Abstract
Over the decades, our understanding of estrogen receptor (ER) function has evolved. Today we are confronted by at least two nuclear ERs: ERα and ERβ; and a number of putative membrane ERs, including ERα, ERβ, ER-X, GPR30 and Gq-mER. These receptors all bind estrogens or at least estrogenic compounds and activate intracellular signaling pathways. In some cases, a well-defined pharmacology, and physiology has been discovered. In other cases, the identity or the function remains to be elucidated. This mini-review attempts to synthesize our understanding of 17β-estradiol membrane signaling within hypothalamic circuits involved in homeostatic functions focusing on reproduction and energy balance.
Keywords: 17β-estradiol, estrogen receptor alpha, estrogen receptor beta, GPR30 (GPER1), Gαq-coupled membrane estrogen receptor, mGluR1 receptor, GnRH neuron proopiomelanocortin (POMC) neuron
Introduction
17β-estradiol (E2) modulates circuits regulating reproduction, energy balance, temperature, circadian rhythms, stress and provides neuroprotection in cases of neurodegenerative diseases and trauma. In addition, ovarian and neurosteroidal E2 are involved in structural plasticity in the hippocampus that influences cognitive behaviors [1]. E2 signaling is one of the most fundamental aspects of reproduction. In females, it is the basis of positive and negative feedback within the hypothalamic-pituitary-ovarian axis. E2 produced by the ovary signals to the brain the endocrine status of gonads and activates circuits that regulate ovulation. Through the activation of a neural-glial network, E2 induces the release of gonadotropin-releasing hormone (GnRH) and luteininzing hormone (LH), and stimulates sexual behavior. Thus, E2 increases the probability that the ovulated egg will be fertilized. To achieve these effects, E2 binds to and activates estrogen receptors (ERs).
Our ideas about what constitutes an ER are constantly evolving as we understand more about the actions of E2, especially in the nervous system. Before ER proteins were characterized and cloned, ERs were defined by their ability to bind estrogens, and elicit a specific response [2]. Early, ER was considered a cytosolic receptor that upon E2 binding underwent a conformational change and translocation to the nucleus where it interacted with DNA to regulate the expression of particular genes through DNA motifs known as the estrogen (receptor) response element (ERE). It became clear that even in the absence of E2, ERs were located in the nucleus, suggesting that ERs were synthesized in the cytoplasm, but were preferentially transported back into the nucleus. Upon cloning, the nuclear ER was determined to be two proteins ERα and ERβ, coded by different genes: ESR1 and ESR2, respectively [3;4]. These proteins share the same modular structure such that both bind E2 and have significant sequence homology, especially in their DNA and ligand binding domains. This bolstered the concept that ERs share a common motif and are members of a nuclear receptor family. To paraphrase Toran-Allerand [5], when there were only the two nuclear receptors, ERα and ERβ, life seemed simple. However, even in those “simple” times, it was known that ERs could interact with other transcription factors, namely Fos and Jun, which bind DNA at the activator protein-1 (AP-1) site, to regulate transcription independent of EREs [6].
Another story was developing in parallel. Early observations implicated E2 in rapid, actions in a number of neuronal and non-neuronal cells. For example, E2 membrane signaling rapidly increased levels of cAMP in the uterus [7], altered neuronal firing of hypothalamic neurons [8] and augmented the release of neuropeptides [9]. In the past two decades, there has been an explosion in the discovery of putative membrane ERs that mediate E2 membrane signaling. Many have been variants of ESR1 and ESR2, but others appear to be completely novel proteins Several of these have been proposed as membrane ERs (mERs), including classical ERα and ERβ or splice variants [10–12], G protein coupled receptor 30 [13], a Gq-mER [14;15] and ER-X [16]. In addition, it has become clear that mERs can interact with other membrane receptors including insulin-like growth factor-1 [17] receptor and metabotropic glutamate receptors [18]. These mER candidates activate a variety of signaling pathways including phospholipase C, protein kinase C (PKC), protein kinase A (PKA) and MAP kinase signaling cascades [19–21]. As a starting point, we will stipulate that all mERs bind 17β-estradiol. While it would be very helpful to have a universal mER antagonist, at present none of the putative antagonists appears to be the “silver bullet” naloxone has been for the opioid receptor field [22]. The most reliable ER antagonist has been ICI 182, 780 (Fulvestrant), but some have noted agonist properties, especially at the ER-X putative mER [16]. This review is an attempt to synthesize our understanding of mERs in hypothalamic functions, to see whether a more complete picture of mERs will emerge.
