Abstract
Abstract. Significant advances in the stem‐cell biology of several tissues, including the mammary gland, have occurred over the past several years. Recent progress on stem‐cell fate determination, molecular markers, signalling pathways and niche interactions in haematopoietic, neuronal and muscle tissue may provide parallel insight into the biology of mammary epithelial stem cells. Taking advantage of approaches similar to those employed to isolate and characterize haematopoietic and epidermal stem cells, we have identified a mammary epithelial cell population with several stem/progenitor cell qualities. In this article, we review some recent data on mammary epithelial stem/progenitor cells in genetically engineered mouse models. We also discuss several potential molecular markers, including stem‐cell antigen‐1 (Sca‐1), which may be useful for both the isolation of functional mammary epithelial stem/progenitor cells and the analysis of tumour aetiology and phenotype in genetically engineered mouse models. In different transgenic mammary tumour models, Sca‐1 expression levels, as well as several other putative markers of progenitors including keratin‐6, possess dramatically altered expression profiles. These data suggest that the heterogeneity of mouse models of breast cancer may partially reflect the selection or expansion of different progenitors.
INTRODUCTION
Breast cancer affects one in eight females in the United States and is the second leading cause of cancer deaths in American women. Women exposed to ionizing radiation as teenagers are more susceptible to breast cancer than those exposed as adults, and these women developed breast cancer several decades following their initial exposure (Land & McGregor 1979). These results, as well as the observation that almost 40% of breast cancers recur after 10 years following the diagnosis and removal of the primary tumour (Rosen et al. 1989), suggest that a population of cells exists with an extremely long half‐life, similar to stem cells, which may be important in the aetiology of breast cancer. Sell and Pierce (1994) originally proposed that the ‘analysis of the cellular origin of carcinomas of different organs indicates that there is in each instance, a determined stem cell required for tissue renewal that is the cell of origin for carcinomas’. With this knowledge, it is surprising that so little is understood about the aetiology of this disease and mammary gland stem cells, even though seminal mammary gland transplantation studies revealed the existence of these cells over 40 years ago (DeOme et al. 1959; Daniel et al. 1968).
Mouse models provide a unique resource for studying the role of mammary gland stem cells in development and tumorigenesis. In the postnatal mouse, mammary gland development undergoes two distinct growth phases, including ductal morphogenesis, which creates the ductal architecture and alveologenesis, which produces functional milk‐producing acini. These pathways are controlled by at least two distinct progenitor cell populations that can give rise to ductal and alveolar cell types (Smith 1996). Defining these differentiation pathways in terms of the molecular signals that drive these processes and the cells that contribute to them is critical for the analyses of transgenic and knockout mouse models with mammary gland phenotypes.
Considerable progress has been made in the field of stem‐cell biology in the past few years, and some of the approaches used in other systems have been applied recently to the mouse mammary gland. The ability to perform transplantation experiments using mammary epithelial cells transplanted into the cleared fat pad, similar to bone marrow transplantation into irradiated hosts, provides a powerful assay to identify and isolate functional stem cells. Our laboratory has used genetically engineered mice as models from which to isolate functional mammary stem cells and progenitors, and to elucidate the mechanisms responsible for stem‐cell renewal and differentiation. This review will focus on those studies.
IDENTIFICATION OF MAMMARY DIFFERENTIATION MARKERS
A crucial limitation to studying the process of mammary gland development and tumorigenesis is the identification of markers for mammary stem cells and progenitors. Using light‐ and electron‐microscopy techniques, morphologically distinct cell populations in the mammary gland have been described (Smith & Medina 1988; Chepko & Smith 1997). These studies have demonstrated a heterogeneous population of epithelial cells with distinct cellular architecture, localization and renewal capacity, associated with different stages of differentiation. Additionally, several molecular markers of differentiated cells have been identified, including intermediate filament proteins, steroid hormone receptors and milk‐protein components. Most of these are intracellular proteins such as keratins, smooth muscle α‐actin, β‐casein, whey acidic protein, progesterone receptor, oestrogen receptor and p63 (Faraldo et al. 1998; Humphreys et al. 1997; Yang et al. 1999). Other differentiation markers recognize unknown antigens and have been generated by inoculation of mice with mammary gland primary culture cells (Dulbecco et al. 1986; Sonnenberg et al. 1986; Soyal et al. 2002). In humans, CALLA and MUC1 are markers for myoepithelial and luminal cells, respectively (Pechoux et al. 1999; Smalley et al. 1999). More recently the NaKCl cotransporter, NKCC1m has been characterized as a potential marker for mammary ductal differentiation, based on its high expression level in luminal cells of ducts and low levels in alveolar cells, while conversely, the sodium–inorganic phosphate (NaPi)‐type IIb cotransporter (Npt2b) appears to be a marker of secretory alveolar cells, and is absent in ducts (2002a, 2002b). These markers identify distinct cell populations based on immunolocalization; however, their usefulness for assessing the process of differentiation, through culturing or transplantation of isolated populations, is limited, owing to either their intracellular localization, or a lack of accessible epitopes on the cell surface. Sorting, enrichment and transplantation of distinct cell populations from the mammary gland will be critical for understanding the process of mammary epithelial differentiation. Similar to the advances in haematopoietic lineage characterization, fluorescence‐activated cell sorter (FACS) analyses of distinct mammary populations may provide a potent tool for studying mammary gland differentiation pathways. However, to take advantage of FACS, it will be necessary to identify cell‐surface molecular markers for mammary epithelial cell populations and then classify these populations for their stem or differentiated qualities.
ANALYSIS OF MAMMARY GLAND PHENOTYPES IN GENETICALLY ENGINEERED MICE USING DIFFERENTIATION MARKERS
Several gene‐targeted and transgenic mouse lines display differentiation phenotypes that may result from deficiencies in stem cell growth or lineage competence (reviewed in Hennighausen et al. 2001). However, the assessment of these phenotypes is limited because of a lack of adequate markers. Typically, anomalous development phenotypes manifest elongation, branching, or alveologenesis defects, which may result from disruption of a differentiation pathway. For example, the ductal marker NKCC1 has been used to demonstrate a differentiation phenotype in Jak2, Stat5ab and PrlR‐deficient mammary epithelium (Miyoshi et al. 2001; Shillingford et al. 2002). Deletion of these genes in the mammary epithelium results in normal ductal architecture, but reduced alveolar development during pregnancy, and failure to produce milk. Interestingly, the ductal marker NKCC1 is expressed in the few alveolar‐like buds that form. These studies correlate an alveologenesis phenotype with the aberrant expression of the ductal marker NKCC1, and suggest that the loss of Jak2, Stat5a or PrlR disrupts the alveolar differentiation pathway. Interestingly, the Jak/Stat pathway has been shown recently to play a major role in the control of stem‐cell renewal in Drosophila spermatogenesis (Tulina & Matunis 2001). Furthermore, a comparative analysis of gene expression in haematopoietic and neural stem cells using microarrays has identified the growth‐hormone receptor gene, a known regulator of the Jak/Stat pathway, as one of a limited number of genes expressed in both lineages (Ivanova et al. 2002; Ramalho‐Santos et al. 2002). Mice in which the C/EBPβ gene has been deleted also have reduced alveolar development during pregnancy (Seagroves et al. 2000), and misexpress NKCC1 in alveolar‐like cells (S. Grimm, personal communication). One unique observation made while analysing the C/EBPβ‐null mice was the aberrant expression of keratin‐6 (K6), as well as a small proline‐rich protein (SPRR2), normally expressed only in the cornified layer of the epidermis, in the luminal cells lining the ducts (Grimm et al. 2002). This is interesting in light of the fact that the mammary gland and epidermis have a common evolutionary origin, as shown by experiments in which the targeted deletion of p63 in the mouse results in defects in development of both organs (Mills et al. 1999; Yang et al. 2001). Keratin‐6 expression is normally restricted to the body cells of the terminal end buds (TEBs) during ductal morphogenesis, and is not usually observed in the mature gland (Smith et al. 1990). During embryonic mammary gland development, K6 expression has also been detected in the mammary anlagen (S. Grimm, personal communication). Thus, K6 may provide a marker for early mammary progenitors.
