Abstract
Background
Schizophrenia has a characteristic onset during adolescence or young adulthood but also tends to persist throughout life. Structural magnetic resonance studies indicate that brain abnormalities are present at onset, but longitudinal studies to assess neuroprogression have been limited by small samples and short or infrequent follow-up intervals.
Methods
The Iowa Longitudinal Study is a prospective study of 542 first-episode patients who have been followed up to 18 years. In this report, we focus on those patients (n = 202) and control subjects (n = 125) for whom we have adequate structural magnetic resonance data (n = 952 scans) to provide a relatively definitive determination of whether progressive brain change occurs over a time interval of up to 15 years after intake.
Results
A repeated-measures analysis showed significant age-by-group interaction main effects that represent a significant decrease in multiple gray matter regions (total cerebral, frontal, thalamus), multiple white matter regions (total cerebral, frontal, temporal, parietal), and a corresponding increase in cerebrospinal fluid (lateral ventricles and frontal, temporal, and parietal sulci). These changes were most severe during the early years after onset. They occur at severe levels only in a subset of patients. They are correlated with cognitive impairment but only weakly with other clinical measures.
Conclusions
Progressive brain change occurs in schizophrenia, affects both gray matter and white matter, is most severe during the early stages of the illness, and occurs only in a subset of patients. Measuring severity of progressive brain change offers a promising new avenue for phenotype definition in genetic studies of schizophrenia.
Keywords: First episode, longitudinal studies, neurodevelopment, neuroprogression, schizophrenia, structural magnetic resonance imaging
Schizophrenia is one of the most important public health problems in the world, ranking as the fourth leading cause of disability among people aged 18 to 45 in developed countries (1). It is a brain disease that typically first manifests itself in young people during their late teens to late 20s, with ongoing signs and symptoms usually occurring throughout the remainder of their lives (2,3). Because the illness begins early but tends to persist throughout life and sometimes worsens, one of the central questions in schizophrenia research is whether the disorder should be conceptualized as a neurodevelopmental disorder, a neuroprogressive disorder, or a combination of the two (4 –9).
Structural magnetic resonance (sMR) imaging provides an opportunity to examine these questions using quantitative measures of brain tissue. One strong piece of evidence indicating that schizophrenia is a neurodevelopmental disorder derives from the fact that many types of brain abnormalities are present in patients who are assessed at the time of their first episode of illness (10 –14). These include decreased cerebral size, decreased frontal and temporal lobe size, decreased thalamic size, decreases in gray matter (GM) and white matter (WM) volume, and increased cerebrospinal fluid (CSF) on the brain surface and in the ventricles. These observations support the likelihood that the illness arises because of aberrations in the complex neurodevelopmental processes that modulate brain maturation during the adolescent and young adult period.
One of the important remaining questions about the nature of the brain abnormalities that characterize schizophrenia concerns their course after disease onset. Do the brain processes that trigger the illness stabilize after onset or does the brain disease continue to progress, producing an additional loss of brain tissue? If changes continue to progress after onset, what is the pattern of change? Is it more severe during early years of the illness or during later years of the illness or does it proceed at the same rate over time? To address these questions, a prospective longitudinal design of long duration with frequent sampling of time points is necessary. Although a few studies have been completed using longitudinal designs, their sample sizes are too small and the follow-up periods too short or infrequent to reach a definitive conclusion about the long-term course of brain changes in schizophrenia (15–23). This report addresses these questions using sMR data collected in the Iowa Longitudinal Study of first-episode schizophrenia, which comprises the largest sample of prospectively followed patients and control subjects collected to date and the longest surveillance period ever examined.
Methods and Materials
Subjects
The Iowa Longitudinal Study was initiated in 1987 and includes a total cohort of 542 first-episode schizophrenia patients who were recruited after their initial presentation for a schizophrenia spectrum disorder. These patients were drawn from consecutive admissions to the University of Iowa Psychiatry Inpatient Service at a rate of 20 to 30 per year; intake into the study ended in 2007. Exclusion criteria included an IQ = 70, history of a significant head injury, or presence of metal implants. They were followed at 6-month intervals after initial intake, with assessment of clinical symptoms, psychosocial function, and treatment received. Intensive assessments (sMR and cognitive testing) were done at intake and at 2, 5, 9, 12, 15, and 18 years. For a study of this length, attrition is remarkably low. Attrition is due to a variety of factors: death by suicide, 15; death because of other factors, 7; being in jail, 2; administrative drop because of change in diagnosis, 29; lost or moved out of the area, 61; refusal to remain in the study, 126. True attrition is probably best defined by those who have been lost or who refused, n = 187, or a rate of 34.5%. Our retention rate is thus a remarkable 65.5% over 19 years. We have compared the subjects who were lost or refused with those who remain in the study, using a variety of variables (e.g., age at onset, age at intake, severity of symptoms at intake, IQ at intake, magnetic resonance [MR] measures at intake). We find no significant differences between those who are in the attrition group and those who continue to participate in the study, suggesting that the sample that we are following is representative of the illness.
