Abstract
A novel surface plasmon resonance (SPR) biosensor which is capable of monitoring proteomic biomarker secretion from living cells is reported here. Vascular endothelial growth factor (VEGF) secretion from living SKOV-3 ovarian cancer cells was measured for concept demonstration.
SPR based biosensing has been a very hot topic drawing substantial research interests in the past decade. Promising biomedical applications of SPR have also been widely studied such as detection of binding activity between cells, proteins, DNA and even small inorganic molecules.1-5 The principle of SPR biosensors is the measurement of refractive index changes at a plane interface between two media with dielectric constants of opposite signs, a dielectric and a metal, such as gold. SPR can be excited when a wedge of polarized light is directed towards the glass face of the sensor surface under the condition of total internal reflection. The resonant angle at which a minimal intensity of reflected light occurs is a function of the local refractive index at or near the gold surface. Such refractive index changes associate intimately with the adsorption or desorption of molecules from the surface, and thus one can expect its great potential in biorecognition measurements.6 This is a newly emerged technique for biomarker detection that is sensitive, fast and realtime. To date, all previously established SPR based sensing platforms have been limited to detection of analyte in a prepared sample.7-9 In these strategies, collection of analytes from cell culture media, purification and pretreatment of analytes are usually required for the purposes of cellular exocytosis and cellular signaling pathways studys.10, 11 These redundant steps are time consuming, and also introduce unpredictable errors to the experiments. Therefore, it is desirable to find an alternative method for direct measurement of secretions from living cells.
Amoung all types of cellular secretions, biomarkers are no doubt the most significant ones for clinical, medical and biochemical applications. Biomarker based cancer diagnosis and treatment on the molecular level have emerged recently. Compared to traditional antineoplastic solutions, such as chemotherapy, radiation therapy, and cryosurgery etc., biomarker therapy revealed mild side effects in clinical studies.12 Proteomic biomarkers are widely involved in the development of many types of cancer. Like all other types of human cells, cancer cells also rely on a constant oxygen supply to maintain their cellular activity. A tumor larger than a millimeter will starve itself of oxygen and energy unless new blood vessels are built to provide a supply. For this reason, many cancer cells employ the normal processes of angiogenesis in order to build their own blood supply.13
Vascular endothelial growth factor (VEGF) is a widely studied angiogenic signal protein biomarker produced by oxygen-hungry cells to promote growth of blood vessels.14 It binds to specialized receptors on the surfaces of endothelial cells and directs them to build new vessels. Some types of tumor cells produce abnormally large amounts of VEGF or block the action of angiogenesis inhibitors. This action is termed as “angiogenic switch”, giving the ability of metastasis to the tumor, since a custom-made blood supply can be constructed wherever new tumors begin to grow.15
In this work, we report a new concept of a SPR biosensing system for realtime VEGF secretion study. A novel design by integrating a mini cell culture module to the SPR system will be introduced. Unlike the traditional configuration of SPR systems for biomarker detection, living cells are cultured on the ceiling of a customized SPR flow cell chamber, and biomarker secretion from cells is rapidly monitored by an immune SPR sensing device (Scheme 1). As a model system, SKOV-3 ovarian cancer cell line is used to demonstrate VEGF secretion livetime measurement. To the best of our knowledge, this new SPR based biosensing strategy for direct measurement of biomarker from living cell has not been reported previously.
Scheme 1.
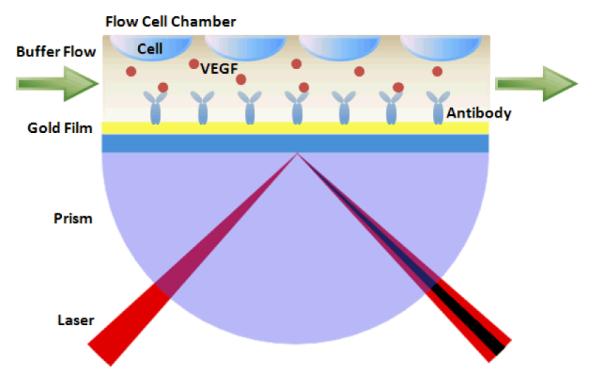
Configuration of the SPR based biosenso r integrating a mini cell culture module for direct measurement of biomarker from living cells.
