Abstract
Obstructive sleep apnea (OSA) is two to three times more common in men as in women. The mechanisms leading to this difference are currently unclear but could include gender differences in respiratory stability [loop gain (LG)] or upper airway collapsibility [pharyngeal critical closing pressure (Pcrit)]. The aim of this study was to compare LG and Pcrit between men and women with OSA to determine whether the factors contributing to apnea are similar between genders. The first group of 11 men and 11 women were matched for OSA severity (mean ± SE apnea-hypopnea index = 43.8 ± 6.1 and 44.1 ± 6.6 events/h). The second group of 12 men and 12 women were matched for body mass index (BMI; 31.6 ± 1.9 and 31.3 ± 1.8 kg/m2, respectively). All measurements were made during stable supine non-rapid eye movement sleep. LG was determined using a proportional assist ventilator. Pcrit was measured by progressively dropping the continuous positive airway pressure level for three to five breaths until airway collapse. Apnea-hypopnea index-matched women had a higher BMI than men (38.0 ± 2.4 vs. 30.0 ± 1.9 kg/m2; P = 0.03), but LG and Pcrit were similar between men and women (LG: 0.37 ± 0.02 and 0.37 ± 0.02, respectively, P = 0.92; Pcrit: 0.35 ± 0.62 and –0.18 ± 0.87, respectively, P = 0.63). In the BMI-matched subgroup, women had less severe OSA during non-rapid eye movement sleep (30.9 ± 7.4 vs. 52.5 ± 8.1 events/h; P = 0.04) and lower Pcrit (–2.01 ± 0.62 vs. 1.16 ± 0.83 cmH2O; P = 0.005). However, LG was not significantly different between genders (0.38 ± 0.02 vs. 0.33 ± 0.03; P = 0.14). These results suggest that women may be protected from developing OSA by having a less collapsible upper airway for any given degree of obesity.
Keywords: pharyngeal critical closing pressure, loop gain, gender
Obstructive Sleep Apnea (OSA) is a disorder characterized by repetitive upper airway collapse during sleep that affects ~4% of adult men and 2% of adult women (48). In addition to the gender difference in prevalence, women with OSA have higher body mass indexes (BMIs) than men with equal-severity OSA (12, 31), suggesting that the pathophysiology of OSA may differ between genders. The pathophysiology of OSA is incompletely understood; however, it appears to involve aspects of both upper airway collapsibility and respiratory control stability (8, 45).
There are multiple variables that contribute to airway collapsibility and respiratory control stability. Airway collapsibility is likely influenced by anatomical factors, such as bony and soft tissue structures as well as airway length, but also by physiological factors such as upper airway dilator muscle activity (24). Similarly, respiratory stability is determined by gas-exchange efficiency, circulatory delays to central and peripheral chemoreceptors, chemoresponsiveness, and potentially by respiratory neural memories such as respiratory after-discharge or long-term facilitation (20). Many of these variables have been compared between men and women in an attempt to explain the male predominance in this disorder (1, 5, 6, 14–17, 22–29, 33, 34, 37–39, 44, 49). Although studies have reported gender differences in some of these variables (24, 28, 29, 39, 49), it is important to note that each variable measured is only one contributor to overall upper airway collapsibility or respiratory control stability. In this study, we wished to compare global measurements of both respiratory control stability and upper airway collapsibility in men and women with OSA to determine whether differences exist and whether the factors contributing to sleep apnea are similar in the two genders.
To assess respiratory control stability, we measured the loop gain (LG) of the respiratory system. LG is a measure of an individual’s susceptibility to periodic breathing and incorporates all of the factors involved in determining the respiratory control system stability previously mentioned. LG is quantified as the ratio of the ventilatory response to a disturbance (47). If LG is < 1, the ventilatory response will be smaller than the initial disturbance and so ventilation will become stable after a few oscillatory cycles. However, if LG is ≥ 1, the response will be equally large (if LG = 1) or larger (if LG > 1) than the initial disturbance, and hence periodic breathing will develop. Recently, Younes et al. (47) developed a method for estimating an individual’s LG by artificially increasing the response to a disturbance using a proportional assist ventilator (PAV). The amount the response needs to be amplified to induce periodic breathing is inversely proportional to the patient’s intrinsic LG. We utilized this technique to measure respiratory control stability in men and women with a range of severity of OSA.
We chose to assess airway collapsibility by measuring the pharyngeal critical closing pressure (Pcrit) (4). This is the nasal pressure below which the upper airway collapses and takes into consideration many anatomical and physiological factors. Sforza and colleagues (36) have previously compared Pcrit between men and women with equally severe OSA using a technique where upper airway dilator muscles are active. These authors reported that, although the women were more obese than men, Pcrit was more negative in women (suggesting a more stable upper airway due to either better anatomy or more effective action of the upper airway dilator muscles). We hypothesized that, for women to have equally severe OSA but a less collapsible airway, they should have a more unstable respiratory controller than men. This hypothesis was tested in the present study by measuring both the Pcrit and LG in men and women with equally severe OSA. However, if LG is an important contributor to apnea-hypopnea index (AHI), then it may be difficult to identify a gender difference in AHI-matched individuals. We therefore also compared LG and Pcrit in a group of BMI-matched men and women. In this group of subjects, we hypothesized that women would have less severe OSA than men and therefore both lower LG and/or Pcrit due to prior reports that both LG and Pcrit are lower in patients with mild to moderate OSA compared with severe OSA (10, 11, 47). The results of some of the subjects reported in this study were also included in another study conducted in our laboratory (40).
METHODS
Subjects
Forty-nine adults (26 men) were recruited and participated in this study. Subjects had either previously been diagnosed with OSA (n = 23), were overweight snorers who were considered likely to have OSA (n = 16), or were unlikely to have OSA (n = 10). No subject smoked or had respiratory disease or a confounding sleep disorder. Nine subjects were taking antihypertensive medications at the time of the study, and four were on oral hypoglycemic agents. No subject was taking any antidepressant or other medications. All subjects gave written, informed consent before participation in this study, which was approved by the Human Research Committee of the Brigham and Women’s Hospital.