In this report, we focus on those subjects for whom we have adequate sMR data to provide a relatively definitive determination of whether progressive brain change occurs over a time interval that is up to 15 years after intake. These comprise a total of 202 patients for whom we have a minimum of 2 scans and a maximum of 5; the total number of scans analyzed is 640; the mean interval between first scan and last available scan = 7.2 years (SD = 3.79; maximum = 15). Their mean age at intake was 24.56 (SD 7.14). One hundred forty-eight were male and 54 were female. Their mean parental education was 13.38 years. Nearly half the patients, or 92, were neuroleptic naive at the time of entry into the study. These patients are compared with a group of healthy normal volunteers (n = 125), recruited from the community by newspaper advertising or word of mouth, and matched to the patients on parental education. The total number of control scans analyzed is 312, with a minimum of 2 and a maximum of 5. Control subjects were screened to rule out a past history of any major psychiatric, neurologic, or general medical disorder, as well as a family history of schizophrenia. Their mean age at intake was 29.69 years (SD 8.37); 66 were male and 59 were female (Supplement 1). Their mean parental education was 13.27 years.
All subjects provided written informed consent, as approved by our Institutional Review Board.
sMR Data Acquisition
In this study, we used two scanning protocols, which we refer to as MR5 and MR6 (Supplement 1). Both are multimodal (i.e., acquire T2 or proton density [PD] sequences in addition to T1), thereby providing optimal discrimination between GM, WM, and CSF. For MR5 subjects, each participant’s data included T1-, T2-, and PD-weighted images collected on a 1.5-T GE Signa scanner (GE Health-care, Waukesha, Wisconsin). MR6 was acquired on a 1.5-T Siemens Avanto scanner (Siemens AG, Muenchen, Germany) using T1 and T2 sequence. Subjects continued in the same sequence throughout the study; those that began with an MR5 protocol remained in it for all scans, and those that began with an MR6 protocol remained in it as well. The two sequences differ primarily in slice thickness and in-plane resolution; both are acquired in the coronal plane. Voxel size for MR5 is 1.0 × 1.0 × 1.5 mm (T1) and 1.0 × 1.0 × 3 mm (T2); voxel size for MR6 is .625 ×.625 × 1.5 mm (T1) and .625 ×.625 × 1.8 mm (T2). Both are multimodal; MR5 acquires T1, T2, and PD sequences, while MR6 uses only T1 and T2. Our multimodal approach permits us to measure surface CSF and delineate the CSF/GM and GM/WM borders more precisely.
Image Analysis
The MR scans were analyzed using BRAINS2 software (created at The University of Iowa Carver College of Medicine by Dr. Andreasen’s IPL staff, Iowa City, Iowa), a locally developed program that now yields automated quantitative measures of multiple brain regions and tissue types (24 –28). Although BRAINS2 analysis was semiautomated for many years, we have recently introduced advanced image processing algorithms that eliminate the need for manual intervention at the stages of image realignment, tissue sampling, and mask editing. In addition, inhomogeneity correction, intensity normalization, and mask cleaning routines have been added to improve the accuracy and consistency of the results. This fully automated image processing routine is known as AutoWorkup (29). To eliminate any measurement artifacts that might be due to rater drift over time and to reduce any that might be due to scanner upgrades, we recently reanalyzed all 952 scans used for this report using this new AutoWorkup program.