E2 membrane signaling and reproduction
E2 membrane signaling and GnRH secretion
An acute, direct effect of E2 on neuronal activity in GnRH neurons from the guinea pig arcuate nucleus of the hypothalamus (ARH) was first described over twenty-five years ago [23] (reviewed in [19]). E2 rapidly hyperpolarizes GnRH neurons in guinea pigs via activation of inwardly-rectifying K+ channels [23;24]. In mice, physiological concentrations (picomolar) of E2 rapidly augment KATP channel activity to hyperpolarize GnRH neurons via PKC and PKA signaling pathways, which are also activated by the selective Gq-mER ligand STX [25]. The K+ channel-mediated hyperpolarization is potentially involved in recruitment of excitatory channels that are critical for burst firing of GnRH neurons including T-type calcium channels [26;27]. Nanomolar concentrations of E2 enhance action potential firing by modulating intrinsic afterhyperpolarizing and afterdepolarizing potentials via a PKA-dependent mechanism involving ERβ [28]. When synaptic input is not blocked, picomolar concentrations of E2 inhibit action potentials via an ERα-dependent mechanisms [28]. In addition, E2 via ERβ and/or GRP30 rapidly potentiates high-voltage-activated Ca2+ currents (L- and R-type Ca2+ channels) suggesting that Ca2+ signaling is also a target for E2 membrane signaling GnRH neurons [29].
In primate and mouse olfactory placode GnRH (immature) neurons, E2 modulates Ca2+ oscillations, which synchronize with a periodicity of approximately 60 minutes [30–32], a rhythm similar to the pulsatile GnRH release [33;34]. Furthermore, nanomolar concentrations of a membrane-delimited E2 (E2-dendrimer) alter the patterns of Ca2+ oscillations in primate GnRH neurons [35]. The E2 membrane signaling modulation of Ca2+ oscillations in primate GnRH neurons is suppressed by pertussis toxin (PTX) treatment, attenuated by knockdown of GPR30 mRNA and partially mimicked by the GPR30 agonist G1 [36]. Ca2+ oscillations are blocked by ICI 182,780, mimicked by E2-BSA and blocked by PTX in immature mouse GnRH neurons [31;32]. However, in adult mouse GnRH neurons, nanomolar concentrations of E2 increase Ca2+ transients via presumably presynaptic GABA input [37]. The E2-mediated effects on Ca2+ oscillations are maintained in ERβKO mice [37]. However, E2 activation of cAMP response element-binding protein (CREB) in mouse GnRH neurons is absent in ERβKO mice [38]. In addition, Terasawa and colleagues have found that GPR30 and the Gq-coupled mER are also involved based on the findings that G1 and STX increase calcium oscillations and GnRH release from monkey placode neurons [36;39].
Although E2 membrane signaling may be involved in negative feedback, it has been difficult to put ERβ-, GPR30- or Gq-mER-dependent signaling into a physiological context of estrogen positive feedback, which is absent in mice with either global or neuronal-specific ERα knockdown [40]. These observations prima facie suggest that activation of female reproduction requires ERα-mediated E2 actions in neurons. However, these gene deletion experiments may not necessarily mean that only ERα is involved. Clearly ERα is a transcription factor affecting hundreds of genes important for cell signaling [41;42]. Some of these genes may be essential for a mER-initiated response that normally contributes to female reproduction but is defective in ERαKO mice.