These studies suggest that germline deletion of certain transcription factors and signal transduction components influences mammary epithelial cell fate. Developing more specific mammary‐cell lineage markers will allow better understanding of the role of each pathway in determining epithelial cell fate.
SIGNALLING PATHWAYS INVOLVED IN STEM‐CELL MAINTENANCE AND DIFFERENTIATION
Our current knowledge in stem‐cell biology originates largely from the study of stem cells in other tissues, such as the haematopoietic and neural systems, epidermis, and intestine. This is largely due to the identification of cell‐surface markers that are employed to isolate these organ‐specific stem cells. These markers include c‐Kit+, Lin−, stem‐cell antigen (Sca‐1)+, and CD34+ for haematopoietic stem cells (Vormoor et al. 1994; Pflumio et al. 1996; Cashman et al. 1997; Hogan et al. 1997; Sakabe et al. 1998), β1‐ and α6‐integrin for epidermis (Jones & Watt 1993), and nestin (Hockfield & McKay 1985; Lendahl et al. 1990)}, Map‐2, gfap (Meltzer et al. 1998), Musashi‐1 (Sakakibara et al. 1996; Sakakibara & Okano 1997; Kaneko et al. 2000), and Sox1 for neural stem cells (Pevny et al. 1998). The identification of specific mammary epithelial stem‐cell markers has lagged behind, and is now the focus of considerable research.
One important issue in stem‐cell biology is to elucidate the common molecular mechanisms governing self‐renewal or differentiation. Several signal‐transduction pathways have been implicated as essential in the self‐renewal of stem cells. For example, it has been determined that the Notch, Sonic hedgehog (Shh) and Wnt signalling pathways may regulate self‐renewal in blood and nervous system (Reya et al. 2001). Notch activation by its ligands, Jagged‐1 and Delta, results in an increase in the number of progenitor cells, suggesting that Notch activation promotes haematopoietic stem‐cell self‐renewal (Karanu et al. 2000; Varnum‐Finney et al. 2000). The Shh pathway has also been implicated in signalling to regulate self‐renewal of stem cells in the haematopoietic system and in the Drosophila ovary (Bhardwaj et al. 2001; Zhang & Kalderon 2001). The Notch pathway and TGF‐β have been shown to maintain haematopoietic stem cells in a quiescent state, whereas members of the BMP signalling pathway and bFGF (FGF‐2) may be involved in their differentiation (Eaves & Eaves 1988; Hatzfeld et al. 1991; Attisano et al. 1994; Maeno et al. 1996; Massague 1996; Bhatia et al. 1999). In the epidermis, β1‐integrin and MAPK cooperate to maintain the epidermal stem cell compartment (Zhu & Watt 1999). Likewise, β‐catenin overexpression has been shown to increase the stem‐cell population, whereas the Delta and Notch pathways may play a dual role in stem‐cell maintenance and induction of differentiation (Artavanis‐Tsakonas et al. 1999; Lowell et al. 2000). In the intestine, the Wnt/β‐catenin pathway and their downstream signalling molecules, such as APC, TCF‐4, Fkh‐6, Cdx‐1 and Cdx‐2, are vital for the maintenance of a stem‐cell compartment and for normal gastrointestinal epithelial cell differentiation (Brittan & Wright 2002). In the neuronal system, the signal‐transduction pathways that result in self‐renewal and prevent differentiation include the Notch pathway, growth factors such as EGF and FGF2, and Musashi. Neurogenesis is controlled by neuronal bHLH transcription factors, including Mash1, neurogenins (Ngn1 and 2), Neuro D and Math. It is thought that Ngn1 inhibits astrocyte differentiation by sequestering the CBP‐Smad1 transcription complex away from the astrocyte differentiation genes, and by inhibiting the activation of the STAT transcription factors that are necessary for gliogenesis (reviewed in Okano 2002). Promotion of astrocytic differentiation results from activation of the gp130/Jak/Stat3 and BMP signalling pathways, and the formation of a ternary complex containing Stat3/CBP/Smad, allowing the expression of differentiation genes (Okano 2002). Additionally, it has been shown that Musashi, an RNA‐binding protein, may inhibit neural differentiation by activating Notch signalling through the repression of mNumb, a Notch inhibitor (Okano et al. 2002). Thus, it is likely that the specification of one cell lineage results in the suppression of alternative fates. In summary, it is likely that the final fate of a progenitor is in the balance between many factors and signal‐transduction pathways that act in concert to regulate the process of differentiation versus self‐renewal.
Although little is known concerning the role of these pathways in mammary stem‐cell renewal or differentiation, it is intriguing that int‐1 and int‐3 (encoding the Wnt‐1 and activated Notch 4 proteins, respectively) were first identified in mammary tumours as a consequence of mouse mammary tumour virus (MMTV) proviral activation. Furthermore, analysis of WAP‐TGFβ transgenic mice has indicated that TGFβ1 overexpression can result in the early senescence of the epithelial stem‐cell population, as well as reduced mammary cancer risk (Kordon et al. 1995; Boulanger & Smith 2001). Thus, it is apparent that the same pathways implicated in the differentiation process of other stem cells may be involved in mammary gland stem‐cell differentiation. Interestingly, many of the same pathways important in the regulation of stem‐cell renewal have been implicated in the aetiology of human cancers (Taipale & Beachy 2001).