Determining the Feasibility of Pooling the Two Sequences
Ensuring that findings are not because of scanner artifacts is a major challenge in sMR research and especially in longitudinal research. To test for comparable reliability and validity of MR5 and MR6, we acquired both types of scans back-to-back in 60 subjects to verify our ability to combine the data from both sequences in our longitudinal analyses. Through the use of minor preprocessing before application of our workup, we are able to process both MR5 and MR6 scans to extract brain measures that are essentially identical. To assess the feasibility of combining data from the two scan sequences, only the modalities common to both (T1 and T2) were used in the analysis. To further reduce the differences, a preprocessing step was included to down-sample the raw images from the MR6 sequence to the same resolution as the raw images from the MR5 sequence. Using AutoWorkup, all scans underwent field inhomogeneity correction and signal intensity normalization, which removes scanner-dependent variation over time and between the two sequences. We found that, using AutoWorkup, the intraclass correlation coefficients were consistently > .96 and usually .98 to .99 for all brain regions, apart from occipital cortex (which is reported in Supplement 1). Therefore, we have concluded that we can pool data that use the two different scanning sequences. Figure 1 illustrates a typical MR5 and MR6 scan from the same individual, as well as a difference map indicating the areas where the two scan types differ.
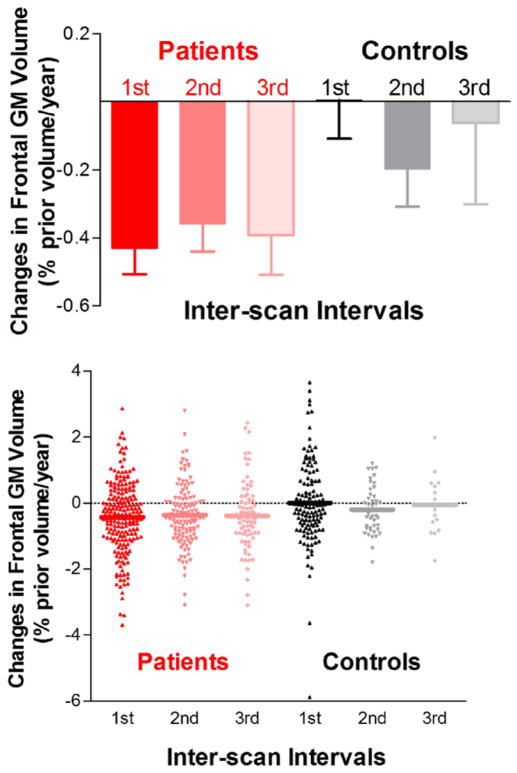
Figure 1.
Bar graphs and scatter plots illustrating the pattern of brain changes over time. Frontal gray matter (GM) changes in schizophrenia patients were most pronounced early in the course of schizophrenia. Frontal GM in schizophrenia patients differed significantly from healthy volunteers during the first interscan interval but not during subsequent intervals.
Cognition
All subjects were evaluated with a comprehensive cognitive battery administered by trained neuropsychologists. To provide comprehensive measurements of cognitive functioning, 36 neuropsychological test variables were grouped into six cognitive domains: verbal learning, attention, problem solving, working memory, verbal fluency, and motor speed (12).
Statistical Analysis
Analyses were performed using SAS (version 9.2; SAS, Cary, North Carolina). General linear mixed models were used to compute changes over time. Intracranial volume at initial MR scan, gender, imaging protocol (MR5 vs. MR6), and age at initial MR scan were included as covariates. To take into account within-subject correlations in brain volumes, subject was entered as random effects (PROC MIXED REPEATED statement). Compound symmetry covariance structure for repeated subject measures was used because its fit statistics were most consistently superior to unstructured correlation matrix. Relationships between brain measures and cognitive and clinical measures were examined using Pearson correlation coefficients; a two-sided p value < .05 was used to determine statistical significance. Because we consider this analysis to be exploratory, we did not correct for multiple comparisons.
To derive the six cognitive domain scores, the age-standardized raw test score from each of the 36 neuropsychological test variables was converted to a Z score (mean = 0, SD = 1) based on norms established using our center’s database of 546 healthy control subjects. This database was used to establish norms for our domain scores. The Z scores were reversed where necessary so that a larger negative score indicates poorer performance below the mean. Using these Z scores, each domain score is the summed average of its component neuropsychological test variables.
