), it follows that any reflux of deoxyglucose implies the persistence of unphosphorylated glucose or deoxyglucose at a finite concentration within the cell. The estimates of γpc from D-glucose and 2-deoxy-D-glucose averaged 12.7 ± 0.94 (n = 18) in dog hearts and 10.1 ± 2.1 (n = 6) in rabbit hearts, or about 0.44 and 0.35 ml/g for . The mere fact that reasonable volumes are estimated indicates that intracellular concentrations are finite. See discussion.
The total of the volumes of distribution, or , was about 0.67 ml/g in dogs and 0.70 in rabbits; both are less than the total water space. This is mainly because the large vessel volume (~0.1 ml/g) is excluded from the computation. If one were to consider the large vessel volume to be zero, then the result could suggest that the arbitrary choice of Vc = 0.035 was too small; a value of 0.0406 ml/g for Vc would give a total water space of 0.777 ml/g, the water space of dog hearts (37). Since large vessel volumes are not zero, a value of 0.0406 is an overestimate of Vc, and the anatomic estimate of 0.035 (6) is physiologically reasonable. Affirming this argument, the sum of the large vessel volume, 0.1 ml/g, plus the total intratissue volume of distribution, 0.67 ml/g, is 0.77 ml/g, almost the expected value of 0.777 ml/g.
Estimates of Gpc
Gpc represents a rate of clearance from the cell cytoplasm into a pool from which there is no return to the cytoplasm. The accuracy of estimation of Gpc is reduced whenever PSpc is low, as it is here. The dog data curves were analyzed with γpc and Gpc free; the estimates of Gpc for D-glucose were 0.2 ± 0.3 ml·g–1·min–1 (n = 11) and for deoxyglucose were 0.2 ± 0.4 (n = 12). The estimates are obviously imprecise but are compatible with calculations from arteriovenous differences and estimates of glucose consumption [e.g., from Rovetto et al. (30)].
DISCUSSION
Importance of Heterogeneity of Flows
Taking into account the heterogeneity of regional myocardial blood flows is critical to the accurate estimation of parameter values. Comparisons between multicapillary and single capillary analysis showed that PSC would be underestimated by 2–50% if a single capillary model were used. PSpc would be estimated with less than 20% error either if PSpc were less than 1 ml·g–1·min–1 or if the heterogeneity of flow were less than 25%, given that PSC is greater than FS/2. At higher values of relative dispersion, greater than 25% or with estimates of PSpc greater than 1 ml·g–1·min–1, overestimates of PSpc could be as large as threefold. In actuality, estimates for PSC using a three-region axially distributed single-capillary model were 21% ± 12% (n = 36 curves in dog hearts) less than those obtained using the multicapillary model. The estimates of PSpc were on the average quite similar for both single and multicapillary modeling; the ratio (single/multiple) was 0.87 ± 0.28 (n = 21). The smallness of the error was due to two offsetting errors: errors in PSC due to single-capillary modeling led to errors in PSpc that were opposite in direction to those occurring when only PSpc is free to err.
Capillary Permeability-Surface Area Products
Most of the data available in the literature on estimating PSC has been obtained by use of the Crone-Renkin equation
| (9) |
where Emax is the apparent maximal extraction during the upslope and the time of the peak of the indicator-dilution curves (cf. Eq. 8). This formula does not account for return flux of tracer from ISF into the effluent blood and so gives an underestimate of the unidirectional flux from the capillary plasma across the capillary membrane into the interstitium. Accordingly, from our data the estimates of PSC (Crone) from this equation averaged 79 ± 14% (n = 36) of the estimates obtained from the multicapillary modeling, which we consider to be the current “gold standard.”