MAMMARY GLAND STEM CELLS
As mentioned previously, the existence of mammary gland stem cells was first confirmed through transplantation studies conducted by DeOme et al. (DeOme 1959), who showed that epithelium isolated from different regions of a mammary gland at different stages of postnatal development was capable of generating fully functional mammary epithelial outgrowths containing ducts, lobules and myoepithelial cells. In 1998, Kordon and Smith showed that progeny of a single multipotent stem cell could produce an entire mammary gland (Kordon & Smith 1998). This was demonstrated by transplanting mammary epithelial fragments from MMTV‐infected female Czech II mice into gland‐free mammary fat pads of syngeneic mice. Unique virus–host restriction fragments were detected in the epithelial cells by Southern analysis, demonstrating that the outgrowths were indeed clonal. In addition, several other important conclusions were inferred from these studies: (1) mammary epithelial stem cells were composed of three distinct progenitor populations, one capable of producing all of the epithelial cells, and two downstream progenitors, with limited potency, that could produce either secretory lobules or branching ducts; (2) mammary epithelial stem cells showed ductal growth senescence upon serial transplantation after six generations; (3) the decisions to form a duct vs. a lobule, the extent of ductal growth, and ductal lateral spacing were made through stem‐cell autonomous mechanisms and these mechanisms were transmitted to the epithelial progeny by the clonogenic progenitors; (4) premalignant hyperplastic alveolar nodules and mammary tumours arose from a single clonogenic progenitor cell.
Smith & Chepko (2001) studied the properties and the location of morphologically distinct stem cells among the more differentiated cells within the mammary gland. By ultrastructural studies they defined four types of cells, SLC (small light cells), ULLC (undifferentiated large light cells), DLLC (differentiated large light cells), and LDC (large dark cells). Among these cells, SLC were described as mammary epithelial stem cells based on the presence of mitotic chromosomes, lack of any specialized organelles, and their basal location within the gland. Their nuclei contained dense clumps of heterochromatin, and organelles were small and showed no evidence of specialized function. Furthermore, these cells were present in side‐by‐side homogenous pairs as well as in heterogeneous pairs, suggesting their ability to undergo symmetric as well as asymmetric division. ULLC were also division‐competent, and contained some secretory granules as well as small lipid droplets. They were also considered competent to undergo both symmetrical and asymmetrical division, a characteristic of mammary progenitor cells. These ULLC were said to be the secondary progenitors to the SLC. Increased numbers of both ULLC and SLC appear to be present in some human breast and mouse mammary tumours, suggesting that they may play a role in the origin of mammary cancer.
Stem cells are found in the mammary gland during all stages of development, however, Kay‐Uwe Wagner et al. (Wagner et al. 2002) have discovered a population of progenitors that was parity‐induced. These experiments were accomplished by breeding mice carrying the WAP‐Cre transgene and the floxed Rosa‐LacZ reporter construct. The WAP‐Cre transgene was activated during pregnancy and allowed the identification of surviving LacZ‐expressing lobular progenitors following involution. These investigators showed that a parity‐induced cell population served as progenitors to lobuloalveolar cells upon subsequent pregnancies. Furthermore, the parity‐induced progenitors gave rise to both ductal and lobuloalveolar cells upon serial transplantation. It was postulated that the parity‐induced progenitors might be responsible for the functional adaptation in genetically engineered mice after subsequent pregnancies, as they were able to reverse a lactation‐deficient phenotype in prolactin‐receptor heterozygous mice after subsequent pregnancies. Therefore, these progenitors may contribute to the biological differences observed in the mammary glands of parous versus nulliparous females.
Other investigators have utilized cell‐culture techniques to identify putative stem cells and to study their clonogenic potential in vitro. Pechoux et al. (1999) studied resting human breast cells obtained from reduction mammoplasties. These investigators showed that under special culture conditions, human luminal epithelial cells could give rise to myoepithelial cells, but myoepithelial cells could not produce luminal epithelial cells under any of the conditions tested. These observations were made by separating luminal and myoepithelial cells immunomagnetically, using antibodies against cell‐surface antigens such as MUC‐1 for luminal epithelial cells and CALLA for myoepithelial cells. A culture medium that supported the preferential expansion of the myoepithelial cells (CDM4) or luminal epithelial cells (CDM6) was devised. Luminal and myoepithelial cells retained their characteristics in their respective culture medium. However, when the culture medium was switched to CDM6, the myoepithelial cells remained unchanged, as revealed by the lack of expression of K19, whereas purified luminal epithelial cells placed in CDM4 showed a distinct focal conversion to myoepithelial cells (2–5%), as judged by the loss of K18, K19 expression and the gain of vimentin, CALLA, smooth muscle α‐actin, and β4 integrin expression. It was concluded that the luminal epithelial cells were precursors of myoepithelial cells, not vice versa. In a follow‐up study by the same group of investigators, Gudjonsson et al. (2002) showed that luminal epithelial cells that expressed epithelial specific antigen and sialomucin (ESA+/MUC+) were restricted in their differentiation repertoire, whereas a subpopulation of luminal cells that expressed ESA and lacked MUC expression (ESA+/MUC−) was able to generate itself as well as myoepithelial cells and terminal duct lobular units.
In another set of studies, Stingl and Emerman (2000) used a combination of in vitro colony assays and flow cytometry to identify and characterize mammary epithelial progenitor cells. This study identified three distinct populations of progenitor cells: luminal restricted, myoepithelial restricted, and bipotent. The luminal‐restricted colonies were identified by their compact morphology with a smooth colony boundary and a hollow centre. Mixed colonies were characterized by the presence of a luminal morphology in addition to a surrounding halo of more dispersed, loosely arranged teardrop‐shaped cells. Likewise, myoepithelial restricted colonies were composed of teardrop‐shaped cells only. These morphologies were further confirmed by the expression of K8, K18, and K19 for luminal cells, and K14 for myoepithelial cells. These investigators concluded that the cells possessing a luminal progenitor potential expressed epithelial cell‐adhesion molecule (EpCAM), α6‐integrin, and MUC‐1. The bipotent progenitors were identified by the expression of EpCAM, α6‐integrin, higher levels of the basal cell marker CALLA and a greater ability to efflux the fluorescent dye, rhodamine 123. The bipotent progenitors expressed lower levels of MUC‐1. The myoepithelial progenitors expressed α6‐integrin and lower levels of EpCAM expression.
The principal limitation of these cell‐culture experiments using human mammary cells has been the inability to validate the results obtained in vitro by transplantation back into the cleared mammary fat pad, but the approaches used should be of value when applied to the murine mammary gland. In addition, by mixing with human stromal cells, it may be possible to functionally evaluate these isolated epithelial cell populations in the future by transplantation into the cleared fat pads of immunocompromised murine hosts.
RECENT ADVANCES IN MAMMARY GLAND DIFFERENTIATION MARKERS
The capacity of stem cells to undergo limited proliferation followed by long quiescent periods has been utilized in long‐term label‐retaining experiments to identify and follow stem cells in vivo (Potten & Morris 1988; Cotsarelis et al. 1990; Welm et al. 2002). The principle for these experiments is that stem cells can be labelled during proliferation and observed after long resting states. To label proliferating cells, DNA analogs such as bromodeoxyuridine (BrdU) or 3H‐thymidine are administered to an animal for a period of time, allowing incorporation into the rarely dividing stem cells. Depending on the rate of stem‐cell proliferation in the target tissue, the period for administering DNA analogues may range from hours to weeks. Subsequent to cell labelling, a retaining period occurs where transit‐amplifying and differentiated cells lose their incorporated proliferation marker either through dilution during continued proliferation or cell death. Cells that contain label after the retaining period (which may be several months) are considered long‐term, label‐retaining cells (LRC). LRC have been identified in several tissues including skin, intestine, mammary gland, cornea and trachea (Borthwick et al. 2001; Dulbecco et al. 1982; MacKenzie & Bickenbach 1985; Cotsarelis et al. 1989; Cotsarelis et al. 1990; Welm et al. 2002). The immunolocalization of LRC can identify the stem‐cell niche in addition to tracking the migratory capacity of transit‐amplifying populations (Taylor et al. 2000). These experiments demonstrate the usefulness of label‐retaining techniques to observe and characterize stem‐cell populations in vivo.