Results
Between-Group Differences Over Time
Table 1 shows a repeated-measures mixed-model analysis of variance that examines amount of brain change over time using all 952 sMR scans from patients and control subjects (i.e., all available time points). Age main effects were highly significant for almost every MR brain volume measure, indicating that GM and WM loss and a related CSF increase are occurring over time in both the patients and the control subjects as a consequence of aging. The age-by-group interactions indicate the brain regions for which schizophrenia patients differ from the normal control subjects over time, reflecting significantly greater progressive brain change in the patients. There are significant age-by-group interaction main effects for multiple GM and WM regions. Significant interactions are also present for multiple CSF measures, generally reflecting the areas in which brain tissue is decreased. Among the subcortical measures, only the thalamus shows an interaction effect. B-coefficients indicate the percent of tissue loss (or increase, in the case of CSF measures) expressed as cubic centimeters per year. Among the regions that differ significantly between patients and control subjects, the actual amount of loss is relatively small.
Table 1.
Comparison of MRI Brain Volume Changes Between Schizophrenia Patients and Healthy Volunteers
| Regions of Interest | B-Coefficient (SE) (CC/year)
|
Age F(1,621) (p) | Age × Group Interaction F(1,621) (p) | |
|---|---|---|---|---|
| Patients (n = 202) | Control Subjects (n = 125) | |||
| Total Cerebral Tissue | −2.34 (.28) | −1.05 (.22) | 259.1 (<.0001) | 24.2 (<.0001) |
| Total Cerebral GM | −1.50 (.20) | −1.62 (.16) | 228.9 (<.0001) | 2.87 (.09) |
| Frontal GM | −.97 (.11) | −.77 (.08) | 313.4 (<.0001) | 20.9 (<.0001) |
| Temporal GM | −.17 (.05) | −.27 (.04) | 48.9 (<.0001) | .02 (.89) |
| Parietal GM | −.31 (.07) | −.37 (.05) | 171.6 (<.0001) | .33 (.57) |
| Total Cerebral WM | −.85 (.27) | .57 (.21) | 15.7 (<.0001) | 12.0 (.0006) |
| Frontal WM | −.56 (.12) | .06 (.09) | 45.8 (<.0001) | 11.4 (.0008) |
| Temporal WM | .01 (.05) | .20 (.04) | 1.4 (.24) | 8.0 (.005) |
| Parietal WM | −.11 (.08) | .23 (.06) | .7 (.41) | 5.4 (.02) |
| Lateral Ventricles | .37 (.07) | .24 (.05) | 119.8 (<.0001) | 7.5 (.006) |
| Sulcal CSF | 1.57 (.22) | .76 (.17) | 273.3 (<.0001) | 31.1 (<.0001) |
| Frontal CSF | 1.19 (.16) | .72 (.13) | 264.7 (<.0001) | 19.0 (<.0001) |
| Temporal CSF | .27 (.03) | .09 (.03) | 189.1 (<.0001) | 38.4 (<.0001) |
| Parietal CSF | .48 (.07) | .34 (.05) | 198.3 (<.0001) | 6.6 (.01) |
| Caudate | −.033 (.006) | −.035 (.005) | 57.2 (<.0001) | .02 (.88) |
| Putamen | −.044 (.008) | −.041 (.007) | 103.5 (<.0001) | .49 (.48) |
| Thalamus | −.064 (.008) | −.036 (.006) | 151.0 (<.0001) | 18.5 (<.0001) |
CSF, cerebrospinal fluid; GM, gray matter; MRI, magnetic resonance imaging; WM, white matter.
Pattern of Changes Over Time
To determine the temporal pattern of these changes and specifically whether they are more virulent during a particular time period over the course of the illness, we conducted an analysis that examined the amount of change that occurred between multiple scan intervals. The results of this analysis are shown in Table 2. In this analysis, we used only the three earliest interscan intervals, because the number of subjects at the later time points in the study is relatively small. The interscan interval in this table is usually 2 years for the first interval and 3 or 4 years for subsequent intervals. As Table 2 indicates, brain tissue volume reductions and CSF volume enlargements in schizophrenia patients were greatest during the first interscan interval; patients had significantly greater reductions than healthy volunteers in nearly all GM and WM measures and a corresponding increase in CSF. Brain volume changes in patients during the second and third interscan intervals did not differ significantly from healthy volunteers. These results are shown graphically in Figure 1.
Table 2.