Protocol
This protocol is comprised of three studies that were performed on 2 or 3 nights in a random order. The first study was an overnight polysomnography without continuous positive airway pressure (CPAP) for determination of current apnea severity. The second and third studies involved measurement of the LG and Pcrit during non-rapid eye movement (NREM) sleep in the supine position. If possible, both LG and Pcrit were determined on the same night. However, if there was insufficient sleep to obtain both measurements on the same night, then the subject returned on a third night to obtain the remaining data. The details of each of the three studies are described below.
Study 1: baseline polysomnography
A full-night polysomnography without CPAP was performed according to the standard laboratory protocol. Sleep was measured with four electroencephalograms, left and right electroocculogram, and submental electromyogram. Respiration was monitored by measurement of nasal pressure (PTAF2, Pro-Tech Services, Woodinville, WA), oronasal airflow (nasal-oral thermistor), arterial oxygen saturation (model 930 Pulse-Oximeter, Respironics, Murrysville, PA), and chest plus abdominal wall motion (piezo-electric bands). A tracheal microphone was used to monitor snoring. Body position and bilateral anterior tibialis electromyogram were also continuously recorded. Recording began between 2200 and 0000 and finished between 0500 and 0700. Subjects were instructed to sleep in the supine body position as much as possible. Data were acquired on Alice data acquisition software (Alice 3 or Alice Host, Respironics), and all studies were scored by a single technician in the standard manner (30), with arousals and respiratory events being scored according to the American Academy of Sleep Medicine guidelines (2, 3). The single technician was blinded to any Pcrit/LG data that had been collected. Subjects were considered to have OSA if the AHI was >15 events/h.
Studies 2 and 3: equipment and measurements
For both the measurement of Pcrit and LG, the subjects were fitted with a nasal mask (Gel Mask, Respironics) attached to a heated pneumotachograph (model 3700A, Hans Rudolf, Kansas City, MO) and a differential pressure transducer (Validyne, Northbridge, CA) for measurement of inspiratory flow. This signal was integrated for measurement of tidal volume (Vt). The pneumotachograph was then connected to a leak valve and then to the ventilator used in each study (details below). Mask pressure and CO2 were continuously measured, as were the electrocardiogram and arterial oxygen saturation. Two channels of electroencephalograms (C3-A2, O2-A1), left and right electroocculogram, and submental electromyogram were recorded for sleep staging. During LG measurements, the additional signals from the PAV of estimated inspiratory flow, volume, and pressure were recorded. All signals were recorded on a 16-channel Grass model 78 polygraph (Grass Instruments, Astro Med, West Warwick, RI). Selected signals (inspiratory flow; Vt; mask pressure; PAV of inspiratory flow, volume, and pressure; CO2; arterial oxygen saturation; electroencephalogram; electrocardiogram) were simultaneously recorded on a personal computer using an analog-to-digital converter (1401 plus, Cambridge Electronic Design, Cambridge, UK) and data acquisition software (Spike 2, version 3.19, Cambridge Electronic Design).
Protocol: Pcrit measurement
For the measurement of Pcrit, a modified CPAP device that could deliver both positive and negative airway pressure (Respironics) was connected to the mask. The airway pressure was initially set at the subject’s prescribed CPAP level or at 4 cmH2O if the subject had not undergone a prior CPAP titration study. After sleep onset, the CPAP level was increased to abolish respiratory events and to normalize the inspiratory flow profile (no flow limitation). This level was used as the holding pressure for each subject. Once 5 min of stable NREM sleep (stage 2 or higher) had been achieved at the holding pressure, CPAP was abruptly reduced by 1–2 cmH2O during expiration and was held at this level for three to five breaths. The pressure was then returned to the holding pressure for 1 min before being dropped a further 1–2 cmH2O for another three to five breaths. This process of progressively dropping CPAP for three to five breaths every minute continued until obstructive apnea occurred during the reduction in CPAP. If arousal occurred during any pressure drop, the CPAP was returned to the holding pressure until stable sleep returned. The entire procedure of progressively dropping CPAP until obstruction was repeated two to five times in each subject.
Data analysis: Pcrit
The third to fifth breaths during a drop in CPAP were used for calculation of Pcrit only if the breaths appeared to be flow limited in shape (7) and were not associated with arousal from sleep or complete apnea. A breath was considered to be flow limited in shape if there was flattening or a plateau of flow throughout inspiration (7). The first and second breaths in a pressure drop were not used for analysis because the maximum flow does not stabilize until the third breath after reduction of CPAP (4). For all flow-limited breaths, mask pressure was plotted against maximum inspiratory flow, and linear regression was used to interpolate the pressure at which zero flow occurred. The measurement of Pcrit without direct measurement of respiratory effort (and therefore flow limitation) has been previously validated in seven patients with OSA and five healthy controls (19) in whom Pcrit was determined with the scorer blinded and not blinded to respiratory drive (epiglottic pressure).