As anticipated from this result, our estimates averaging 0.72 ml·g–1·min–1 for PSC are therefore somewhat higher than those obtained for the heart by earlier investigators. Values of PSC for glucose in isolated, blood-perfused, nonworking dog hearts obtained by Alvarez and Yudilevich (1) were 0.31 ± 0.09 ml·g–1·min–1 (n = 14), by Yipintsoi et al. (38) were 0.31 ± 0.13 (n = 23), and by Duran and Yudilevich (10) at the highest flows were 0.64 ± 0.09 (n = ll), all of which can be interpreted as underestimates because of lack of accounting for tracer reflux from ISF to capillary (“back diffusion”). In rabbit hearts perfused with Krebs-Henseleit solution, Bassingthwaighte et al. (4) found a correction factor for the calculation of the “true PSC” for ascorbate and glucose from the PSC (Crone); i.e., true PSC = 1.39 PSC (Crone), with a standard deviation of 34%. The dog data of this study would suggest a smaller error
| (10) |
with a standard deviation of 18% (n = 26). There is a good reason for the difference, namely that blood-perfused hearts, with substantially lower PSCs, have smaller errors because there is less back diffusion. One can expect the error in the Crone expression to be larger with larger values of PSc/Fs, as Cousineau et al. (7) observed.
We should emphasize that the data on PSC for the dogs and those from rabbits do differ significantly, the rabbit having the higher values. This is presumably partly due to the absence of albumin in the perfusate for the rabbit hearts and also to the presence of higher flows and virtually complete vasodilation. The isolated Tyrode-perfused rabbit heart is certainly not a normal physiological preparation in that the PSC is abnormally high, although it seems otherwise to be a perfectly adequate model for testing our methods of analysis and for studying a variety of phenomena in which interstitial edema does not interfere.
Having concluded that glucose is transported passively via clefts across myocardial capillaries, one may contrast this with the situation at the tight endothelial barrier in the brain. For blood-brain exchange the present models would have to be augmented to include another barrier and concentration-dependent permeabilities. Transport across brain capillaries is by a facilitated, or carrier-mediated, transport mechanism (8), and the transcapillary conductances PSC are concentration dependent. The conductance for tracer is governed by the concentration of nonlabeled glucose at each point in the capillary in each particular steady state; the steady state is not changed by the presence of tracer so Eqs. 2 are appropriate locally, but the PSs change by even a quarter or a third, increasing as concentrations of nontracer diminish toward the capillary outflow. Brain capillary PSC is not low because of the carrier facilitation (8, 39), and the degree of underestimation by the Crone-Renkin expression (Eq. 9) may not be any less than in the heart.
Estimation of
The true volume of the ISF space, VISF, can be estimated from γISF (from the modeling), the hematocrit, and the assumed total capillary volume of the heart of 0.035 ml/g (6)
| (11) |
This assumes that there is no binding of glucose in the ISF space and that all of the ISF is freely accessible to the glucose. The estimates of averaged 0.19 ± 0.04 (n = 18) for the dog hearts and 0.31 ± 0.03 (n = 23) for the rabbit hearts. For comparison, an estimate of the total extracellular space of 19% or 0.179 ml/g was obtained by Polimeni (25) using sulfate in quickly frozen hearts from intact rats. Guller et al. (11) found a value of 0.21 ml/g to be their best estimate of in blood-perfused isolated dog hearts. Unpublished data from our own laboratory on for sucrose and cobaltic EDTA in anaesthetized intact rabbit hearts quickly frozen in their in vivo state averaged 0.21 ± 0.032 ml/g (n = 432). Thus the estimates obtained from the dog heart data from the modeling in our present study agree reasonably well with data obtained in vivo and from isolated blood-perfused hearts and again affirm the reasonableness of using a VC of 0.035 ml/g to estimate the other spaces. As for the Tyrode-perfused rabbit hearts, it is clear that they become swollen because of the absence of albumin from the perfusate, and the values for γISF are significantly higher. Our values for of 0.30 ± 0.03 ml/g (n = 26) are not as high as those of Schafer and Johnson (32) of 0.42 ml/g for total sucrose space, from which the estimated capillary plasma space should be subtracted. This is in spite of the fact that our perfusion system for the isolated rabbit hearts is fairly similar to what they used. The difference may be that we avoided any perfusion at high pressures which causes the hearts to swell more.