Comparison of Brain Volume Changes with Each Successive Interscan Intervala Between Healthy Volunteers and Schizophrenia Patients
| First Interscan Interval Control
|
T (p) | Second Interscan Interval Control
|
T (p) | Third Interscan Interval Control
|
T (p) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Subjects (n = 125) | Patients (n = 202) | Subjects (n = 42) | Patients (n = 126) | Subjects (n = 15) | Patients (n = 79) | ||||
| Cerebral Tissue | .02 (.64) | −.31 (.82) | 3.86 (.0001) | −.01 (.46) | −.00 (.72) | .05 (.96) | −.12 (.39) | −.32 (.47) | 1.55 (.13) |
| Cerebral GM | .10 (1.15) | −.16 (.88) | 2.33 (.02) | −.13 (.67) | −.27 (.70) | 1.17 (.24) | −.01 (.62) | −.30 (.81) | 1.34 (.19) |
| Frontal GM | .01 (1.28) | −.43 (1.08) | 3.30 (.001) | −.20 (.72) | −.36 (.92) | 1.04 (.30) | −.06 (.93) | −.39 (1.04) | 1.14 (.26) |
| Temporal GM | .12 (1.02) | −.08 (.81) | 2.01 (.04) | .10 (.82) | −.16 (1.08) | 1.42 (.16) | .00 (.65) | −.14 (.77) | .65 (.52) |
| Parietal GM | −.00 (1.36) | −.37 (1.17) | 2.56 (.01) | −.28 (.91) | −.24 (1.24) | .19 (.85) | −.10 (.57) | −.27 (1.05) | .60 (.55) |
| Cerebral WM | −.07 (1.31) | −.49 (1.98) | 2.11 (.04) | .16 (.85) | .43 (1.45) | 1.16 (.25) | −.24 (.98) | −.36 (1.23) | .35 (.73) |
| Frontal WM | −.10 (1.35) | −.63 (1.99) | 2.62 (.009) | .05 (.95) | .26 (1.56) | .79 (.43) | −.35 (.98) | −.44 (1.27) | .22 (.83) |
| Temporal WM | .34 (1.83) | −.25 (1.95) | 2.69 (.008) | .13 (.98) | .50 (1.53) | 1.48 (.14) | −.25 (.79) | −.42 (1.31) | .48 (.63) |
| Parietal WM | −.01 (1.38) | −.03 (1.98) | .09 (.92) | .19 (.98) | .43 (1.59) | .91 (.36) | −.29 (1.02) | −.43 (1.32) | .38 (.71) |
| Sulcal CSF | 4.29 (9.80) | 7.08 (14.22) | 1.93 (.05) | 2.89 (5.57) | 3.65 (8.34) | .56 (.58) | 3.92 (4.80) | 7.16 (11.00) | 1.12 (.27) |
| Lateral Ventricles | 2.08 (6.27) | 2.43 (6.42) | .48 (.63) | 1.23 (4.25) | 1.40 (4.19) | .22 (.82) | 1.08 (1.97) | 1.86 (4.07) | .72 (.47) |
| Caudate | 1.24 (3.05) | .27 (2.13) | 3.32 (.001) | −.24 (1.91) | .33 (2.32) | 1.41 (.16) | −.55 (1.17) | −.74 (1.41) | .49 (.63) |
| Putamen | −.17 (1.50) | .69 (2.84) | 3.13 (.002) | −.83 (1.21) | −.24 (1.69) | 2.06 (.04) | −.29 (.82) | −.87 (1.39) | 1.54 (.13) |
| Thalamus | .16 (1.79) | −.41 (2.00) | 2.61 (.009) | −.21 (1.15) | −.34 (1.52) | .53 (.60) | .02 (1.14) | −.75 (1.27) | 2.20 (.03) |
Brain tissue volume reductions and CSF volume enlargements in schizophrenia patients were greatest during the first interscan interval. Patients had significantly greater reductions than healthy volunteers on cerebral tissue, total cerebral GM, frontal GM, temporal GM, and parietal GM volumes (T ≥ 2.01, p ≤ .04) at time 2 scan compared with time 1 scan. Sulcal CSF volume enlargements in patients during the first interscan interval were significantly greater than healthy volunteers (T = 1.93, p = .05). Lateral ventricle changes in patients during the first interscan interval did not differ significantly from those in control subjects (T = .48, p = .63). Although brain tissue volume reductions and CSF enlargements in patients during the second and third interscan intervals were greater than those in control subjects, these differences were not statistically significant (T ≤ 1.55, p ≥ .13).
CSF, cerebrospinal fluid; GM, gray matter; WM, white matter.