Protocol: LG measurement
A PAV (BiPAP Vision, model 582059, Respironics) was attached to the mask for the measurement of LG. The PAV provides both a constant expiratory pressure and a variable inspiratory pressure that is proportionate to the subject’s respiratory drive. If the subject takes a large breath, they will receive a large pressure support. However, if they take a small breath, they will only receive a small amount of pressure during inspiration. Thus PAV can be used to amplify the subjects’ fluctuations in respiratory drive and, given there is a delay in the feedback circuit (due to gas exchange, circulation time to chemoreceptors, etc.) PAV can induce periodic breathing. The amount the subjects’ intrinsic respiratory drive needs to be amplified to induce periodic breathing is inversely proportional to their intrinsic LG (47). The protocol for measurement of LG is very similar to that originally described by Younes and colleagues (47) and is outlined below. The ventilator was set in the PAV/T Custom mode with the backup rate set at 0 breaths/min and the initial settings being volume assist (elastance component) = 12 cmH2O/l, flow assist (resistance component) = 4 cmH2O·l–1·s, and %assist = 0. With 0% assist, the inspiratory and expiratory positive airway pressure (EPAP) are the same (i.e., CPAP). The EPAP level was initially set at the subject’s prescribed CPAP level or at 4 cmH2O if the subject had not undergone a prior CPAP titration study. After sleep onset, the EPAP was increased to abolish respiratory events and to normalize the flow profile in the same manner as described for Pcrit measurement. When the subject was in stable NREM sleep (>5 min stage 2 or deeper), the %assist was gradually increased (10% increments every 20–30 s) to 60%. The %assist was then increased more gradually (5% every 30–60 s) until small cyclical oscillations in breathing were observed following an increase in %assist. After stable breathing resumed at this level of %assist, the Vt amplification factor (VtAF) was determined by dropping the %assist to 0% for one breath and dividing the Vt with no assist by the average Vt over the last three breaths during assisted breathing (VtAF = assisted Vt/unassisted Vt). VtAF measurement was repeated several times at each level of assist, with each repeat separated by at least 30 s of stable breathing. The %assist was then increased in 5% increments until sustained periodic breathing developed (defined as 4 or more cycles of waxing and waning Vt with the nadir Vt being <50% of the peak Vt with a cycle length of 20–90 s). VtAF was recalculated after every increase in %assist. If 100% assist was reached without periodic breathing, the volume-assist component was increased until periodic breathing began or volume runaway occurred (see Ref. 46 for review). If at any time the subject developed flow or volume runaway, the level of assist was reduced by 5% and the component (flow or volume assist) that did not reach runaway was increased before the %assist was again increased. If at any time the subject briefly aroused, the %assist was held constant until at least 60 s of stable sleep and breathing had occurred. If full awakening occurred, the %assist was reduced to 0 or 10% until stable sleep (>5 min stage 2 or higher) resumed.
Data analysis: LG measurement
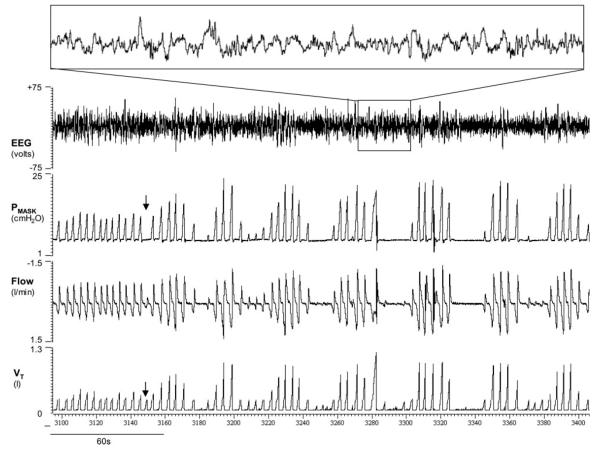
Each subject’s intrinsic LG was calculated in the standard manner (47) as the inverse of the average VtAF measured two to six times at the level of assist immediately before the development of periodic breathing (LG = 1/average VtAF). If arousals occurred during periodic breathing (this occurred in only 1 subject), then the VtAF was averaged at the highest level of assist without arousals (because it is unclear whether periodic breathing would still have existed without arousals), and LG was assumed to be less than the inverse of this VtAF. Similarly, if periodic breathing was not induced, the LG was assigned a value less than the inverse of the highest VtAF obtained. For statistical comparisons, the LG was assumed to be equal to this value in these individuals. An example of periodic breathing induced with proportional assist ventilation is shown in Fig. 1.
Fig. 1.
An example of periodic breathing induced by proportional assist ventilation in a female patient with obstructive sleep apnea [apnea-hypopnea index (AHI) = 23 events/h]. The patient had stable breathing at 100% assist with volume assist = 16 cmH2O/l and flow assist = 4 cmH2O·l–1·s–1 for 3 min before tidal volume (Vt) amplification factor was measured by dropping the %assist to 0% for 1 breath (solid arrow). After this disturbance to ventilation, sustained periodic breathing developed. There was no sign of electroencephalogram (EEG) arousal during cyclical breathing, as indicated by inset. Pmask, mask pressure.
Statistical analysis
Statistical comparisons were performed with SigmaStat software (version 3.0, SPSS, Chicago, IL). All comparisons between genders were performed with paired, two-tailed Student’s t-tests with pairing based on BMI or AHI as appropriate. Linear regression was used to calculate Pcrit-adjusted AHI. All values are means ± SE, and P < 0.05 was considered significant.
RESULTS
Thirteen subjects failed to sleep sufficiently to obtain all of the required data, and another subject dropped out, leaving 35 subjects (16 men) in whom all data were obtained. Of the 35 subjects with complete data, 11 men and 11 women were well matched for severity of OSA (AHI within 5 events/h; Table 1) and 12 men and 12 women were well matched for BMI (within 2 kg/m2; Table 2).
Table 1.
Subject characteristics in men and women matched for OSA severity
| Men | Women | |
|---|---|---|
| Age, yr | 45.1±2.2 | 47.8±3.1 |
| BMI*, kg/m2 | 30.0±1.9 | 38.0±2.4 |
| Total AHI, events/h | 43.8±6.1 | 44.1±6.6 |
| NREM AHI, events/h | 46.7±7.3 | 43.1±7.4 |
| REM AHI, events/h | 52.9±6.3 | 60.4±4.5 |
| Supine AHI, events/h | 50.4±8.1 | 47.2±7.9 |
| Obstructive AI, events/h | 13.9±6.1 | 6.0±2.8 |
| Central AI, events/h | 0.2±0.1 | 0.5±0.2 |
| Mixed AI, events/h | 0.7±0.4 | 0.4±0.4 |
| Hypopnea index, events/h | 29.1±3.1 | 36.0±5.2 |
Values are means ± SE. Age, body mass index (BMI), and apnea-hypopnea index (AHI) measured in total sleep non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep, and sleep while only in the supine body position, obstructive apnea index (Obstructive AI), central apnea index (Central AI), mixed apnea index (Mixed AI), and hypopnea index in 11 men and 11 women with OSA.