Estimation of PSpc for D-glucose and Deoxyglucose
Values of PSpc are high enough that reflux of untransformed tracer from cells occurs, which is to say that the intracellular concentration is above zero. Our estimates are based on the assumption that L-glucose serves as an extracellular reference that is not taken up by the cells. The stereospecificity of the glucose transport system was demonstrated clearly by LeFevre and Marshall (15) and by Park et al. (22). As reviewed by these authors, the dextroglucoses exhibit the behavior expected of a carrier transport mechanism, i.e., inhibition, competition, and countertransport. The process is also facilitated by insulin and by anoxia, as reviewed by Morgan et al. (19), who also observed that the volume of distribution of L-glucose was not significantly different from that of an inert molecule, sorbitol. While they apparently did not test the effects of L-glucose on counter flow of nonmetabolized glucose analogues, they did observe an acceleration of D-glucose transport by insulin or anoxia when there was no effect on L-glucose transport.
The estimates of PSpc, are compatible with the maximal glucose uptake rates of 2 μmol·g–1·min–1 in isolated Krebs-Henseleit-perfused rat hearts without insulin, in reports summarized by Randle and Tubbs (27). The transcapillary and transsarcolemmal fluxes must both exceed that by much of an order of magnitude to allow consumption at this rate and also the return of some tracer to the efflux as D-glucose. The transmembrane unidirectional fluxes J are given by J = PS · C, where C is the concentration on the side of origin of the flux. With CC equal to 5 mmol and PSC equal to 0.8 ml·g–1·min–1, the unidirectional flux across the capillary wall would be 4 μmol·g–1·min–1, which is more than adequate. Calculating a combined or total PST for transport across capillary and sarcolemmal membranes in series, PST = 1/(1/PSC + 1/PSpc) = 1/(1/0.8 + 1/0.5) = 0.29 ml·g–1·min–1 gives a maximum flux at a normal glucose concentration of 5 mM of 5 × 0.29 = 1.5 μmol·g–1·min–1, which is again adequate and exceeds normal metabolic utilization. However, a heart undergoing work stress and lacking fatty acid as a substrate would have greater demands, and delivery of glucose might well be transport limited unless PSpc, increased to compensate.
Intracellular Reaction
While it is evident that to estimate Gpc, accurately the experimental data must be acquired with higher accuracy and for longer periods than was accomplished in these experiments, an interpretation of Gpc is nevertheless worthwhile. Conservation requires that the consumption be defined by the flow times the arteriovenous difference in the steady state, as well as by the intracellular reaction; therefore
| (12) |
The unidirectional fluxes at the membranes must exceed this, as discussed in the previous section, i.e.,
| (13) |
Taking values of FS of 1 ml·g–1·min–1 and Cart – Cvein = 0.2 mM, then GpcCpc = 0.2 μmol·g–1·min–1. If Gpc were actually 0.2 ml·g–1·min–1, then Cpc would be 1 mM. Using Eq. 13 with CC = 5 mM, then PST should be ≥Gpc·Cpc/CC = 0.2 Gpc. This inequality is fulfilled with values of Gpc as high as 1.45 ml·g–1·min–1 even if PST is only 0.29 ml·g–1·min–1, as calculated in the preceding paragraph.
In the steady state, several features of the system must show compatibility: the consumption is measured by FS(Cart – Cvein), by the intracellular consumption Gpc · Cpc and by the net flux from blood into cell. If there is 3% arteriovenous extraction (the value obtained in 2 rabbit hearts) at a flow of 2 ml·g–1·min–1, the D-glucose consumption is 2 ml·g–1·min–1 × 5 mM × 0.03 or 0.3 μmol·g–·min–1. The net intracellular reaction must match this exactly (assuming no net flux into or from glycogen), so that Cpc = consumption/Gpc = 0.3 μmol·g–1·min–1/0.2 ml·g–1·min–1 = 1.5 mM. The conductance from capillary lumen to inside the cell must be high enough to allow this consumption, so that the value PST·(CC – Cpc) must exceed 0.3 μmol·g–1·min–1, where PST is the overall conductance. If CC – Cpc = 5 – 1.5 = 3.5 mM, then PST must exceed 0.3/3.5 or 0.1 ml·g–1·min–1. With PSC = 0.7 and PSpc = 0.6 ml·g–1·min–1, PST = 1/(1/O.6 + 1/0.7) = 0.32 ml·g–1·min–1, a threefold excess over the minimal required conductance. This PST would permit a unidirectional flux PST(CC – Cpc) of 0.32 × 3.5 = 1 μmol·g–1·min–1. The conclusion is that the membrane barriers are only a mild impedance to glucose transport, and the permeabilities are high enough that bidirectional fluxes must occur, i.e., back diffusion, at both membranes. Insulin availability can be expected to raise, PSpc, further.