The mouse mammary gland undergoes a 5–7‐week growth phase in the immature animal that establishes the ductal architecture (Daniel & Silberstein 1987). This phase of growth, called ductal morphogenesis, occurs at around 3 weeks of age and terminates when the animal becomes sexually mature at around 8–10 weeks of age. Following ductal morphogenesis, the mammary gland remains quiescent until the onset of pregnancy. In the mammary gland, we have used label‐retaining experiments to identify a cell population with proliferative capacity during ductal morphogenesis and long‐term quiescent characteristics in the mature gland (Welm et al. 2002). These experiments have allowed us to characterize the expression of differentiation markers, such as progesterone receptors, in LRC by double‐labelling immunofluorescence. Mice were surgically implanted with minipumps that delivered BrdU continuously for 2 weeks, and tissue was removed periodically for 9 weeks after removal of the pumps. We observed that only a small fraction of mammary gland LRC (9 weeks after removal of BrdU minipumps) expressed the progesterone receptor, whereas 40% of cells that were BrdU+ at the time minipumps were removed were additionally PR+ (Welm et al. 2002). Furthermore, most proliferating cells observed after a short (2 h) BrdU pulse were PR− (Seagroves et al. 2000). These data are similar to those observed for the relationship between DNA labelling and oestrogen receptor (OR) expression (Zeps et al. 1998). Assuming that mammary epithelial cells undergo a linear differentiation pathway, these data would suggest that proliferating cells (PR/OR−) either commit to a PR/OR‐positive pathway and terminally differentiate, or commit to a PR/OR‐negative pathway and remain quiescent. Since PR‐positive cells rarely undergo proliferation, it is feasible that PR is a marker for a committed pathway that gives rise to mature ductal or alveolar cell types (Fig. 1). However, a small fraction of PR+ cells have been observed to proliferate, and a few LRC were PR+. Therefore, it cannot be ruled out that a subpopulation of PR‐expressing cells have stem‐cell activity. It will be necessary to study the regulation of PR during mammary gland differentiation further to determine its utility as a marker of differentiation. These studies may be facilitated by developing mouse models that express fluorescent proteins, such as GFP or CFP under the regulation of the PR promoter.
Figure 1.
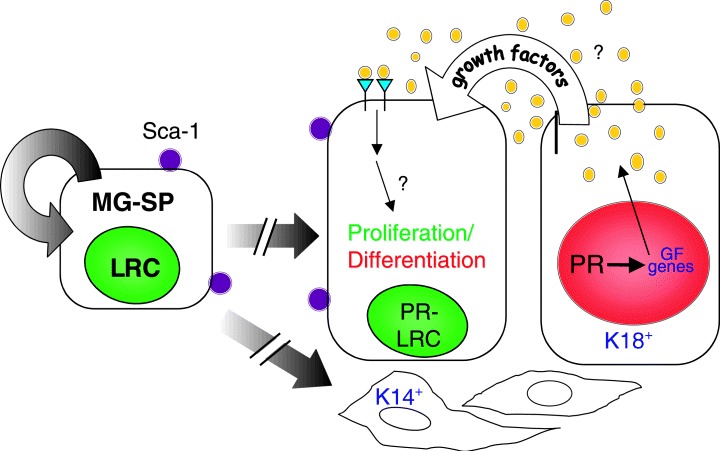
A simplified mammary gland cell lineage model. Mammary gland side population (MG‐SP) cells, defined by their ability to efflux Hoechst dye, are estimated to represent approximately 0.5–3% of the epithelial cells in the virgin mammary gland. These cells also have been identified by their ability to retain BrdU during a longterm labelling and chase protocol, and are therefore designated as label‐retention cells (LRC). By FACS analysis, approximately 75% of the SP cells are stem‐cell antigen (Sca‐1) positive. The SP/Sca‐1/LRC‐positive mammary stem cells have the capability to either self‐renew to generate more stem cells (symmetric proliferation) or to undergo asymmetric proliferation to generate differentiated cells including keratin‐18 progesterone receptor (K18‐PR)‐positive mammary luminal epithelial and keratin 14 (K14)‐positive myoepithelial cells. The broken arrows indicate the presence of a number of potential intermediate progenitors for these cell lineages. The luminal epithelial cells are organized in the mature mammary ducts into nondividing steroid receptor‐positive cells (red nucleus, designated PR+) representing approximately 25% of the ductal epithelium, and proliferative cells (green nucleus, designated PR−, LRC). Local growth factors including IGF‐II, amphiregulin, Wnt 4 and RankL, induced by oestrogen, progesterone and prolactin, act to regulate proliferation of adjacent cells via a paracrine/juxtacrine mechanism. Co‐localization of oestrogen receptor‐alpha, the progesterone receptor and prolactin receptor has been observed in the cells designated PR+. The long‐term labelling and chase experiments suggest that the majority of the stem/progentior cells are steroid receptor‐negative and that the proliferative cells may then give rise to the steroid receptor‐positive cells.
The ability of many stem‐cell populations to actively efflux chemicals out of their cytoplasm allows for another potential method to isolate viable mammary cell populations. This effluxing mechanism results from expression of multidrug resistance proteins that can actively pump several drugs such as the vital dye Hoechst‐33342, mitoxantrone and chemotherapeutics out of a cell (Goodell et al. 1996; Jackson et al. 2002). The effluxing capacity in stem cells may be a result of expression of ABCG, a member of the family of proteins called ATP‐binding cassette (ABC) transporters (Goodell et al. 1996; Zhou et al. 2001). In particular, ABCG2 (also called breast cancer related protein 1 or BCRP1) was first discovered in breast cancer cells resistant to topoisomerase inhibitors (Chen et al. 1990), and is overexpressed in breast cancer and in breast cancer cell lines that have been treated with the antiproliferative agent mitoxantrone (Allen et al. 1999). Bone marrow cells that have the greatest capacity to efflux Hoechst‐33342 from their cytoplasm are known as ‘side population’ (SP) cells because they are observed as a small but distinct population adjacent to the Hoechst‐33342 bright population on FACS profiles (Goodell et al. 1996). SP cells isolated from bone marrow are lin−/Sca+/c‐Kit+ stem cells that can efficiently reconstitute the bone marrow when transplanted into lethally irradiated mice (Goodell et al. 1996). The ability of stem cells to efflux fluorescent dye provides a powerful and inexpensive assay to isolate viable stem cells using FACS. An SP population has been observed in mouse mammary gland primary cultures, and these cells can reconstitute the mammary gland and contribute to ductal and alveolar epithelial populations (Alvi et al. 2002; Welm et al. 2002). Side‐population cells have also been observed in human breast tissue (Alvi et al. 2002). However, due to the cytotoxicity of the Hoechst dye observed in dissociated primary mammary epithelial cells, it has not yet been possible to establish that the MG‐SP population is enriched for a functional stem/progenitor population compared with the non‐SP cells upon transplantation. Both limiting dilution transplantation and clonal analyses are required to prove definitively that isolated SP cells are true mammary stem cells. While the cytotoxicity of Hoechst‐33342 on mammary primary cultures makes this compound a poor candidate for isolating a viable stem‐cell population, other compounds that are also efficiently effluxed from cells by ABC transporters have been described (Robey et al. 2001). These fluorescent compounds include mitoxantrone, BIODPY‐prazosin and rhodamine‐123. Thus, characterizing potential ABC transporter effluxing compounds could facilitate the isolation of mammary epithelial stem cells.