P < 0.05, different between genders.
Table 2.
Subject characteristics in men and women matched for BMI
| Men | Women | |
|---|---|---|
| Age, yr | 44.8±1.9 | 45.6±3.2 |
| BMI, kg/m2 | 31.3±1.8 | 31.6±1.9 |
| Total AHI†, events/h | 49.4±7.6 | 32.9±7.3 |
| NREM AHI*, events/h | 52.5±8.1 | 30.9±7.4 |
| REM AHI, events/h | 49.8±6.5 | 50.7±9.2 |
| Supine AHI*, events/h | 56.7±9.4 | 32.5±7.0 |
| Obstructive AI*, events/h | 15.2±5.8 | 2.8±1.1 |
| Central AI, events/h | 0.2±0.1 | 0.3±0.2 |
| Mixed AI, events/h | 0.8±0.3 | 0.03±0.02 |
| Hypopnea index, events/h | 33.2±4.6 | 28.6±6.3 |
Values are means ± SE. Anthropometric and sleep-related variables in 12 men and 12 women matched for BMI. See Table 1 for definition of terms.
P < 0.05, different between genders.
P = 0.09.
Pcrit and LG in men and women matched for AHI
The AHI-matched women were significantly heavier than men (Table 1). There was no gender difference in OSA severity when separated into rapid eye movement or NREM sleep, or when restricted to the supine body position (Table 1). The type of respiratory events (obstructive/central/mixed apnea or hypopnea) were also not different between genders (Table 1). During stable NREM sleep on therapeutic CPAP, the AHI-matched men and women did not differ in terms of minute ventilation (8.2 ± 0.7 and 7.6 ± 0.4 l/min, respectively; P = 0.48), Vt (0.52 ± 0.03 and 0.49 ± 0.02 liter, respectively; P = 0.45), breathing frequency (17.3 ± 1.9 and 15.6 ± 0.7 breaths/min, respectively; P = 0.40), or end-tidal CO2 (40.8 ± 1.7 and 39.7 ± 1.1 Torr, respectively; P = 0.61). Neither mean (0.31 ± 0.02 and 0.33 ± 0.04 l/s, P = 0.76) nor peak inspiratory flow (0.45 ± 0.03 and 0.44 ± 0.02 l/s, P = 0.74) differed between genders.
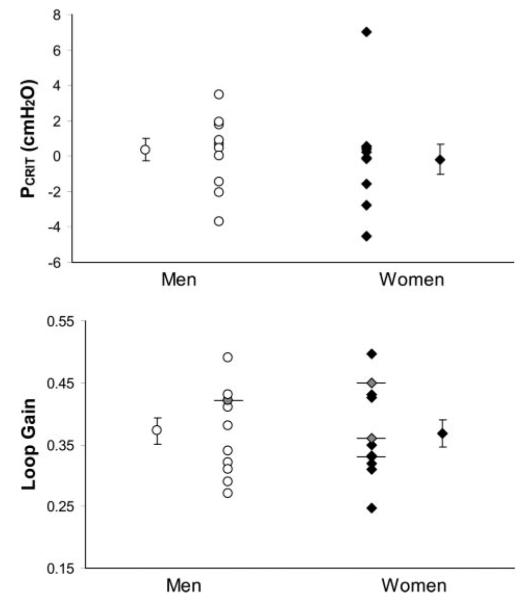
Pcrit in the AHI-matched men and women did not differ between genders (0.35 ± 0.62 cmH2O in men vs. –0.18 ± 0.87 cmH2O in women, P = 0.63; Fig. 2). In four subjects (3 women), periodic breathing could not be induced, and thus a LG value of less than the reciprocal of the highest VtAF was obtained. These subjects are indicated in Fig. 2 (see legend). No gender difference existed in LG in the men and women with equally severe OSA (0.372 ± 0.02 vs. 0.368 ± 0.02, respectively, P = 0.92; Fig. 2).
Fig. 2.
Pharyngeal critical closing pressure (Pcrit; top) and loop gain (bottom) in 11 men and 11 women matched for severity of obstructive sleep apnea (within 5 events/h). Individual and group means (±SE) are presented. Subjects identified by horizontal bars and shaded symbols are individuals who did not develop periodic breathing, and thus their loop gain may be lower than the value indicated. There were no significant differences between genders in either variable.
Pcrit and LG in men and women matched for BMI
The BMI-matched men and women were similarly aged; however, the women had less severe OSA than men in NREM sleep and in the supine position (Table 2). The women in this comparison also had fewer obstructive apneas and a trend (P = 0.055) to fewer mixed apneas (Table 2). The BMI-matched men and women had similar resting respiratory characteristics during stable NREM sleep; minute ventilation (8.1 ± 0.6 and 6.8 ± 0.4 l/min, respectively; P = 0.10), Vt (0.52 ± 0.03 and 0.46 ± 0.02 liter, respectively; P = 0.08), breathing frequency (15.3 ± 0.8 and 14.9 ± 0.9 breaths/min, respectively; P = 0.70), end-tidal CO2 (40.7 ± 1.6 and 38.9 ± 0.8 Torr, respectively; P = 0.34), mean inspiratory flow (0.31 ± 0.02 and 0.29 ± 0.04 l/s, respectively; P = 0.67), and peak inspiratory flow (0.46 ± 0.03 and 0.39 ± 0.02 l/s, respectively; P = 0.09).