Mean percent of prior scan per year (SD); first interscan interval: (time 2 scan volume minus time 1 scan volume) divided by time 1 scan volume per year (df = 325); second interscan interval: (time 3 scan volume minus time 2 scan volume) divided by time 2 scan volume per year (df = 166); third interscan interval: (time 4 scan volume minus time 3 scan volume) divided by time 3 scan volume per year (df = 92).
Magnitude and Variation of Tissue Loss in Individual Patients
These results provide information about between-group differences over time, the average annualized percent tissue loss over time through the beta coefficients, and the more specific timing of the changes during the early years after onset. However, they do not indicate the magnitude and variation of the tissue loss in individual patients. That is, does the process of neuroprogression after onset affect everyone diagnosed with schizophrenia or does it affect only a subgroup of patients who have neuroprogressive schizophrenia? Therefore, we also examined how many individual patients showed tissue decreases and the nature of the amount, using measurements from the first and second interscan intervals and dividing by the number of years between scans, so that we could have an individual measure of percent tissue loss per year during the time period with greatest severity. In this analysis, we found that 34% of the patients were losing overall cerebral tissue at a rate of at least .5% per year and 14% at a rate of 1% (as compared with 19% and 3% in control subjects). In the case of frontal GM, 47% were losing at least .5% and 34% were losing at least 1% (as compared with 36% and 23% in control subjects). In the case of frontal WM, 55% were losing at least .5% and 40% were losing at least 1% (as compared with 38% and 26% in control subjects).
Relationship to Clinical Measures
We examined three kinds of clinical correlates to determine whether the severity of brain tissue loss during the early years after onset may predict later clinical outcomes. We examined three outcomes: performance on cognitive tests, symptom patterns, and overall clinical improvement as measured by the amount of time that subjects achieved symptomatic remission. In these analyses, we examine the relationship between brain tissue loss during the first interscan interval, expressed as percent change, and scores at the most recent clinical follow-up.
Cognitive Impairment
Correlations between changes in brain measures and cognitive test performance are shown in Table 3. The strongest relationships are observed for the domains that assess verbal learning, attention, problem solving, and working memory. Progressive brain changes are weakly related to verbal fluency and have no relationship with motor speed. White matter loss has a much stronger relationship with cognitive function than GM loss. In terms of regional patterns, frontal tissue loss (WM or total frontal tissue) is associated with poorer performance on tests of verbal learning, attention, and working memory; temporal tissue loss is associated with poorer performance on the domains of verbal learning, attention, problem solving, verbal fluency, and working memory; parietal loss is associated with all domains except for motor speed.
Table 3.
Correlations Between Brain Tissue Change and Cognitive Measures
| Verbal Learning
|
Attention
|
Problem Solving
|
Verbal Fluency
|
Working Memory
|
Motor Speed
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Total Cerebral Tissue | .173 | .016 | .230 | .001 | .149 | .039 | .116 | .110 | .232 | .001 | .030 | .686 |
| Total Cerebral GM | −.016 | .828 | −.004 | .954 | .021 | .768 | .076 | .292 | .027 | .712 | .030 | .676 |
| Frontal GM | .068 | .347 | .088 | .225 | .031 | .666 | .062 | .392 | .054 | .452 | .044 | .553 |
| Temporal GM | −.014 | .843 | −.028 | .701 | .014 | .844 | −.020 | .784 | .046 | .529 | .057 | .436 |
| Parietal GM | .014 | .845 | .047 | .516 | .033 | .648 | .137 | .058 | .025 | .728 | .049 | .506 |
| Total Cerebral WM | .169 | .019 | .212 | .003 | .126 | .082 | .051 | .479 | .191 | .008 | .015 | .839 |