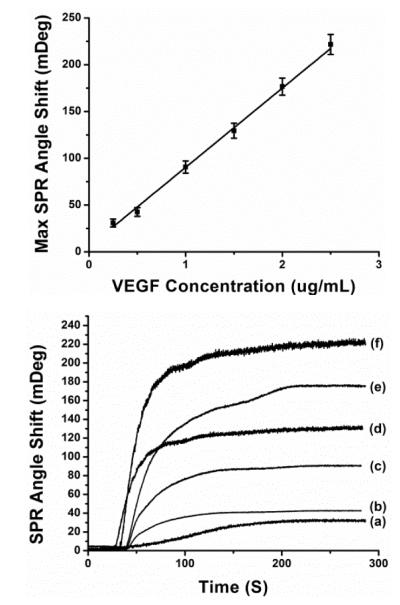
Initially, reproducibility and linearity of the functionalized Au sensing surface were examined using different concentrations of VEGF sample. Experimentally, carboxylic groups were immobilized on the sensing surface and activated in the SPR microfluidic system using a previously reported method.16 Following this, activated SPR chip was immobilized by injecting 50μg/mL protein G solution to capture the Fc portions of the antibody in order to assure proper antibody orientation.17 50μg/mL monoclonal anti-VEGF was bounded on top of the protein G layer as the biorecognition ligand. All injections to the SPR microfluidic system were performed with a 20 μL/min rate. SPR experiments were performed in phosphate buffered saline (PBS) as flowing buffer, and each solution was also prepared in PBS (pH=7.4) unless otherwise specified. We investigated the sensor performance for reproducibility and linearity using VEGF samples with 8 different concentrations (0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4μg/mL). Fig. 1A shows the time resolved SPR spectra in response to VEGF binding (3, 4μg/mL samples not shown). VEGF binding can be clearly characterized by the SPR angle shift upon introduction of VEGF sample solution. Fig. 1B depicts the calibration curve obtained by linear fit of SPR response to different concentrations of VEGF samples. The SPR sensor allows real-time and sensitive VEGF detection within a linear dynamic range 0.1-2.5μg/mL. The maximum value of the inter-assay relative standard deviations was 13.6% (n = 4). This indicates that our detection strategy offers an acceptable reproducibility towards the detection of VEGF.
Fig. 1.
(A. upper figure) SPR sensor responses to VEGF sol ution at different concentration levels: (a) at 0.25 μg/mL, (b) at 0.5 μg/mL, (c) at 1 μg/mL, (d) at 1.5 μg/mL, (e) at 2 μg/mL and (f) at 2.5 μg/mL. (B. lower figure) Calibration curve: the linear relationship between change of SPR angle shift and VEGF concentration.
For the purpose of measuring VEGF secretion directly from living cancer cells, we first demonstrated cell viability in the SPR flow chamber. The PDMS chamber gasket (Biosensing Instrument Inc.) was detached from the SPR flow chamber. Several drops of 0.1% w/v gelatin solution made by boiling distilled water was applied onto the PDMS gasket to cover the whole surface.18 The gasket was then dried for 12h in a biological fume hood to prevent contamination. Human ovarian carcinoma SKOV-3 cells were cultured on the gelatin coated gasket in McCoy’s 5A medium added with 1% penicillin and 10% fetal bovine serum, and kept in a 37°C cell incubator with a humidified atmosphere of 5% CO2 and 95% air. SKOV-3 cells were then stained with MitoTracker Red CMXRos dye (579/599 nm) and Hoechst 33342 dye (350/461 nm) after 48h incubation. Same cell culture was repeated on uncoated PDMS gasket and tissue culture plate as control experiments. Fluorescent imaging was performed using a previously reported method in order to examine cell confluency on each substrate.19 As shown in Fig. S1, cell count on the tissue culture plate, gelatin coated gasket and uncoated gasket are 174, 218 and 76, respectively. Since the gelatin coated gasket showed significant enhancement of cell attachment compared to the uncoated gasket, it is a suitable substrate for the living cell experiment in the SPR flow chamber.