Although sorting by Hoechst efflux ability does not efficiently produce viable mammary epithelial cells, it has been observed that the SP cells are enriched for the cell surface protein Ly‐6a/e or Sca‐1. Sca‐1 is a GPI‐anchored membrane protein that is expressed by murine bone marrow and muscle stem‐cell populations, and may function in T‐cell activation or cell adhesion (Rock et al. 1989). We reasoned that, as in bone‐marrow reconstitution experiments, Sca‐1 should be a useful marker for enriching stem cells from mouse mammary primary cultures. By performing limiting dilution–reconstitution experiments, we observed that a population of cells expressing Sca‐1 in mouse mammary gland primary cultures is required for outgrowth. One thousand Sca‐1‐enriched mouse primary‐culture cells were able to reconstitute the mammary glands of host mice cleared of endogenous tissue (Welm et al. 2002). Moreover, transplantation of Sca‐1‐depleted primary‐culture cells resulted in poor outgrowth rates. These results established that stem or progenitors cells express Sca‐1. As Sca‐1 is expressed on about 20–30% of freshly prepared mouse mammary primary cells (Welm et al. 2002), the Sca‐1‐positive cells in the mammary gland probably comprise a mixed population containing stem, progenitor and possibly some differentiated cells.
In addition to transplantation studies, using a Sca‐1–GFP knock‐in mouse, highly GFP‐positive cells have been visualized in the TEBs at the tips of growing ducts in what appears to be the highly proliferative cap cell layer, and weaker GFP‐expressing cells were scattered along the mature ducts. Consistent with Sca‐1 expression in stem cells, the GFP‐positive cells in the mature virgin gland did not overlap with the progesterone receptor‐positive cells, which represent approximately 25% of the luminal cells (Fig. 1). Further refinement of the Sca‐1 population into smaller populations is possible by double and triple fluorescent cell sorting similar to the methods employed to isolate brain and bone marrow stem cells (Welm B, unpublished data). Eventually, it may be possible not only to define stem and progenitor cells by differential cell surface markers but also to isolate highly enriched populations for further in vitro and in vivo analyses.
One caveat for future experiments is that the percentage of both SP‐ and Sca‐1‐positive cells observed in primary mammary epithelial cells can change as a function of cell‐culture conditions. Thus, it has been reported that the ability to isolate SP cells is lost in primary MECs cultured for 8 days on plastic in the presence of 10% fetal calf serum, growth factors and cholera toxin (Alvi et al. 2002), while the fraction of SP‐ and Sca‐1‐positive cells may actually increase under other conditions where cells are cultured on specific substrata in the presence of different growth factors (Rosen JM, unpublished observations).
MAMMARY GLAND STEM CELLS AND TUMORIGENESIS
When a mammary gland stem cell acquires a mutation, this mutation can be passed on to all of its progeny, resulting in a chimeric ductal network of normal and mutant cells (Tsai et al. 1996). If the mutation results in oncogene activation, then the mutant tissue (whether a stem cell or differentiated cell) is more susceptible to tumorigenesis. In addition to increasing the tumorigenic susceptibility of the gland by propagating a mutation, stem cells may play a role in tumour phenotype. In rats, tumours that arise from carcinogen treatment, such as DMBA and NMU, are molecularly heterogeneous and express luminal and myoepithelial cell markers (Haslam & Bern 1977; Dulbecco et al. 1986). Moreover, rats are more susceptible to tumorigenesis when carcinogens are administrated during ductal outgrowth when stem cells in the terminal end buds are proliferating (Russo & Russo 1978). These data suggest that, in the rat mammary gland, carcinogen‐induced tumorigenesis targets an early progenitor or stem cell. Consistent with this hypothesis we have observed an expansion of the mammary epithelial Sca‐1 progenitor cell population in DMBA‐induced tumours (unpublished observation).
Transgenic mouse lines that are predisposed to mammary tumorigenesis can give rise to morphologically and genetically distinct tumour phenotypes dependent on the oncogenic stimulus used (Cardiff et al. 2000; Desai et al. 2002; http://ccm.ucdavis.edu/tgmouse/colo/lect1c.htm). For example, tumours that arise in MMTV‐PyMT and ‐Neu mice are molecularly more related to each other than they are to tumours induced by MMTV‐c‐Myc (Desai et al. 2002; Rosner et al. 2002). Furthermore, by immunolocalization of mammary differentiation markers, tumours induced by MMTV‐Neu and ‐PyMT express more homogenously the keratin‐18 luminal epithelial marker, while tumours induced by MMTV‐c‐Myc and ‐Wnt‐1 express differentiation markers for luminal, myoepithelial and stem/progenitor cells (Li et al. submitted, and unpublished data). These data suggest that specific oncogenic stimuli can differentially affect stem, progenitor and differentiated cells, resulting in distinct tumour phenotypes. Interestingly, the percentage of Sca‐1‐expressing cells in tumours, as measured by FACs, correlates with stem‐cell cytokeratin markers in several transgenic mammary tumour‐inducing lines (Li et al. submitted, and unpublished data). For example, freshly prepared tumour cells from MMTV‐neu and ‐PyMT mice exhibit a small Sca‐1 population as observed by FACS; however, MMTV‐Wnt1 hyperplasias and tumours contain an abundant Sca‐1 population compared with normal mammary tissue (Li et al. submitted). Loss of heterozygosity for both p53 and PTEN tumour suppressor genes has been observed in MMTV‐Wnt 1 × p53+/– or PTEN+/– mice in both myoepithelial and luminal cells, strongly supporting the concept that tumours arise in a common progenitor (Li et al. submitted). The Sca‐1 tumour population can also be divided into several subpopulations with distinct tumorigenic potential when isolated by FACS and transplanted (unpublished observation). This suggests that the oncogenic event may target subpopulations of mammary epithelial cells and subsequently control tumour growth and morphology (Fig. 2). If, for example, a stem or early progenitor cell is targeted by the Wnt‐1 oncogene, this could explain the presence of myoepithelial, luminal and stem/progenitor cell markers observed in hyperplasias and tumours induced by MMTV‐Wnt‐1 (Fig. 2). Similarly, Neu or PyMT may target a luminal‐committed progenitor cell, resulting in homogeneous keratin‐18 expression and low numbers of Sca‐1‐positive cells (Fig. 2). One possibility is the presence of a tumour cell renewing population that functions in maintaining the organization of the tumour and its cellular constituents (Al‐Hajj et al. 2003). If a specific tumour cell constituent is the primary renewing population, then this has important implications in the design of therapeutics for targeting specific tumour cell populations rather than the whole tumour.