In addition to the idea that E2 directly influences neurons to elicit both positive and negative feedback on GnRH neuronal activity, recent evidence supports the idea that E2 acts through a glial-neuronal network that provides an appropriate local hormonal environment for an LH surge [43;44]. This hypothesis rests on the observation that E2 facilitates progesterone synthesis in mature hypothalamic astrocytes, which if prevented abrogates estrogen positive feedback of the LH surge in rodents [43–46]. In astrocytes, E2 membrane signaling is dependent on the interaction of ERα and mGluR1a, which induces a rapid release of IP3 receptor-sensitive Ca2+ stores required for progesterone synthesis [47–49]. At physiologically relevant (picomolar) concentrations, E2 elevation of [Ca2+]i is blocked with LY367,385 ((S)-(+)-a-Amino-4-carboxy-2-methylbenzeneacetic acid), a mGluR1a antagonist. While glutamate is not required for E2 action, DHPG ((S)-3,5-dihydroxyphenylglycine), an mGluR1a agonist, augments the Ca2+ and progesterone responses to E2 stimulation in vitro [49;50]. These results suggest that elevation of local glutamate, presumably through neuronal activity, increases the amount of progesterone released. This would potentially generate a larger estrogen positive feedback response. Interestingly, STX also increases Ca2+ release and stimulates progesterone synthesis in primary adult hypothalamic cultures, mimicking the actions of E2 [51]. The activation of a novel Gq-mER signaling pathway by STX is blocked by the mGluR1a antagonist, LY367,385, suggesting convergent E2 membrane signaling in astrocytes (reviewed in [44]).
Estrogen membrane signaling and sex behavior
Traditionally nuclear-initiated E2 signaling was considered sufficient for female sexual receptivity - lordosis in rodents. This behavior characterized by the ventral arching of the spine in response to mounting by a male is displayed during behavioral estrus and is controlled by E2 through a complex hypothalamic circuitry involving the ARH, medial preoptic nucleus (MPN) and the ventromedial nucleus of the hypothalamus (VMH) [50;52]. Within the past decade, it has become clear that in addition to the direct nuclear actions of E2 in these various nuclei, membrane-initiated signaling is important for sexual behavior. One thought is that the initial E2 membrane signaling activates a signaling cascade (PKA and PKC) in VMH neurons that augments the nuclear-mediated E2 signaling and lordosis behavior [53]. One mechanism highlights the potential importance of growth factors mediating actions of E2. E2 activates IGF-I, IGF-I receptors and induces the association between IGF-1 receptors and ERα [17;54–56], leading to phosphatidylinositol 3-kinase (PI3K) and protein kinase B/Akt activation and facilitation of lordosis behavior [55–57].
A model of E2 membrane signaling that explains both facilitatory and inhibitory actions proposes that classical ERα or ERβ at the cell membrane transactivate mGluRs to initiate cell signaling (Figure 1) [18;21]. This ERα-mGluR interaction regulates the hypothalamic circuitry controlling female sexual receptivity [58]. ERα and mGluR1a are co-expressed in ARH cells and can be co-immunoprecipitated from membrane fractions [49;58;59]. Blocking either ERs or mGluR1a in the ARH prevents the rapid PKC phosphorylation and activation of MPN projecting proopiomelanocortin (POMC, the precursor of β-endorphin) neurons that are responsible for activating/internalizing μ-opioid receptors (MOR) [60–62]. Similarly, activation of ARH neurons with a membrane-impermeant E2 or DHPG stimulates MOR internalization [58]. These MPN MOR neurons, in turn, project to the VMH (Sinchak et al., unpublished findings), and the transient activation of MOR is necessary for full sexual receptivity that is apparent 30–48 hrs after E2 treatment [63]. While full sexual receptivity also requires E2-induced gene transcription, one question about membrane-initiated E2 action is how the transient, E2-induced activation and internalization of MOR in the MPN facilitate full sexual receptivity at the later time points? One possible explanation is that MOR activation produces a transient inhibition of the MPN neurons that project to the VMH. These neurons rebound from a hyperpolarized state with facilitated firing that excites VMH neurons, ultimately facilitating sexual receptivity.