| Frontal WM | .145 | .044 | .242 | .001 | .136 | .060 | .085 | .240 | .213 | .003 | .016 | .831 |
| Temporal WM | .167 | .020 | .164 | .023 | .166 | .021 | .102 | .158 | .228 | .001 | −.015 | .840 |
| Parietal WM | .215 | .003 | .181 | .012 | .145 | .044 | .076 | .292 | .173 | .016 | .014 | .850 |
| Total Cerebral CSF | −.137 | .057 | −.141 | .051 | −.083 | .253 | −.114 | .113 | −.108 | .134 | −.043 | .556 |
| Frontal CSF | −.086 | .236 | −.100 | .167 | −.047 | .516 | −.090 | .213 | −.067 | .352 | −.055 | .451 |
| Temporal CSF | −.133 | .065 | −.130 | .071 | −.085 | .241 | −.146 | .043 | −.090 | .214 | −.046 | .532 |
| Parietal CSF | −.180 | .013 | −.204 | .005 | −.156 | .031 | −.129 | .077 | −.228 | .002 | .017 | .820 |
| Total Frontal Lobe | .163 | .024 | .256 | .000 | .126 | .080 | .113 | .119 | .212 | .003 | .032 | .666 |
| Total Temporal Lobe | .140 | .053 | .124 | .087 | .161 | .025 | .077 | .286 | .244 | .001 | .026 | .721 |
| Total Parietal Lobe | .211 | .003 | .198 | .006 | .152 | .034 | .163 | .023 | .177 | .014 | .043 | .555 |
| VBR | −.191 | .008 | −.183 | .011 | −.146 | .043 | −.108 | .136 | −.185 | .010 | −.063 | .387 |
| Putamen | .133 | .065 | .086 | .235 | .165 | .022 | .075 | .298 | .065 | .370 | −.108 | .139 |
| Thalamus | .099 | .172 | .106 | .144 | .112 | .121 | .137 | .058 | .156 | .029 | .011 | .877 |
| Caudate | .103 | .168 | .177 | .017 | .177 | .017 | .093 | .210 | .185 | .013 | .017 | .819 |
Correlations are between the amount of percent change and the cognitive dimension score at the most recent follow-up visit; a negative correlation indicates a greater percent change score associated with a worse cognitive dimension score, while a positive correlation indicates a lower percent change and a better cognitive dimension score. Bold indicates variables that are statistically significant.
CSF, cerebrospinal fluid; GM, gray matter; VBR, ventricular-brain ratio; WM, white matter.
Symptom Dimensions
Correlations between changes in brain measures and the three symptom dimensions of schizophrenia (negative symptoms, psychotic symptoms, and disorganization) are shown in Table 4 (30,31). Relationships are relatively sparse. The strongest are with the psychotic dimension, which has significant negative correlations with total cerebral tissue, WM in the cerebrum and the frontal and temporal lobes, and total size of the frontal and temporal lobes. Negative symptoms have an association with increases in total CSF and in the ventricles as indicated by the ventricular-brain ratio.
Table 4.
Correlations Between Brain Tissue Change and Symptom Dimensions
| Negative
|
Psychotic
|
Disorganized
|
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Total Cerebral Tissue | −.026 | .726 | −.189 | .009 | −.058 | .428 |
| Total Cerebral GM | .134 | .067 | .087 | .234 | .004 | .951 |
| Frontal GM | .033 | .651 | −.006 | .936 | −.045 | .541 |
| Temporal GM | .087 | .233 | .024 | .739 | −.067 | .363 |
| Parietal GM | .132 | .071 | .104 | .148 | .008 | .910 |
| Total Cerebral WM | −.120 | .101 | −.227 | .002 | −.055 | .453 |
| Frontal WM | −.124 | .091 | −.234 | .001 | −.052 | .480 |
| Temporal WM | −.115 | .117 | −.279 | .000 | −.063 | .387 |
| Parietal WM | −.057 | .434 | −.137 | .061 | −.040 | .585 |
| Total CSF | .161 | .027 | −.120 | .101 | −.121 | .099 |
| Frontal CSF | .038 | .607 | .115 | .116 | .015 | .842 |
| Temporal CSF | .024 | .749 | .108 | .139 | −.029 | .691 |
| Parietal CSF | .049 | .511 | .194 | .008 | .126 | .088 |
| Frontal Total | −.083 | .260 | −.203 | .005 | −.072 | .325 |
| Temporal Total | −.036 | .628 | −.236 | .001 | −.116 | .112 |
| Parietal Total | .044 | .553 | −.053 | .472 | −.032 | .665 |
| VBR | .148 | .043 | .191 | .009 | .020 | .786 |
Correlations are between the amount of percent change and the symptom dimension score at the most recent follow-up visit; a negative correlation indicates a greater percent change associated with a worse symptom severity, while a positive correlation indicates a lower percent change and a better symptom severity. Bold indicates variables that are statistically significant.
CSF, cerebrospinal fluid; GM, gray matter; VBR, ventricular-brain ratio; WM, white matter.