Figure 2.
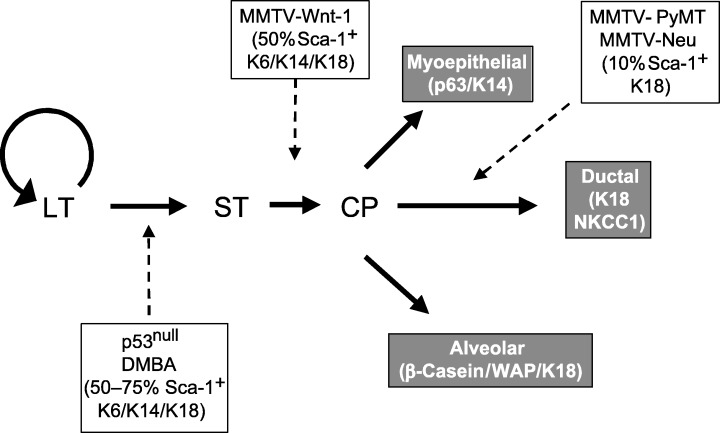
Cellular heterogeneity of mouse models of breast cancer may partially reflect the selection of different progenitors. Analysis of different tumour models has indicated the differential expression of Sca‐1 and K6 as well as K14 in hyperplasias and tumours of differing aetiologies. By analogy to haematopoietic stem cells, LT refers to a long‐term stem‐cell progenitor population, which is capable of self‐renewal, and ST to a short‐term progenitor, which can differentiate into a committed progenitor (CP). The dashed lines indicate the putative origins of mouse mammary tumours induced by the loss of p53, treatment with the carcinogen, DMBA, or overexpression of Wnt‐1, Neu and PyMT under the control of the MMTV‐LTR (Li et al. submitted for publication).
OBSTACLES, UNRESOLVED ISSUES AND FUTURE DIRECTIONS
Many transgenic mouse models for mammary tumorigenesis rely on the use of the MMTV long‐terminal repeat, whey acidic protein or beta‐lactoglobulin promoters to drive expression of an oncogene (Hennighausen 2000). These models have provided important in vivo data on tumorigenesis and normal mammary gland development and are a vital part of breast cancer research. However, when expressing intracellular oncogenes, many of these promoters target differentiated cell types, and not progenitor or stem‐cell populations, which are likely to be the early targets in breast cancer. Oncogenes that are expressed in different cell subpopulations may give rise to tumours with unique characteristics including histology, metastasis potential and proliferative rates. Identifying mammary subpopulation markers and utilizing their promoters in transgenic mice will be critical to study the in vivo function of stem cells in mammary development and tumorigenesis. Thus, the development of new, improved preclinical mouse models for breast cancer may depend on the ability to target specific genetic events to stem cells.
The composition of the mammary gland, containing adipocytes, collagen‐impregnated stroma surrounding ductal epithelium, basement membrane, and tightly interacting epithelial cells does not make it an ideal tissue to produce single‐cell suspensions efficiently. Furthermore, preparing single‐cell suspensions from the mammary gland requires long digestive procedures that may destroy potential cell‐surface epitopes that could be used for sorting. Culturing mammary epithelial cells for several days prior to sorting allows for more rapid dissociation, lower cell death, and better population distinction; however, expression profiles can dramatically change compared with freshly prepared cells. Transplantation of isolated cell populations into cleared fat pads of syngeneic mice remains the main method to validate stem‐cell enrichment from mouse mammary glands. Nevertheless, culturing freshly isolated cell populations and observing differentiation in 3D culture models may provide an alternative technique (Gudjonsson et al. 2002). Extrapolating knowledge from other stem‐cell‐driven organs may identify potential candidate markers more efficiently, and reduce the number of transplantation experiments required to screen enriched mammary epithelial cell populations. An area of potential information is determining any commonality between tissue‐specific stem‐cell pathways including niche interactions and lineage markers (Ivanova et al. 2002; Ramalho‐Santos et al. 2002). At least one common epitope that has been validated, Sca‐1, is found on other stem‐cell populations including blood and muscle, but it will be important to elucidate other cell‐surface markers (unique or common) that may be used to isolate mammary stem cells.
In summary, identification of mammary gland stem‐cell markers should help in our understanding of normal development and provide tools to characterize the role of stem cells in breast cancer. In addition, these studies may provide new therapeutic and diagnostic targets for treatment.
ACKNOWLEDGEMENTS
The authors thank Alana Welm for critical review of the manuscript. These studies were supported by grant U01 CA84243 from the National Cancer Institute. The p53 null Balb/c and DMBA‐induced tumours were kindly provided by Dr Daniel Medina.
The first two authors contributed equally to this review article.