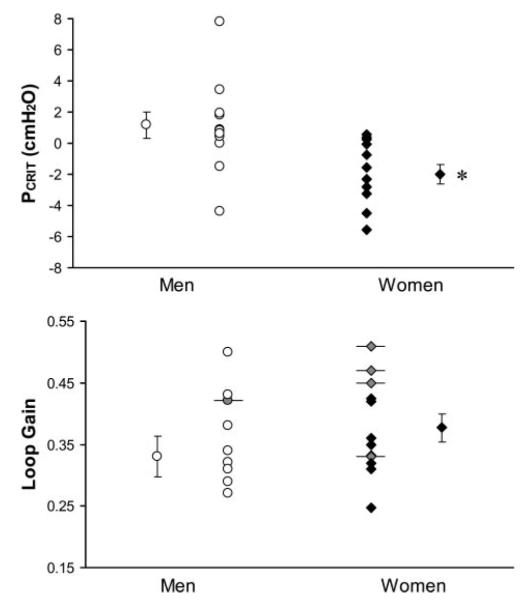
LG could not be determined precisely in five subjects in this comparison (4 women), and therefore a less than value for LG was used. LG did not differ in men and women matched for BMI (0.33 ± 0.03 in men and 0.38 ± 0.02 in women, P = 0.14; Fig. 3). Pcrit was significantly lower in women than in BMI-matched men (–2.01 ± 0.62 and 1.16 ± 0.83 cmH2O, respectively, P = 0.005; Fig. 3). To explore gender effects on AHI independent of Pcrit, the Pcrit-adjusted AHI was computed for the BMI-matched men and women by regressing AHI on Pcrit. No significant difference was found between men and women (adjusted AHI = 41.4 ± 5.4 and 42.1 ± 6.1 events/h, respectively, P = 0.93). This suggests that, after accounting for potential differences in BMI, the only effect of gender on AHI occurs via effects on Pcrit.
Fig. 3.
Pcrit (top) and loop gain (bottom) in 12 men and 12 women with obstructive sleep apnea who were matched for body mass index (BMI; within 2 kg/m2). Individual and group means (±SE) are presented. Subjects identified by horizontal error bars and shaded symbols are individuals who did not develop periodic breathing, and thus their loop gain may be lower than the value indicated. *P = 0.005.
The difference in Pcrit between genders appeared to persist over the full range of BMIs studied (Fig. 4). In multivariate regression using all 35 subjects, every 1 kg/m2 increase was associated with a 0.17-cmH2O increase in Pcrit (P = 0.006), whereas female gender was associated with a 2.01-cmH2O decrease in Pcrit (P = 0.043). A BMI-by-gender interaction term was nonsignificant (P = 0.94), supporting the hypothesis that the gender effect on Pcrit is constant across the range of BMI studied. In other words, female gender was equivalent to a 11.7-kg/m2 reduction in BMI.
Fig. 4.
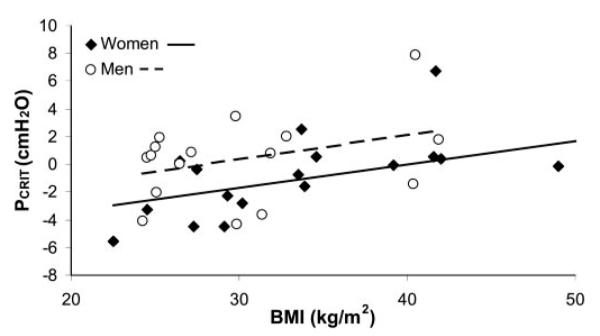
Relationship between Pcrit and BMI in all 35 subjects studied (16 men and 19 women). Although gender and BMI were both independently significantly associated with Pcrit (P = 0.043 and 0.006, respectively), an interaction term was not (P = 0.94), indicating that the gender effect on Pcrit is constant across the range of BMI studied.
DISCUSSION
In this study, we have measured two variables (respiratory control stability and upper airway collapsibility) that we believe are major contributors to the pathogenesis of OSA to determine whether the factors contributing to OSA are the same in men and women. The main findings were that the stability of the respiratory controller and the passive collapsibility of the upper airway did not differ between men and women with equally severe OSA but that the upper airway was more collapsible in men than in equally overweight/obese women. Although the difference in LG was not significantly different between the BMI-matched men and women, this comparison was underpowered (see below). However, we do not believe that LG contributed to the gender difference in AHI between BMI-matched men and women because the Pcrit-adjusted AHIs were near identical between genders. Of note, the difference in Pcrit between genders appeared to persist across the full range of BMIs studied (Fig. 4). How Pcrit contributes to the severity of OSA is unclear, and whether the relationship between Pcrit and AHI is the same between genders is unknown, yet it is a fairly weak relationship (r = 0.23, 0.51, and 0.66) when both genders are considered (35, 36, 40). However, our findings would suggest that gender differences in Pcrit are a possible mechanism for the male predisposition to airway collapse during sleep.
Gender and airway collapsibility
Several previous studies have investigated gender differences in airway collapsibility and respiratory control in an attempt to explain the male predisposition to OSA. Sforza et al. (36) compared the Pcrit between 89 men and 17 women with equally severe OSA from a clinic population. The women in this comparison were significantly heavier than the men but had significantly lower Pcrit (1.6 vs. 2.2 cmH2O). This finding differs from the AHI-matched comparison in the present study. However, the magnitude of the difference between genders is very similar at 0.53 cmH2O. Given the standard deviation and number of subjects in the present study, power calculations indicate that we did not have sufficient power to detect such a small difference (0.53 cmH2O) in Pcrit between the AHI-matched men and women. Thus it is possible that the lack of a gender difference in Pcrit found in the current comparison of equally severe men and women is a result of a type II error. It is important to note that the techniques for Pcrit measurement in the study by Sforza et al. (36) differed from the present study in that the upper airway muscle activity was not minimal in the Sforza et al. study. Therefore, the differences in findings between these studies may be interpreted to indicate that the effectiveness of the upper airway muscles in reducing the collapsibility of the airway is greater in women than in men. This, however, is not supported by the study of Younes (45) where the compensatory effectiveness is reported to be similar in men and women.