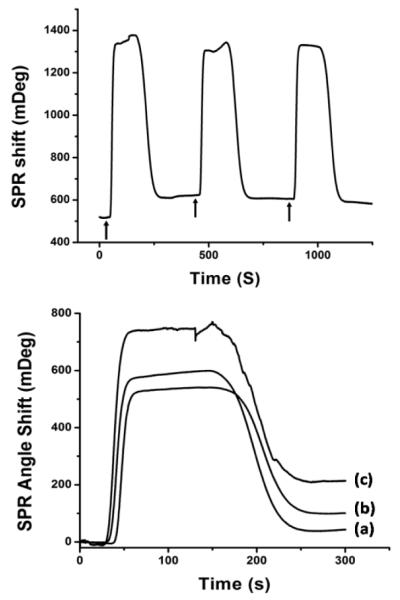
In order to demonstrate direct measurement of VEGF from living carcinoma cells, SKOV-3 cells were cultured for 48h on gelatin coated gaskets using the aforementioned method. Same functionalization protocol described above will be used for immobilization of monoclonal anti-VEGF antibody on the SPR sensing chip. The PDMS flow chamber gasket with SKOV-3 cell culture was then removed from cell culture media and throughly rinsed with Kreb’s buffer to remove cell culture media and unattached cells. Following this, the PDMS gasket with cells was mounted on the SPR flow chamber, and SPR flowing buffer was changed from PBS (pH=7.4) to Kreb’s buffer (pH=7.4) in order to maintain cell viability during experiments. After resuming buffer flow, 500μm Ca2+ ionophore (A23187) was injected to induce rapid exocytosis of VEGF from the SKOV-3 cells. Fig. 2A shows the time resolved SPR response upon Ca2+ ionophore injections. Each arrow in the figure indicates an injection of Ca2+ ionophore. An 87 ± 6 mDeg SPR angle shift was observed after the first injection. However, the following two injections did not induce obvious angle shift. It seems that all intracellular VEGF was released during the first stimulation. According to our calibration curve for different concentration VEGF samples, SKOV-3 cells released about 1μg/mL VEGF during the stimulation.20
Fig. 2.
(A. upper figure) SPR sensor responses to living SKOV-3 cells secretion stimulated by injections of 500μm Ca2+ ionophore (A23187). Each arrow indicates an injection of Ca2+ ionophore. (B. lower figure) SPR sensor responses to stimulated VEGF secretion from groups of cells with different cell number. VEGF secretion from: (a) 8×104 cells induced 43.4 mDeg SPR angle shift; (b) 16×104 cells induced 100.6 mDeg SPR angle shift; (c) 32×104 cel ls induced 214.2 mDeg SPR angle shift.
We also performed a cell number study to investigate the relationship between the amount of VEGF secretion and total cell number. Different numbers of cells (5×104, 10×104, 20×104) were seeded on three similar PDMS gaskets and cultured under the same conditions as previously mentioned. After 48h, cell numbers reached 8×104, 16×104 and 32×104, respectively. SPR measurements were then performed on three samples with different cell numbers. Fig. 2B shows the SPR responses to induced VEGF secretion from different numbers of cells. From the VEGF concentration detected by SPR, cell number and flow chamber volume (2μL), we calculated that the amount of VEGF released from each cell is 0.0137 ± 0.0012 pg. Due to the capacity limitation of our SPR flow chamber, the sample of 32×104 cells had reached 100% confluency inside the chamber. Therefore, we were not able to further investigate VEGF release amount from larger group of SKOV-3 cells. However, this sensing platform has provided a strategy for accurate prediction of carcinoma cell number.