REFERENCES
- Al‐Hajj M, Wicha M, Benito‐Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH (1999) The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 59, 4237. [PubMed] [Google Scholar]
- Alvi AJ, Clayton H, Joshi C, Enver T, Ashworth A, Vivanco MDM, Dale TC, Smalley MJ (2002) Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 5, R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis‐Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science. 284, 770. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL, Lopez‐Casillas F, Massague J (1994) TGF‐beta receptors and actions. Biochim. Biophys. Acta 1222, 71. [DOI] [PubMed] [Google Scholar]
- Barbareschi M, Pecciarini L, Cangi MG, MacRi E, Rizzo A, Viale G, Doglioni C (2001) p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am. J. Surg. Pathol. 25, 1054. [DOI] [PubMed] [Google Scholar]
- Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN, Bhatia M (2001) Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat. Immunol. 2, 172. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, Dick JE (1999) Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J. Exp. Med. 189, 1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH (2001) Evidence for stem‐cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 24, 662. [DOI] [PubMed] [Google Scholar]
- Boulanger CA, Smith GH (2001) Reducing mammary cancer risk through premature stem cell senescence. Oncogene. 20, 2264. [DOI] [PubMed] [Google Scholar]
- Brittan M, Wright NA (2002) Gastrointestinal stem cells. J. Pathol. 197, 492. [DOI] [PubMed] [Google Scholar]
- Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE (2000) The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 19, 968. [DOI] [PubMed] [Google Scholar]
- Cashman JD, Lapidot T, Wang JC, Doedens M, Shultz LD, Lansdorp P, Dick JE, Eaves CJ (1997) Kinetic evidence of the regeneration of multilineage hematopoiesis from primitive cells in normal human bone marrow transplanted into immunodeficient mice. Blood 89, 4307. [PubMed] [Google Scholar]
- Chen YN, Mickley LA, Schwartz AM, Acton EM, Hwang JL, Fojo AT (1990) Characterization of adriamycin‐resistant human breast cancer cells which display overexpression of a novel resistance‐related membrane protein. J. Biol. Chem. 265, 10073. [PubMed] [Google Scholar]
- Chepko G, Smith GH (1997) Three division‐competent, structurally‐distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell. 29, 239. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM (1989) Existence of slow‐cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57, 201. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM (1990) Label‐retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329. [DOI] [PubMed] [Google Scholar]
- Daniel CW, De Ome KB, Young JT, Blair PB, Faulkin LJ, Jr (1968) The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc. Natl Acad. Sci. USA 61, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB (1987) Postnatal development of the rodent mammary gland In: Neville MC, Daniel CW, eds. The Mammary Gland. New York: Plenum Press, p. 3. [Google Scholar]
- Deome Kb FI, Bern HA, Blair PB (1959) Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland free mammary fat pads of female C3H mice. J. Natl Cancer Inst. 78, 751. [PubMed] [Google Scholar]
- Desai KV, Xiao N, Wang W, Gangi L, Greene J, Powell JI, Dickson R, Furth P, Hunter K, Kucherlapati R, Simon R, Liu ET, Green JE (2002) Initiating oncogenic event determines gene‐expression patterns of human breast cancer models. Proc. Natl Acad. Sci. USA 99, 6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R, Allen WR, Bologna M, Bowman M (1986) Marker evolution during the development of the rat mammary gland: stem cells identified by markers and the role of myoepithelial cells. Cancer Res. 46, 2449. [PubMed] [Google Scholar]
- Dulbecco R, Armstrong B, Allen WR, Bowman M (1986) Distribution of developmental markers in rat mammary tumors induced by N‐nitrosomethylurea. Cancer Res. 46, 5144. [PubMed] [Google Scholar]
- Dulbecco R, Henahan M, Armstrong B (1982) Cell types and morphogenesis in the mammary gland. Proc. Natl Acad. Sci. USA 79, 7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves AC, Eaves CJ (1988) Maintenance and proliferation control of primitive hemopoietic progenitors in long‐term cultures of human marrow cells. Blood Cells 14, 355. [PubMed] [Google Scholar]
- Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA (1998) Perturbation of beta1‐integrin function alters the development of murine mammary gland. EMBO J. 17, 2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo . J. Exp. Med. 183, 1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm SL, Seagroves TN, Kabotyanski EB, Hovey RC, Vonderhaar BK, Lydon JP, Miyoshi K, Hennighausen L, Ormandy CJ, Lee AV, Stull MA, Wood TL, Rosen JM (2002) Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobuloalveolar development. Mol. Endocrinol. 16, 2675. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Ronnov‐Jessen L, Bissell MJ, Petersen OW (2002) Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 16, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam SZ, Bern HA (1977) Histopathogenesis of 7,12‐diemthylbenz (a) anthracene‐induced rat mammary tumors. Proc. Natl Acad. Sci. USA 74, 4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld J, Li ML, Brown EL, Sookdeo H, Levesque JP, O'Toole T, Gurney C, Clark SC, Hatzfeld A (1991) Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor beta 1 or Rb oligonucleotides. J. Exp. Med. 174, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L (2000) Mouse models for breast cancer. Breast Cancer Res. 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW (2001) Signaling pathways in mammary gland development. Dev. Cell. 1, 467. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RD (1985) Identification of major cell classes in the developing mammalian nervous system. J. Neurosci. 5, 3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan CJ, Shpall EJ, McNulty O, McNiece I, Dick JE, Shultz LD, Keller G (1997) Engraftment and development of human CD34(+)‐enriched cells from umbilical cord blood in NOD/LtSz‐scid/scid mice. Blood 90, 85. [PubMed] [Google Scholar]
- Humphreys RC, Lydon JP, O'Malley BW, Rosen JM (1997) Use of PRKO mice to study the role of progesterone in mammary gland development. J. Mammary Gland Biol. Neoplasia 2, 343. [DOI] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR (2002) A stem cell molecular signature. Science 298, 601. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Majka SM, Wulf GG, Goodell MA (2002) Stem cells: a minireview. J. Cell Biochem. Suppl. Suppl. 38, 1. [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM (1993) Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Sakakibara S, Imai T, Suzuki A, Nakamura Y, Sawamoto K, Ogawa Y, Toyama Y, Miyata T, Okano H (2000) Musashi‐1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev. Neurosci. 22, 139. [DOI] [PubMed] [Google Scholar]
- Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, Bhatia M (2000) The notch ligand jagged‐1 represents a novel growth factor of human hematopoietic stem cells. J. Exp. Med. 192, 1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH (1995) Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev. Biol. 168, 47. [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125, 1921. [DOI] [PubMed] [Google Scholar]
- Land CE, McGregor DH (1979) Breast cancer incidence among atomic bomb survivors: implications for radiobiologic risk at low doses. J. Natl Cancer Inst. 62, 17. [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell. 60, 585. [DOI] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, Tan LK, Rosen JM, Varmus HE (2003) Differential expression of developmental markers in transgene‐induced mouse mammary cancers correlates with activation of different signaling pathways. Genes Develop. Submitted.