The difference in Pcrit was more marked and statistically significant when comparing BMI-matched men and women (3.2 cmH2O). Previously, Pcrit has been reported to differ between individuals with the upper airway resistance syndrome and mild to moderate OSA by 2.4 cmH2O (11), suggesting that the difference observed between BMI-matched men and women in the present study is likely physiologically important. Pcrit has also been compared between healthy men and women without OSA (34). In this study, Rowley and colleagues measured Pcrit in a similar manner to the present study, with drops in airway pressure being applied to the nose for two breaths during sleep. Between the negative pressure breaths, the subjects in the study of Rowley and colleagues breathed at atmospheric pressure rather than having CPAP, as in the present study. The eight men and eight women who Rowley and colleagues studied had similar BMIs and age, and the Pcrit was not different between genders. This is surprising based on the observations in the present study that the gender difference in airway collapsibility appeared to be relatively constant over the range of BMIs studied (Fig. 4). Clearly, there were fewer subjects in the present study at the lower end of the BMI range, and thus we may have been underpowered. However, it is possible that the subjects in the study of Rowley and colleagues had greater activation of the upper airway dilator muscles (because they were not studied on CPAP), resulting in more negative closing pressures than would have been the case had upper airway dilator muscles been passive. This effect may have been more pronounced in the slightly heavier men (BMI = 26.3 and 23.8 kg/m2 in the men and women). Further evidence supporting the idea that healthy young nonobese men have a more collapsible upper airway than women is provided by a study of inspiratory resistive loading during sleep in which Pillar and colleagues (28) reported that men had larger increases in airway resistance and more flow limitation than women during resistive load application. Importantly, upper airway muscle activity was measured in this study and was not found to be different between genders during stable sleep or resistive loading. Further studies are needed to assess the collapsibility of the upper airway and the effect of upper airway dilator muscles on collapsibility in men and women across the full range of BMI.
Respiratory control in men and women and OSA
Respiration has been found to be less stable in severe OSA patients than in individuals with less severe OSA (47) and healthy controls (13), and so respiratory stability has been proposed as an important contributor to OSA pathogenesis. Thus the finding in the present study that a measure of respiratory control stability (LG) is not different between men and women with either equally severe OSA or equal BMIs is important because it indicates that respiratory control stability contributes to OSA similarly in men and women. Given this negative finding, it is important to assess whether there was sufficient power to detect physiologically important differences in LG. We believe that a difference in LG of 0.1 is physiologically relevant, given that the difference between severe and mild/moderate OSA has been previously reported to be 0.16 (47). With the standard deviation measured in this study, we could detect a difference of this magnitude between the 11 men and 11 women who were matched for AHI with 88% power and an α = 0.05. Thus the lack of a gender difference in the LG measured in this comparison is unlikely to be due to a type II statistical error. However, a difference in LG of 0.1 between BMI-matched men and women could only be detected with 68% power. It is therefore possible that the lack of a gender difference in LG between BMI-matched men and women may have been the result of a type II error. Our laboratory has previously shown that respiratory stability is not different between young healthy men and women without OSA (41), providing further evidence that LG is not different between genders. Interestingly, periodic breathing could not be induced in 6 of 19 women but only 1 of the 16 men in the present study. This may indicate that a subtle gender difference exists in respiratory control that was not detected by the LG measurement, or this may simply reflect different arousal thresholds between genders.
Although a number of investigators have assessed the impact of gender on sleep apnea pathogenesis (1, 5, 6, 14–17, 22–29, 33, 34, 37–39, 44, 49), only a few studies have attempted to combine multiple variables in clinical sleep apnea populations. In one recent study, Younes (45) reported that men (n = 66) had more severe OSA than women (n = 16) after controlling for airway mechanics but that this difference did not persist after correction for baseline flow and ventilatory demand, suggesting that differences in OSA severity were likely related to ventilatory drive differences rather than control mechanisms. In the present study of either AHI- or BMI-matched men and women, no differences were observed in baseline flow, ventilatory demand, or LG. Although methodological differences between these two studies preclude rigorous conclusions, we would hypothesize that the higher BMI seen clinically in women with OSA likely results in upper airway collapsibility that more closely resembles men. Clearly, further research is required to verify this suggestion.