Among the highest consumptions under insulin stimulation are the values reported by Penpargkul, Kuziak, and Scheuer (23) of 0.67 μmol·g–1·min–1 and by Rovetto, Whitmer, and Neely (30) of 2 μmol·g–1·min–1. In the latter studies on Tyrode-perfused hearts the perfusate glucose level was 22 mM, so that the minimum PST had only to exceed 0.09 ml·g–1·min–1 to allow the observed flux, presuming that Gpc was also increased in this stimulated situation where the insulin level was 25 mU/ml. They estimated that Cpc might be 21 mM, so that with CC equal to 22 mM, the minimum PST would have to be 2 μmol·g–1·min–1/1 μmol·ml–1 ≈ 2 ml·g–1·min–1 and Gpc could be as low as 0.1 ml·g–1·min–1. The high PST could be explained if insulin stimulation increased PSpc, but capillary PSC at the level observed in our rabbit hearts would be at a borderline level.
Gpc and its Relationship to the Glycolytic Series
The clearance or the “first-order sequestration process” may provide a measure of the rate of metabolic reaction so long as there is no further diffusional resistance inside the cell. Naturally, the first reaction in the series, the hexokinase reaction in which glucose is phosphorylated to glucose 6-phosphate, is a prime candidate. The reverse reaction, catalyzed by glucose-6-phosphatase is considered to be slow, if it occurs at all in the heart. The formation of fructose 6-phosphate via isomerase is not paralleled by a similar reaction for 2-deoxy-D-glucose because the transformation requires the availability of an –OH group in the two position. Thus 2-deoxy-D-glucose tends to be retained in the cell as the -6-phosphate unless dephosphorylated and cannot be further metabolized or stored as glycogen. Of consequence to the interpretation of images of [11C]2-deoxy-D-glucose obtained by positron emission tomography is the likelihood of washout from the cell rather than 100% retention in the phosphorylated form. Given that PSpc and Gpc are similar for D-glucose and 2-deoxy-D-glucose and that phosphatase action is negligible, then the rate of uptake into 2-deoxyglucose-6-phosphate is related to the hexokinase reaction but is nevertheless dominated by flow and membrane transport. To interpret uptake data in terms of the hexokinase reaction, one must have good estimates of FSi, PSC and PSpc, and of .
Gpc would represent the hexokinase reaction exactly in the absence of dephosphorylation or other reactions of glucose 6-phosphate or deoxyglucose 6-phosphate. However, for glucose, if the hexokinase and glucose 6-phosphatase reactions were both rapid compared with the further metabolic reactions (via hexosephosphate isomerase, phosphoglucomutase, or glucose-6-phosphate dehydrogenase), then the specific activity of the glucose 6-phosphate pool would rise so that there would be tracer return to the free glucose pool (mathematically equivalent to back diffusion); this effect of tracer return to the glucose increases with time at a rate inversely proportional to the pool size of glucose 6-phosphate.