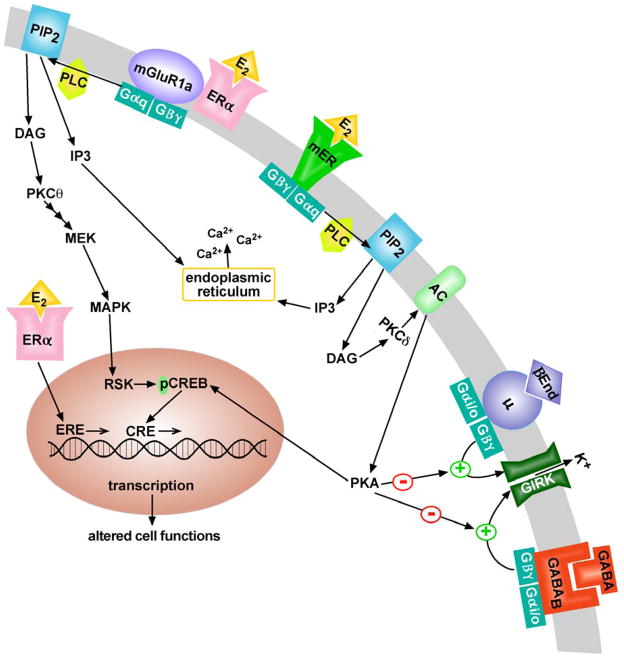
Figure 1.
Proposed schema of E2 signaling in POMC neurons: As in other non-neural cells, E2 binds to intracellular ERα to activate genes via estrogen-response elements (EREs). In addition, E2 binds to ERα localized at the neuronal (plasma) membrane and activates group 1 metabotropic glutamate receptor (mGluR1a) signaling. Activation of mGluR1a causes Gαq stimulation of phospholipase C (PLC), which leads to the hydrolysis of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) to inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). IP3 releases internal calcium (Ca2+) stores, while DAG activates protein kinase C-theta (PKCθ) leading to mitogen-activated kinase kinase (MEK) to mitogen-activated kinase (MAPK) to ribosomal S6 kinase (RSK)-induced phosphorylation of cAMP response element-binding protein (CREB). pCREB will activate genes expressing CREB-response elements (CREs). Also, E2 activates a membrane-associated ER (mER) that is Gαq -coupled (Gq-mER) to activate PLC generating IP3 and DAG. Ca2+ is released from the endoplasmic reticulum by IP3, and DAG activates protein kinase C-delta (PKCδ). Through phosphorylation, adenylyl cyclase (AC) activity is upregulated by PKCδ. The generation of cAMP activates PKA, which through phosphorylation can rapidly uncouple GABAB and μ-opioid (μ) receptors from G protein-coupled, inwardly rectifying K+ (GIRK) channels. Gq-mER-mediated activation of PKA can also phosphorylate CREB. Therefore in addition to the direct effects on membrane excitability, both mGluR1 and mER signaling pathways can alter gene transcription through CREB response elements (CREs) on genes.
In the rat ARH, E2 activation of POMC neurons involves the activation of the NPY-Y1 receptor [62]. While a direct E2 action on POMC neurons cannot be ruled out, one hypothesis is that E2 activates the ERα-mGluR1a complex in the ARH initiating multiple signaling pathways, including a Ca2+-independent PKCθ [64]. Activating PKCθ in the ARH appears to be necessary for lordosis behavior since blocking PKCθ in the ARH abrogates MOR internalization, and prevents lordosis behavior [64]. Activation of protein kinases is critical for not only lordosis behavior [53;64–66] but also for feeding behavior (described below). These data collectively suggest that the membrane-initiated interactions of the ERα-mGluR1a complex via a PKC-mediated pathway are a component of the E2 control of MOR internalization in the MPN and the regulation of lordosis behavior.