There are methodological issues in this study that deserve comment. First, in most subjects, the three measurements (AHI, LG, and Pcrit) were obtained on separate study nights. Although we restricted subjects to the supine body position for Pcrit and LG measures and requested subjects to sleep supine during the polysomnography, it is still possible that the severity of OSA, upper airway collapsibility, or respiratory control stability varied between nights and contributed to the variability of our measurements. This was, however, unavoidable, given the time required to make accurate measures of each variable of interest. Second, the women studied varied in terms of their menstrual status (8 premenopausal and 11 postmenopausal). The prevalence of OSA changes across menopause, and it is also possible that the pathogenic mechanisms resulting in OSA vary between pre- and postmenopausal women. This may have increased the variability of our measurements in women and made it more difficult to identify gender differences. Third, there are likely factors (such as upper airway dilator muscle activity and lung volume) that contribute to airway collapsibility in natural sleep that were altered by CPAP in the measurement of Pcrit in this study. Therefore, the measured Pcrit may differ from the actual airway collapsibility that occurs during normal sleep. However, we do not have any reason to suspect that this effect would be different between genders. Fourth, although measurement of LG includes many variables that contribute to overall respiratory stability, there are factors that would normally contribute to respiratory stability/instability during sleep that were excluded in our measurement. LG was measured during stable sleep without arousals, such that variable arousal thresholds and ventilatory responses to arousal did not confound our measurement. However, the ventilatory changes associated with arousal have been shown with modeling studies to destabilize breathing (21), and thus arousal may be an important variable that was not included in this study. Also, there is a prior report of a gender difference in the hypocapnic-apneic threshold (49), which when combined with the increased ventilatory response to arousal in men (17, 18) may make men more prone to ventilatory instability following arousal from sleep. Clearly, this effect was not measured in our LG measurement of respiratory stability. Finally, upper airway collapsibility was excluded from the current measurements of respiratory stability by studying the patients on EPAP sufficient to maintain airway patency. It is possible, if not likely, that central respiratory instability and upper airway collapsibility act synergistically in contributing to OSA severity. Further work is required to determine how these variables interact. In addition, recent data from our laboratory suggests that respiratory stability may only importantly contribute to OSA severity in individuals with Pcrit close to atmospheric pressure (40). By studying individuals with a range of collapsibility, we may have made it difficult to identify gender differences in LG that importantly contribute to the gender difference in OSA severity.
In conclusion, this study demonstrated that men have a more positive pharyngeal Pcrit and more severe OSA than women who are matched for BMI. The difference in Pcrit appeared to completely explain the difference in AHI between BMI-matched men and women. In addition, the difference in Pcrit appeared to persist across a wide range of BMIs. Respiratory control stability did not differ between either BMI- or AHI-matched men and women. These findings suggest that women are less prone to OSA than men because of an anatomically more stable upper airway, not because of a more stable respiratory control system.
Acknowledgments
GRANTS This work was supported by National Institutes of Health Grants RO1 HL-48531, P50 HL-60292, F32 HL-072560–01, MO1 RR-02635, and RR-01032 and American Heart Association Grant 0425786T. A. S. Jordan is a recipient of the Thoracic Society of Australia and New Zealand/Allen and Hanbury’s respiratory research fellowship. Both A. Malhotra and S. R. Patel have received Scientific Development Grants from the American Heart Association.
REFERENCES
- 1.Aitken ML, Franklin JL, Pierson DJ, Schoene RB. Influence of body size and gender on control of ventilation. J Appl Physiol. 1986;60:1894–1899. doi: 10.1152/jappl.1986.60.6.1894. [DOI] [PubMed] [Google Scholar]
- 2.American Sleep Disorders Association Task Force EEG arousals: scoring rules and examples. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 3.American Sleep Disorders Association Task Force Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 4.Boudewyns A, Punjabi N, Van de Heyning PH, De Backer WA, O’Donnell CP, Schneider H, Smith PL, Schwartz AR. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–1041. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 5.Brooks LJ, Strohl KP. Size and mechanical properties of the pharynx in healthy men and women. Am Rev Respir Dis. 1992;146:1394–1397. doi: 10.1164/ajrccm/146.6.1394. [DOI] [PubMed] [Google Scholar]
- 6.Buyse B, Markous N, Cauberghs M, Van Klaveren R, Muls E, Demedts M. Effect of obesity and/or sleep apnea on chemosensitivity: differences between men and women. Respir Physiol Neurobiol. 2003;134:13–22. doi: 10.1016/s1569-9048(02)00202-1. [DOI] [PubMed] [Google Scholar]
- 7.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:475–480. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 8.Deegan PC, McNicholas WT. Pathophysiology of obstructive sleep apnoea. Eur Respir J. 1995;8:1161–1178. doi: 10.1183/09031936.95.08071161. [DOI] [PubMed] [Google Scholar]
- 9.Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis. 1982;126:758–762. doi: 10.1164/arrd.1982.126.5.758. [DOI] [PubMed] [Google Scholar]
- 10.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 11.Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121:1531–1540. doi: 10.1378/chest.121.5.1531. [DOI] [PubMed] [Google Scholar]
- 12.Guilleminault C, Quera-Salva MA, Partinen M, Jamieson A. Women and the obstructive sleep apnea syndrome. Chest. 1988;93:104–109. doi: 10.1378/chest.93.1.104. [DOI] [PubMed] [Google Scholar]
- 13.Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158:1142–1149. doi: 10.1164/ajrccm.158.4.9712105. [DOI] [PubMed] [Google Scholar]
- 14.Jordan AS, Catcheside PG, O’Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present in healthy men or women. J Appl Physiol. 2002;93:2129–2136. doi: 10.1152/japplphysiol.00135.2002. [DOI] [PubMed] [Google Scholar]