These arguments demonstrate some of the difficulties in deriving kinetic interpretations from observations of multiple indicator-dilution curves, even with carefully chosen sets of glucose analogues, and lead us to conclude that observations on the retention of a tracer such as [18F]2-deoxy-D-glucose, are similarly handicapped. A compartmental approach to interpreting the regional deposition of labeled glucoses in brain was worked out by Raichle et al. (26) and by Sokoloff et al. (33) in terms of the chemical reactions but omitting consideration of the influences of flow and of permeation through the capillary and cell membranes. Our present study demonstrates the permeation strongly influences the intracellular availability of glucose but is not the sole determinant of consumption rate. We would therefore hazard the warning that interpreting the rates of local tracer deposition as the measure of the rates of glucose metabolism, independent of flow and permeation, is inaccurate and that estimates of flow and particularly of permeabilities (which requires knowing flow) should be made and their influences taken into account. It is probably impossible to do this with observations of a single tracer, so multiple tracer studies should be devised for such situations. Moreover, the rate of glucose entry into the cell is equal to the rate of glycolysis only if there is no reflux of glucose from the cell and no incorporation into glycogen or activity of the hexose monophosphate shunt, so we conclude that tracer uptake normally overestimates the rate of glycolysis.
Acknowledgments
Marta Chaloupka, Joseph Chan, and Craig Althen assisted with the data analysis and the illustrations and Geraldine Crooker with the manuscript preparation.
This work was supported by National Heart, Lung, and Blood Institute Grants HL-19135 and HL-19139 and by National Institutes of Health Division of Research Resources Grant RR-01243. J. Kuikka was supported by Fogarty International Fellowship TW-02480-01 from the National Institutes of Health.
Footnotes
For the methods of computation available in detail, order NTIS Document UW/BIOENG-82/1 from National Technical Information Services, Dept. of Commerce, 5285 Port Royal Rd., Springifleld, VA 22161.
References
- 1.Alvarez OA, Yudilevich DL. Heart capillary permeability to lipid-insoluble molecules. J. Physiol. Lond. 1969;202:45–58. doi: 10.1113/jphysiol.1969.sp008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassingthwaighte JB. A concurrent flow model for extraction during transcapillary passage. Circ. Res. 1974;35:483–503. doi: 10.1161/01.res.35.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassingthwaighte JB, Goresky CA. Handbook of Physiology. The Cardiovascular System. Microcirculation. Vol. 4. Am. Physiol. Soc.; Bethesda, MD: 1984. Modeling in the analysis of solute and water exchange in the microvasculature. pp. 549–626. sect. 2. chapt. 13. [Google Scholar]
- 4.Bassingthwaighte JB, Kuikka JT, Chan IS, Arts T, Reneman RS. A comparison of ascorbate and glucose transport in the heart. Am. J. Physiol. 1985;249:H141–H149. doi: 10.1152/ajpheart.1985.249.1.H141. Heart Circ. Physiol. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassingthwaighte JB, Lenhoff AM, Stephenson JL. A sliding-element algorithm for rapid solution of spatially distributed convection-permeation models (Abstract). Biophys. J. 1984;45:175a. [Google Scholar]
- 6.Bassingthwaighte JB, Yipintsoi T, Harvey RB. Microvasculature of the dog left ventricular myocardium. Microvasc. Res. 1974;7:229–249. doi: 10.1016/0026-2862(74)90008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousineau D, Rose CP, Lamoureaux D, Goresky CA. Changes in cardiac transcapillary exchange with metabolic coronary vasodilation in the intact dog. Circ. Res. 1983;53:719–730. doi: 10.1161/01.res.53.6.719. [DOI] [PubMed] [Google Scholar]