Estrogen membrane signaling and energy homeostasis
Besides reproduction, E2 controls a number of hypothalamic-regulated autonomic functions including energy homeostasis, the balance between energy intake and energy expenditure. E2 signaling via ERα is a necessary component of the regulation of energy homeostasis [67]. A loss-of-function mutation in ERα has a clear metabolic phenotype in man with expression of type 2 diabetes, hyperinsulinemia and obesity [68]. However, global reinstatement of an ERα that is lacking the ERE targeting domain is sufficient for “rescuing” the metabolic deficits in mice [69]. These findings suggest an important role for non-ERE mediated E2 signaling. Moreover, brain-specific knockout of ERα causes hyperphagia and hypometabolism, and selective knockdown of ERα in POMC neurons recapitulates the hyperphagic phenotype in female mice [70]. A caveat is that POMC-Cre is expressed also in progenitor neurons that are destined to become NPY and perhaps other hypothalamic neurons [71]. Thus, the neuronal site of action of ERα in the control of energy homeostasis remains ambiguous.
Besides the nuclear-initiated ERα signaling, compelling results point to a role for estrogen membrane signaling in the control of energy homeostasis. For example, E2 attenuates food intake within 4–6 hrs of administration into the third ventricle in fasted, ovariectomized rats and mice [72;73]. A Gq-mER signaling pathway has been elucidated in hypothalamic POMC neurons that is activated within minutes [14;15;74]. This pathway was first characterized in guinea pigs, and later shown in wild type (C57BL/6), ERα/β double-knockout and GPR30 knockout mice [14;74;75]. This Gq-mER signaling pathway is blocked by the anti-estrogen ICI 182,780 [14;15], which at present is a sine qua non for an ER-mediated response. Gq-mER through PLC-PKC-PKA pathways attenuates the GABAB receptors and MOR activation of G protein-coupled inwardly rectifying K+ (GIRK) channels (Figure 1). The resulting attenuation of inhibitory presynaptic inputs increases the excitability of the anorectic POMC neurons.
Moreover, the high affinity (subnanomolar affinity for E2 [14]) Gq-mER is selectively activated by STX, an analogue of 4-OH tamoxifen, which has an even greater (20-fold) affinity for Gq-mER and no measureable binding affinity for classical ERs [76]. STX, similar to E2, reduces post-ovariectomy weight gain, inhibits food intake by reducing meal frequency, reduces abdominal fat accumulation, maintains bone density and reduces core body temperature in ovariectomized female guinea pigs [74;77;78]. Furthermore, the STX-initiated signaling pathway alters the transcription of a plethora of ARH genes involved in the control of homeostatic functions [77] (Figure 1). Therefore, Gq-mER appears to be a key player in mediating the effects of E2 on hypothalamic neurons (POMC, NPY, etc.) controlling homeostatic functions.
Summary and Conclusions
The hypothalamic regulation of homeostatic functions has traditionally been regarded as mediated by direct nuclear actions of E2, which has been the dogma for decades. However, recent results indicate that E2 actions in the hypothalamus are a composite of membrane-initiated signaling and direct nuclear effects. Although somewhat unusual for the nuclear receptor field, the idea of multiple receptors, using multiple signaling strategies is commonplace among other receptor families. For example, glutamate has several families of ionotropic and metabotropic receptors. E2 activates a cornucopia of different membrane-associated molecules that affect cell function. It is unusual however, that the same molecules are used as both nuclear and membrane ERs. In many ways, the membrane ER field is still in its infancy: which of the putative receptors are present on the membrane and how do they signal? For example, although ERα and ERβ have been localized in signaling complexes associated with the cell membrane [12;47;49;59], some controversy exists whether GPR30 (GPER1) is localized to the cell membrane. This is in spite of the fact that GRP30 resembles the canonical G protein-coupled receptor structure. One possibility is that GRP30 is localized on smooth endoplasmic reticulum, and E2 activation directly releases intracellular Ca2+ stores. In this way GRP30 mediates rapid E2 action but not at the cell membrane [79].