- 15.Jordan AS, Catcheside PG, O’Donoghue FJ, Saunders NA, McEvoy RD. Selected Contribution: Genioglossus muscle activity at rest and in response to brief hypoxia in healthy men and women. J Appl Physiol. 2002;92:410–417. doi: 10.1152/japplphysiol.00461.2001. [DOI] [PubMed] [Google Scholar]
- 16.Jordan AS, Catcheside PG, Orr RS, O’Donoghue FJ, Saunders NA, McEvoy RD. Ventilatory decline after hypoxia and hypercapnia is not different between healthy young men and women. J Appl Physiol. 2000;88:3–9. doi: 10.1152/jappl.2000.88.1.3. [DOI] [PubMed] [Google Scholar]
- 17.Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. The ventilatory response to brief arousal from NREM sleep is greater in men than in women. Am J Respir Crit Care Med. 2003;168:1512–1519. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- 18.Jordan AS, McEvoy RD, Edwards JK, Schory K, Yang CK, Catcheside PG, Fogel RB, Malhotra A, White DP. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol. 2004;558:993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan AS, Wellman DA, Fogel RB, Pierce RJ, Edwards JK, Schory K, Malhotra A, White DP. Pharyngeal critical closing pressure measurement without respiratory effort: a validation study (Abstract) Am J Respir Crit Care Med. 2003;167:A600. [Google Scholar]
- 20.Khoo MC. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 21.Khoo MC, Koh SS, Shin JJ, Westbrook PR, Berry RB. Ventilatory dynamics during transient arousal from NREM sleep: implications for respiratory control stability. J Appl Physiol. 1996;80:1475–1484. doi: 10.1152/jappl.1996.80.5.1475. [DOI] [PubMed] [Google Scholar]
- 22.Lee JJ, Ramirez SG, Will MJ. Gender and racial variations in cephalometric analysis. Otolaryngol Head Neck Surg. 1997;117:326–329. doi: 10.1016/S0194-5998(97)70121-9. [DOI] [PubMed] [Google Scholar]
- 23.Lowe AA, Ono T, Ferguson KA, Pae EK, Ryan CF, Fleetham JA. Cephalometric comparisons of craniofacial and upper airway structure by skeletal subtype and gender in patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1996;110:653–664. doi: 10.1016/s0889-5406(96)80043-6. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, Loring SH, White DP. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 25.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10:2087–2090. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 26.Mohsenin V. Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest. 2001;120:1442–1447. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell CP, Schwartz AR, Smith PL. Upper airway collapsibility: the importance of gender and adiposity. Am J Respir Crit Care Med. 2000;162:1606–1607. doi: 10.1164/ajrccm.162.5.ed11-00b. [DOI] [PubMed] [Google Scholar]
- 28.Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Respir Crit Care Med. 2000;162:1627–1632. doi: 10.1164/ajrccm.162.5.2003131. [DOI] [PubMed] [Google Scholar]
- 29.Popovic RM, White DP. Influence of gender on waking genioglossal electromyogram and upper airway resistance. Am J Respir Crit Care Med. 1995;152:725–731. doi: 10.1164/ajrccm.152.2.7633734. [DOI] [PubMed] [Google Scholar]
- 30.Rechtschaffen A, Kales AE. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. National Institutes of Health; Los Angeles, CA: 1968. [Google Scholar]
- 31.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 32.Regensteiner JG, Woodard WD, Hagerman DD, Weil JV, Pickett CK, Bender PR, Moore LG. Combined effects of female hormones and metabolic rate on ventilatory drives in women. J Appl Physiol. 1989;66:808–813. doi: 10.1152/jappl.1989.66.2.808. [DOI] [PubMed] [Google Scholar]
- 33.Rowley JA, Sanders CS, Zahn BR, Badr MS. Gender differences in upper airway compliance during NREM sleep: role of neck circumference. J Appl Physiol. 2002;92:2535–2541. doi: 10.1152/japplphysiol.00553.2001. [DOI] [PubMed] [Google Scholar]
- 34.Rowley JA, Zhou X, Vergine I, Shkoukani MA, Badr MS. Influence of gender on upper airway mechanics: upper airway resistance and Pcrit. J Appl Physiol. 2001;91:2248–2254. doi: 10.1152/jappl.2001.91.5.2248. [DOI] [PubMed] [Google Scholar]
- 35.Sforza E, Bacon W, Weiss T, Thibault A, Petiau C, Krieger J. Upper airway collapsibility and cephalometric variables in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:347–352. doi: 10.1164/ajrccm.161.2.9810091. [DOI] [PubMed] [Google Scholar]
- 36.Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med. 1999;159:149–157. doi: 10.1164/ajrccm.159.1.9804140. [DOI] [PubMed] [Google Scholar]
- 37.Sin DD, Jones RL, Man GC. Hypercapnic ventilatory response in patients with and without obstructive sleep apnea: do age, gender, obesity, and daytime PaCO2 matter? Chest. 2000;117:454–459. doi: 10.1378/chest.117.2.454. [DOI] [PubMed] [Google Scholar]
- 38.Thurnheer R, Wraith PK, Douglas NJ. Influence of age and gender on upper airway resistance in NREM and REM sleep. J Appl Physiol. 2001;90:981–988. doi: 10.1152/jappl.2001.90.3.981. [DOI] [PubMed] [Google Scholar]
- 39.Trinder J, Kay A, Kleiman J, Dunai J. Gender differences in airway resistance during sleep. J Appl Physiol. 1997;83:1986–1997. doi: 10.1152/jappl.1997.83.6.1986. [DOI] [PubMed] [Google Scholar]
- 40.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz E, Schory K, Ranieri J, MacDonald M, White DP. Ventilatory control stability predicts apnea severity in patients with an atmospheric pharyngeal critical pressure. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellman A, Malhotra A, Fogel RB, Edwards JK, Schory K, White DP. Respiratory system loop gain in normal men and women measured with proportional-assist ventilation. J Appl Physiol. 2003;94:205–212. doi: 10.1152/japplphysiol.00585.2002. [DOI] [PubMed] [Google Scholar]
- 42.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Hypoxic ventilatory response during sleep in normal premenopausal women. Am Rev Respir Dis. 1982;126:530–533. doi: 10.1164/arrd.1982.126.3.530. [DOI] [PubMed] [Google Scholar]
- 43.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Sexual influence on the control of breathing. J Appl Physiol. 1983;54:874–879. doi: 10.1152/jappl.1983.54.4.874. [DOI] [PubMed] [Google Scholar]
- 44.White DP, Lombard RM, Cadieux RJ, Zwillich CW. Pharyngeal resistance in normal humans: influence of gender, age, and obesity. J Appl Physiol. 1985;58:365–371. doi: 10.1152/jappl.1985.58.2.365. [DOI] [PubMed] [Google Scholar]
- 45.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 46.Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory Am Rev Respir Dis. 1992;145:114–120. doi: 10.1164/ajrccm/145.1.114. [DOI] [PubMed] [Google Scholar]
- 47.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 48.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 49.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol. 2000;89:192–199. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]