- 8.Crone C. Facilitated transfer of glucose from blood into brain tissue. J. Physiol. Lond. 1965;181:103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crone C, Thompson AM. Comparative studies of capillary permeability in brain and muscle. Acta Physiol. Scand. 1973;87:252–260. doi: 10.1111/j.1748-1716.1973.tb05388.x. [DOI] [PubMed] [Google Scholar]
- 10.Duran WN, Yudilevich DL. Estimate of capillary permeability coefficients of canine heart to sodium and glucose. Microvasc. Res. 1978;15:195–205. doi: 10.1016/0026-2862(78)90018-3. [DOI] [PubMed] [Google Scholar]
- 11.Guller B, Yipintsoi T, Orvis AL, Bassingthwaighte JB. Myocardial sodium extraction at varied coronary flows in the dog: estimation of capillary permeability by residue and outflow detection. Circ. Res. 1975;37:359–378. doi: 10.1161/01.res.37.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkins RA, Mans AM, Davis DW, Viña JR, Hibbard LS. Cerebral glucose use measured with [14C]glucose labeled in the 1, 2, or 6 position. Am. J. Physiol. 1985;248:C170–C176. doi: 10.1152/ajpcell.1985.248.1.C170. Cell Physiol. 17. [DOI] [PubMed] [Google Scholar]
- 13.King RB, Bassingthwaighte JB. Radioactivity. In: Reneman RS, Strackee J, editors. Data in Medicine: Collection, Processing and Presentation. Instrumentation and Techniques in Clinical Medicine. I. Nijhoff; The Hague, Netherlands: 1979. pp. 79–113. [Google Scholar]
- 14.King RB, Bassingthwaighte JB, Hales JRS, Rowell LB. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ. Res. 1985;57:285–295. doi: 10.1161/01.res.57.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeFevre PG, Marshall JK. Conformational specificity in a biological sugar transport system. Am. J. Physiol. 1958;194:333–337. doi: 10.1152/ajplegacy.1958.194.2.333. [DOI] [PubMed] [Google Scholar]
- 16.Lenhoff AM, Lightfoot EN. The effects of axial diffusion and permeability barriers on the transient response of tissue cylinders. II. Solution in time domain. J. Theor. Biol. 1984;106:207–238. doi: 10.1016/0022-5193(84)90020-1. [DOI] [PubMed] [Google Scholar]
- 17.Levin M, Kuikka J, Bassingthwaighte JB. Sensitivity analysis in optimization of time-distributed parameters for a coronary circulation model. Med. Prog. Technol. 1980;7:119–124. [PMC free article] [PubMed] [Google Scholar]
- 18.Macchia DD, Page E, Polimeni PI. Interstitial anion distribution in striated muscle determined with [35S]sulfate and [3H]sucrose. Am. J. Physiol. 1979;237:C125–C130. doi: 10.1152/ajpcell.1979.237.3.C125. Cell Physiol. 6. [DOI] [PubMed] [Google Scholar]
- 19.Morgan HE, Regen DM, Park CR. Identification of a mobile carrier-mediated sugar transport system in muscle. J. Biol. Chem. 1964;239:369–374. [PubMed] [Google Scholar]
- 20.Opie LH. Effects of regional ischemia on metabolism of glucose and fatty acids: relative rates of aerobic and anaerobic energy production during myocardial infarction and comparison with effects of anoxia. Circ. Res., Suppl. I. 1976;38:I–52-I-74. [PubMed] [Google Scholar]
- 21.Paris S, Pouysségur J, Ailhaud G. Analysis by counter-transport, relationship to phosphorylation and effect of glucose starvation. Biochim. Biophys. Acta. 1980;602:644–652. doi: 10.1016/0005-2736(80)90342-9. [DOI] [PubMed] [Google Scholar]
- 22.Park CR, Rinewein D, Henderson MJ, Cadenas E, Morgan HE. The action of insulin on the transport of glucose through the cell membrane. Am. J. Med. 1959;26:674–684. doi: 10.1016/0002-9343(59)90227-x. [DOI] [PubMed] [Google Scholar]