It is expected that these various ERs will have some measure of interaction. However, how and at what level these receptors interact is at present unclear. Certainly, activation of ERα-mGluR1a or Gq-mER stimulates signaling cascades that can phosphorylate CREB and thus regulate gene expression independent of ERE (Figure 1). But how is this transcriptional activity integrated with activation of ERE and stabilization of the AP-1 site – hallmarks of direct nuclear action? Is there an adaptive advantage of estrogen membrane signaling-mediated gene expression? Over the years, the steroid receptor field has been hindered by technical limitations in addition to the dogma about nuclear-initiated signaling. However, with the advent of new technologies we realize that E2 alters the activity of a large number of proteins – most of whose functions, in a physiological sense, are unknown. Integrating these global changes is the next big challenge for the field. The localization of ERs, broadly defined, throughout the nervous system suggests that their functions are critical. Indeed, the ability of nervous tissue to synthesize steroids, and in particular E2, also argues that these messengers are vital.
Acknowledgments
Research in the author’s laboratories was funded by grants from the National Institutes of Health: DA13185 (PEM), HD04263 (PEM), DK68098 (MJK), NS38809 (MJK). The authors would like to thank Dr. Oline K. Ronnekleiv for her comments on earlier drafts of this review and also Martha A. Bosch for her help in preparing Figure 1.
References
- 1.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 2.Jensen EV, DeSombre ER. Estrogen-receptor interaction. Science. 1973;182:126–134. doi: 10.1126/science.182.4108.126. [DOI] [PubMed] [Google Scholar]
- 3.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA. 1985;23:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JÅ. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 6.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 7.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 9.Micevych PE, Matt DW, Go VL. Concentration of cholecystokinin, substance P, and bombesin in discrete regions of male and female rat brain: sex differences and estrogen effects. Exp Neurol. 1988;100:416–425. doi: 10.1016/0014-4886(88)90119-7. [DOI] [PubMed] [Google Scholar]
- 10.Skipper JK, Young LJ, Bergerson JM, Tetzlaff MT, Osborn CT, Crews D. Identification of an isoform of the estrogen receptor messenger RNA lacking exon four and present in the brain. Proc Natl Acad Sci USA. 1993;90:7172–7175. doi: 10.1073/pnas.90.15.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorosito S, Lorenzo AG, Cambiasso MJ. Estrogen receptor a is expressed on the cell-surface of embryonic hypothalamic neurons. Neurosci. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-a trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filardo EJ, Quinn JA, Bland KI, Frackelton ARJ. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 14.Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- 15.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quesada A, Etgen AM. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of a1B-adrenoceptors and female reproductive function. J Neurosci. 2002;22:2401–2408. doi: 10.1523/JNEUROSCI.22-06-02401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rønnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendo. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Kelly MJ, Rønnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein A, Naidu A. Multiple opioid receptors: Ligand selectivity profiles and binding site signatures. Mol Pharm. 1989;36:265–272. [PubMed] [Google Scholar]
- 23.Kelly MJ, Rønnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 24.Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17b and m-opioid peptides rapidly hyperpolarize GnRH neurons: A cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Kelly MJ, Rønnekleiv OK. 17β-estradiol rapidly increases adenosine 5′-triphosphate-sensitive potassium channel activity in gonadotropin-releasing hormone neurons via a protein kinase signaling pathway. Endocrinology. 2010;151:4477–4484. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin- releasing hormone neurons express KATP Channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17b-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29:10552–10562. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estadiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terasawa E, Schanhofer WK, Keen KL, Luchansky LL. Intracellular Ca2+ oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci. 1999;19:5898–5909. doi: 10.1523/JNEUROSCI.19-14-05898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004;24:6326–6333. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temple JL, Wray S. BSA-estrogen compounds differentially alter gonadotropin-releasing hormone-1 neuronal activity. Endocrinology. 2005;146:558–563. doi: 10.1210/en.2004-1117. [DOI] [PubMed] [Google Scholar]
- 33.Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated Rhesus macaques. Endocrinology. 1985;117:711–721. doi: 10.1210/endo-117-2-711. [DOI] [PubMed] [Google Scholar]