- Lowell S, Jones P, Le Roux I, Dunne J, Watt FM (2000) Stimulation of human epidermal differentiation by delta‐notch signalling at the boundaries of stem‐cell clusters. Curr. Biol. 10, 491. [DOI] [PubMed] [Google Scholar]
- MacKenzie IC, Bickenbach JR (1985) Label‐retaining keratinocytes and Langerhans cells in mouse epithelia. Cell Tissue Res. 242, 551. [DOI] [PubMed] [Google Scholar]
- Maeno M, Mead PE, Kelley C, Xu RH, Kung HF, Suzuki A, Ueno N, Zon LI (1996) The role of BMP‐4 and GATA‐2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis . Blood 88, 1965. [PubMed] [Google Scholar]
- Massague J (1996) TGFbeta signaling: receptors, transducers, and Mad proteins. Cell 85, 947. [DOI] [PubMed] [Google Scholar]
- Meltzer H, Hatton JD, Sang UH (1998) Cell type‐specific development of rodent central nervous system progenitor cells in culture. J. Neurosurg. 88, 93. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A (1999) p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, Rosen JM, Robinson GW, Hennighausen L (2001) Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J. Cell Biol. 155, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H (2002) Stem cell biology of the central nervous system. J. Neurosci. Res. 69, 698. [DOI] [PubMed] [Google Scholar]
- Okano H, Imai T, Okabe M (2002) Musashi: a translational regulator of cell fate. J. Cell Sci. 115, 1355. [DOI] [PubMed] [Google Scholar]
- Pechoux C, Gudjonsson T, Ronnov‐Jessen L, Bissell MJ, Petersen OW (1999) Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev. Biol. 206, 88. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell‐Badge R (1998) A role for SOX1 in neural determination. Development 125, 1967. [DOI] [PubMed] [Google Scholar]
- Pflumio F, Izac B, Katz A, Shultz LD, Vainchenker W, Coulombel L (1996) Phenotype and function of human hematopoietic cells engrafting immune‐deficient CB17‐severe combined immunodeficiency mice and nonobese diabetic‐severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood 88, 3731. [PubMed] [Google Scholar]
- Potten CS, Morris RJ (1988) Epithelial stem cells in vivo . J. Cell Sci. Suppl 10, 45. [DOI] [PubMed] [Google Scholar]
- Ramalho‐Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA (2002) ‘Stemness’: transcriptional profiling of embryonic and adult stem cells. Science 298, 597. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105. [DOI] [PubMed] [Google Scholar]
- Robey RW, Honjo Y, Van De Laar A, Miyake K, Regis JT, Litman T, Bates SE (2001) A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2). Biochim. Biophys. Acta 1512, 171. [DOI] [PubMed] [Google Scholar]
- Rock KL, Reiser H, Bamezai A, McGrew J, Benacerraf B (1989) The LY‐6 locus: a multigene family encoding phosphatidylinositol‐anchored membrane proteins concerned with T‐cell activation. Immunol. Rev. 111, 195. [DOI] [PubMed] [Google Scholar]
- Rosen PP, Groshen S, Saigo PE, Kinne DW, Hellman S (1989) Pathological prognostic factors in stage I (T1N0M0) and stage II (T1N1M0): breast carcinoma: a study of 644 patients with median follow‐up of 18 years. J. Clin. Oncol. 7, 1239. [DOI] [PubMed] [Google Scholar]
- Rosner A, Miyoshi K, Landesman‐Bollag E, Xu X, Seldin DC, Moser AR, MacLeod CL, Shyamala G, Gillgrass AE, Cardiff RD (2002) Pathway pathology: histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am. J. Pathol. 161, 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo IH, Russo J (1978) Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12‐dimethylbenz[a]anthracene. J. Natl Cancer Inst. 61, 1439. [PubMed] [Google Scholar]
- Sakabe H, Yahata N, Kimura T, Zeng ZZ, Minamiguchi H, Kaneko H, Mori KJ, Ohyashiki K, Ohyashiki JH, Toyama K, Abe T, Sonoda Y (1998) Human cord blood‐derived primitive progenitors are enriched in CD34+c‐kit‐cells: correlation between long‐term culture‐initiating cells and telomerase expression. Leukemia 12, 728. [DOI] [PubMed] [Google Scholar]
- Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K, Yasutomi D, Nagata T, Kurihara Y, Uesugi S, Miyata T, Ogawa M, Mikoshiba K, Okano H (1996) Mouse Musashi‐1, a neural RNA‐binding protein highly enriched in the mammalian CNS stem cell. Dev. Biol. 176, 230. [DOI] [PubMed] [Google Scholar]
- Sakakibara S, Okano H (1997) Expression of neural RNA‐binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development. J. Neurosci. 17, 8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM (2000) C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol. Endocrinol. 14, 359. [DOI] [PubMed] [Google Scholar]
- Sell S, Pierce GB (1994) Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab. Invest. 70, 6. [PubMed] [Google Scholar]
- Shillingford JM, Miyoshi K, Flagella M, Shull GE, Hennighausen L (2002a) Mouse Mammary epithelial cells express the NaKCl cotransporter, NKCC1. Characterization, localization, and involvement in ductal development and morphogenesis. Mol. Endocrinol. 16, 1309. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Miyoshi K, Robinson GW, Grimm SL, Rosen JM, Neubauer H, Pfeffer K, Hennighausen L (2002b) Jak2 is an essential tyrosine kinase involved in pregnancy‐mediated development of mammary secretory epithelium. Mol. Endocrinol. 16, 563. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Titley J, Paterson H, Perusinghe N, Clarke C, O'Hare MJ (1999) Differentiation of separated mouse mammary luminal epithelial and myoepithelial cells cultured on EHS matrix analyzed by indirect immunofluorescence of cytoskeletal antigens. J. Histochem. Cytochem. 47, 1513. [DOI] [PubMed] [Google Scholar]
- Smith GH (1996) Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res. Treat. 39, 21. [DOI] [PubMed] [Google Scholar]
- Smith GH, Chepko G (2001) Mammary epithelial stem cells. Microsc. Res. Techn. 52, 190. [DOI] [PubMed] [Google Scholar]
- Smith GH, Medina D (1988) A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J. Cell Sci. 90, 173. [DOI] [PubMed] [Google Scholar]
- Smith GH, Mehrel T, Roop DR (1990) Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ. 1, 161. [PubMed] [Google Scholar]
- Sonnenberg A, Daams H, Van Der Valk MA, Hilkens J, Hilgers J (1986) Development of mouse mammary gland: identification of stages in differentiation of luminal and myoepithelial cells using monoclonal antibodies and polyvalent antiserum against keratin. J. Histochem. Cytochem. 34, 1037. [DOI] [PubMed] [Google Scholar]
- Soyal S, Ismail PM, Li J, Mulac‐Jericevic B, Conneely OM, Lydon JP (2002) Progesterone receptors – animal models and cell signaling in breast cancer: Progesterone's role in mammary gland development and tumorigenesis as disclosed by experimental mouse genetics. Breast Cancer Res. 4, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl JEC, Emerman JT (2000) Characterization of Normal Human Breast Epithelial Cell Subpopulations Isolated by Fluorescence Activated Cell Sorting and Their Clonogenic Growth in vitro. New York: Kluwer Academic/Plenum Publishers. [Google Scholar]
- Taipale J, Beachy PA (2001) The Hedgehog and Wnt signalling pathways in cancer. Nature 411, 349. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM (2000) Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Lu Y, Nichols PW, Zlotnikov G, Jones PA, Smith HS (1996) Contiguous patches of normal human mammary epithelium derived from a single stem cell: implications for breast carcinogenesis. Cancer Res. 56, 402. [PubMed] [Google Scholar]
- Tulina N, Matunis E (2001) Control of stem cell self‐renewal in Drosophila spermatogenesis by JAK‐STAT signaling. Science 294, 2546. [DOI] [PubMed] [Google Scholar]
- Varnum‐Finney B, Xu L, Brashem‐Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID (2000) Pluripotent, cytokine‐dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 6, 1278. [DOI] [PubMed] [Google Scholar]
- Vormoor J, Lapidot T, Pflumio F, Risdon G, Patterson B, Broxmeyer HE, Dick JE (1994) Immature human cord blood progenitors engraft and proliferate to high levels in severe combined immunodeficient mice. Blood 83, 2489. [PubMed] [Google Scholar]
- Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH (2002) An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 129, 1377. [DOI] [PubMed] [Google Scholar]
- Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA (2002) Sca‐1 (pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev. Biol. 245, 42. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F (1999) p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714. [DOI] [PubMed] [Google Scholar]
- Zeps N, Bentel JM, Papadimitriou JM, D’Antuono MF, Dawkins HJ (1998) Estrogen receptor‐negative epithelial cells in mouse mammary gland development and growth. Differentiation 62, 221. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kalderon D (2001) Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side‐population phenotype. Nat. Med. 7, 1028. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Watt FM (1999) Beta‐catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development 126, 2285. [DOI] [PubMed] [Google Scholar]