- 23.Penpargkul S, Kuziak J, Scheuer J. Effect of uremia upon carbohydrate metabolism in isolated perfused rat heart. J. Mol. Cell. Cardiol. 1975;7:499–511. doi: 10.1016/0022-2828(75)90166-2. [DOI] [PubMed] [Google Scholar]
- 24.Phelps ME, Hoffman EJ, Huang SC, Kuhl DE. Positron tomography: in vivo autoradiographic approach to measurement of cerebral hemodynamics and metabolism. Acta Neurol. Scand, Suppl. 1977;56:446–447. [PubMed] [Google Scholar]
- 25.Polimeni PI. Extracellular space and ionic distribution in rat ventricle. Am. J. Physiol. 1974;227:676–683. doi: 10.1152/ajplegacy.1974.227.3.676. [DOI] [PubMed] [Google Scholar]
- 26.Raichle ME, Larson KB, Phelps ME, Grubb RL, Jr., Welch MJ, Ter-Pogossian MM. In vivo measurement of brain glucose transport and metabolism employing glucose-11C. Am. J. Physiol. 1975;228:1936–1948. doi: 10.1152/ajplegacy.1975.228.6.1936. [DOI] [PubMed] [Google Scholar]
- 27.Randle PJ, Tubbs PK. Handbook of Physiology. The Cardiovascular System. The Heart. Vol. 1. Am. Physiol. Soc.; Bethesda, MD: 1979. Carbohydrate and fatty acid metabolism. pp. 805–844. sect. 2. chapt. 23. [Google Scholar]
- 28.Rose CP, Goresky CA. Vasomotor control of capillary transit time heterogeneity in the canine coronary circulation. Circ. Res. 1976;39:541–554. doi: 10.1161/01.res.39.4.541. [DOI] [PubMed] [Google Scholar]
- 29.Rose CP, Goresky CA, Bach GG. The capillary and sarcolemmal barriers in the heart. An exploration of labeled water permeability. Circ. Res. 1977;41:515–533. doi: 10.1161/01.res.41.4.515. [DOI] [PubMed] [Google Scholar]
- 30.Rovetto MJ, Whitmer JT, Neely JR. Comparison of the effects of anoxia and whole heart ischemia on carbohydrate utilization in isolated working rat hearts. Circ. Res. 1973;32:699–711. doi: 10.1161/01.res.32.6.699. [DOI] [PubMed] [Google Scholar]
- 31.Sacks W, Schechter DC, Sacks S. A difference in the in vivo cerebral production of [1-14C]lactate from D-[3-14C]glucose in chronic mental patients. J. Neurosci. Res. 1981;6:225–236. doi: 10.1002/jnr.490060209. [DOI] [PubMed] [Google Scholar]
- 32.Schafer D, Johnson JA. Permeability of mammalian heart capillaries to sucrose and insulin. Am. J. Physiol. 1964;206:985–991. doi: 10.1152/ajplegacy.1964.206.5.985. [DOI] [PubMed] [Google Scholar]
- 33.Sokoloff L, Reivich M, Kennedy C, des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 34.Yipintsoi T. Single-passage extraction and permeability estimation of sodium in normal dog lungs. Circ. Res. 1976;34:523–531. doi: 10.1161/01.res.39.4.523. [DOI] [PubMed] [Google Scholar]
- 35.Yipintsoi T, Bassingthwaighte JB. Circulatory transport of iodoantipyrine and water in the isolated dog heart. Circ. Res. 1970;27:461–477. doi: 10.1161/01.res.27.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yipintsoi T, Dobbs WA, Scanlon PD, Knopp TJ, Bassingthwaighte JB. Regional distribution of diffusible tracers and carbonized microspheres in the left ventricle of isolated dog hearts. Circ. Res. 1973;33:573–587. doi: 10.1161/01.res.33.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yipintsoi T, Scanlon PD, Bassingthwaighte JB. Density and water content of dog ventricular myocardium. Proc. Soc. Exp. Biol. Med. 1972;141:1032–1035. doi: 10.3181/00379727-141-36927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yipintsoi T, Tancredi RG, Richmond DR, Bassingthwaighte JB. Myocardial extractions of sucrose, glucose, and potassium. In: Crone C, Lassen NA, editors. Capillary Permeability. Proceedings of Alfred Benzon Symposium II. Munksgaard; Copenhagen: 1970. pp. 153–156. [Google Scholar]
- 39.Yudilevich DL, De Rose N. Blood-brain transfer of glucose and other molecules measured by rapid indicator dilution. Am. J. Physiol. 1971;220:841–846. doi: 10.1152/ajplegacy.1971.220.3.841. [DOI] [PubMed] [Google Scholar]