- 34.Gearing M, Terasawa E. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method inb the rhesus monkey. Brain Res Bull. 1988;21:117–121. doi: 10.1016/0361-9230(88)90126-8. [DOI] [PubMed] [Google Scholar]
- 35.Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1neurons. Endocrinology. 2008;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-protein couple receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endo. 2009;3:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanò N, Lee K, Ábrahám IM, Jasoni CL, Herbison AE. Non-classical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2008;149:5335–5344. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenealy BP, Keen KL, Rønnekleiv OK, Terasawa E. STX, a novel nonsteroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology. 2011;152:182–191. doi: 10.1210/en.2011-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne H-J, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari C, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endo. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 42.Malyala A, Pattee P, Nagalla SR, Kelly MJ, Rønnekleiv OK. Suppression subtractive hybridization and microarray identification of estrogen regulated hypothalamic genes. Neurochem Res. 2004;29:1189–1200. doi: 10.1023/b:nere.0000023606.13670.1d. [DOI] [PubMed] [Google Scholar]
- 43.Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendo. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- 44.Micevych P, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Frontiers in Endocrinology. 2011;2:1–11. doi: 10.3389/fendo.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;25:343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- 46.Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290:44–50. doi: 10.1016/j.mce.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 48.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JKH, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 49.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-α interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendo. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff D, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, CA U.S.A: Academic Press; 2002. pp. 139–214. [Google Scholar]
- 53.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quesada A, Etgen AM. Insulin-like growth factor-1 regulation of a1-adrenergic receptor signaling is estradiol dependent in the preoptic area and hypothalamus of female rats. Endocrinology. 2001;142:599–607. doi: 10.1210/endo.142.2.7946. [DOI] [PubMed] [Google Scholar]
- 55.Cardona-Gomez GP, Mendez P, DonCarlos L, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and isulin-like growth factor-I in the brain: molecular mechanishms and functional implications. J Ster Bioc Mol Biol. 2003;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- 56.Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phospatidylinositol 3-kinase in the adult rat brain. Mol Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 57.Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology. 2003;144:3828–3835. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- 58.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych PE. Membrane estrogen receptor-a interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor levels in hypothalamic neurons. J Neurosci. 2010;30:12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of m-opoid receptor immunoreactivity in the medial preoptic nucleas and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinchak K, Shahedi K, Dewing P, Micevych PE. Sexual receptivity is reduced in the female mu-opioid receptor knockout mouse. NeuroReport. 2005;15:1697–1700. doi: 10.1097/01.wnr.0000181585.49130.93. [DOI] [PubMed] [Google Scholar]
- 64.Dewing P, Christensen A, Bondar G, Micevych PE. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kow L-M, Mobbs CV, Pfaff DW. Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: A review. Neurosci Biobehav Rev. 1994;18:251–268. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 66.Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 68.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 69.Park CJ, Zhao Z, Glidewell-Kenny C, Lazic M, Chambon P, Drust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. J Clin Invest. 2011;121:604–612. doi: 10.1172/JCI41702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Y, Nedugadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct Hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Mol Pharm. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- 73.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao X-B, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Med. 2006;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 74.Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tobias SC, Qiu J, Kelly MJ, Scanlan TS. Synthesis and biological evaluation of SERMs with potent nongenomic estrogenic activity. ChemMedChem. 2006;1:565–571. doi: 10.1002/cmdc.200500098. [DOI] [PubMed] [Google Scholar]
- 77.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlová-Wuttke D, Wuttke W, Scanlan TS, Rønnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151:4926–4937. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prossnitz ER, Arteburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]