In conclusion, this work demonstrates the new concept of a SPR biosensor for biomarker study. On the basis of integration of a mini cell culture system within the traditional SPR sensing platform, this biosensor is capable of direct measurement of VEGF biomarker secretion from living SKOV-3 carcinoma cells. Because the configuration of this SPR biosensor mimics the in vivo microenvironment of the VEGF signaling pathway, this platform possesses great potential on cellular signaling pathways study and antineoplastic drug development. By modifying the surface functionalization of the SPR assay, this biosensor might open up new horizons for detection and analysis of biomarker from living cells and tissue for different diseases.
Supplementary Material
Acknowledgments
We thank FIU Graduate School Doctoral Evidence Acquisition Fellowship for financial support. This research and development project was partially supported by the grant NIH R15 ES021079-01 and W81XWH-10-1-0732 by U.S. Army Medical Research & Materiel Command (USAMRMC) and the Telemedicine & Advanced Technology Research Center (TATRC).
Notes and references
- 1.Ly N, Foley K, Tao N. Anal. Chem. 2007;79:2546. doi: 10.1021/ac061932+. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AD, Ladda J, Yu Q, Chen S, Homola J, Jiang S. Biosens. Bioelectron. 2006;22:752. doi: 10.1016/j.bios.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Su X, Wu Y, Knoll W. Biosens. Bioelectron. 2005;21:719. doi: 10.1016/j.bios.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto K, Torimaru A, Ishitobi S, Sakai T, Ishikawa H, Toko K, Miura N, Imato T. Talanta. 2005;68:305. doi: 10.1016/j.talanta.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 5.Forzani ES, Zhang H, Chen W, Tao N. Environ. Sci. Technol. 2005;39:1257. doi: 10.1021/es049234z. [DOI] [PubMed] [Google Scholar]
- 6.Beyene HT, Chakravadhanula VSK, Hanisch C, Elbahri M, Strunskus T, Zaporojtchenko V, Kienle L, Faupel F. J. Mater. Sci. 2010;45:5865. [Google Scholar]
- 7.Huang X, Gottstein C, Brekken RA, Thorpe PE. Biochem. Bioph. Res. Co. 1998;252:643. doi: 10.1006/bbrc.1998.9717. [DOI] [PubMed] [Google Scholar]
- 8.Tiedemann BV, Bilitewski U. Biosens. Bioelectron. 2002;17:983. doi: 10.1016/s0956-5663(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Lee HJ, Corn RM. Anal. Chem. 2007;79:1082. doi: 10.1021/ac061849m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy PJ, Sadhu S, Ray S, Srivastava S. Clin. Lab. Med. 2012;32:47. doi: 10.1016/j.cll.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Altintas Z, Uludag Y, Gurbuz Y, Tothill IE. Talanta. 2011;86:377. doi: 10.1016/j.talanta.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Cancer. Res. 1997;57:4593. [PubMed] [Google Scholar]
- 13.Folkman J. Nat. Med. 1996;1:27. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N, Gerber HP, LeCouter J. Nat. Med. 2003;9:669. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Cell. 2000;100:57. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 16.Su X, Wu YJ, Robelek R, Knoll W. Langmuir. 2005;21:348. doi: 10.1021/la047997u. [DOI] [PubMed] [Google Scholar]
- 17.Oh BK, Kim YK, Park KW, Lee WH, Choi JW. Biosens. Bioelectron. 2004;19:1497. doi: 10.1016/j.bios.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Leclerc E, Sakai Y, Fujii T. Biomed. Microdevices. 2003;5:109. [Google Scholar]
- 19.Lei T, Srinivasan S, Tang Y, Manchanda R, Nagesetti A, Fernandez-Fernandez A, McGoron AJ. Nanomed-Nanotechnol. 2011;7:324. doi: 10.1016/j.nano.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesiano S, Ferrara N, Jaffe RB. Am. J. Pathol. 1998;153:1249. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.