Time Spent in Remission
Remission of symptoms is an important clinical outcome measure. Remission is defined as having symptoms that are rated as mild or less using the Scale for the Assessment of Negative Symptoms and Scale for the Assessment of Positive Symptoms (32). More than half of the patients (n = 103) were in remission for at least 1 year during the follow-up period. Among those who achieved remission, the average total length of time in remission was 6.4 years. As shown in Table 5, remission status is significantly correlated with CSF measures, such that patients who achieve longer periods of remission have less expansion of CSF, which serves as a marker of generalized and regional brain tissue loss.
Table 5.
Correlations Between Brain Tissue Change and Remission
| r | p | |
|---|---|---|
| Total Cerebral Tissue | .083 | .245 |
| Total Cerebral GM | .031 | .661 |
| Frontal GM | .039 | .588 |
| Temporal GM | .003 | .972 |
| Parietal GM | .032 | .656 |
| Total Cerebral WM | .098 | .168 |
| Frontal WM | .111 | .119 |
| Temporal WM | .080 | .263 |
| Parietal WM | .078 | .272 |
| Total Cerebral CSF | −.169 | .017 |
| Frontal CSF | −.146 | .040 |
| Temporal CSF | −.112 | .117 |
| Parietal CSF | −.184 | .010 |
| Total CSF | −.147 | .038 |
| Total Frontal | .121 | .088 |
| Total Temporal | .073 | .309 |
| Total Parietal | .043 | .550 |
| VBR | −.239 | .001 |
Correlations are between the amount of percent change and the total amount of time spent in remission by the time of the most recent follow-up visit; a negative correlation indicates a larger percent change and a shorter remission time. Bold indicates variables that are statistically significant.
CSF, cerebrospinal fluid; GM, gray matter; VBR, ventricular-brain ratio; WM, white matter.
Discussion
This study indicates that some schizophrenia patients are undergoing a process of progressive brain change, defined as a decrement in brain tissue volume that is occurring at a more rapid rate in patients than in control subjects. Thus, schizophrenia has a neuro-progressive component, defined as tissue volume decrease occurring after onset. The changes include both GM and WM and manifest their greatest severity in the frontal lobes.
The GM loss is consistent with the known neuropathology of the illness, which involves cortical thinning, due primarily to a shrinkage in neuropil, and without either the neuronal loss or gliosis that characterize neurodegenerative processes (33,34). The GM loss could be explained by several different neurodevelopmental mechanisms. One is pathologically extended pruning. It is well-established that normal adolescent brain development is characterized by reduction of GM and expansion of WM, processes that sculpt the brain into maturity and expand its capacity for high-level reasoning and problem solving (35–37). The typical onset of schizophrenia during this time period is thought to occur as a consequence of a defect in this normal maturational process (9), which causes abnormalities in connectivity of brain networks that, in turn, lead to the characteristic features of the illness, such as abnormalities in perception (hallucinations), inferential thinking (delusions), decreased fluency of thought and speech (alogia), or decreased emotional expression (affective blunting). If the normal process of pruning was not only defective during adolescence (overpruning) but also continued its defective actions into a later time period, such as the 20s and 30s (i.e., pathologically extended pruning), a single neurodevelopmental mechanism might explain the observation that GM is decreased at the time of onset and that it also continues to decrease during the years immediately after onset. Another mechanism that may explain the GM decreases that occur after the onset of schizophrenia is diminished neuroplasticity, an impairment in activity-dependent neuroplasticity that affects spines and synapses, leading to a shrinkage of neuropil. In this scenario, the driving force would be an impairment in the processes that regulate activity-dependent modeling of the pattern and strength of synaptic connections, with associated reduction in spines and even dendritic arbors. This would also manifest itself neuropathologically as a shrinkage in neuropil and cortical thinning without gliosis or neuronal loss. Genes that regulate neurodevelopment and neuroplasticity (e.g., BDNF, DISC1, ERBB4, NRG1) are candidate causal agents for the GM findings (38,39).
Another possible explanation for the brain tissue loss is that it is a consequence of some confounder, such as neuroleptic treatment. Studies of neuroleptic-treated animals and of human postmortem tissue have been used to explore this possibility (40 – 43). While the postmortem studies have generally not shown a relationship between treatment intensity and tissue change, controlled animal studies have indicated that neuroleptic-treated animals have brain volume reductions. We have examined treatment effects in the current sample and have found that both follow-up duration and neuroleptic treatment contribute significantly and independently to the tissue volume reductions, while other possible confounders such as substance misuse do not (44). Therefore, while neuroprogression may be partially accounted for by a medication effect, it also reflects an intrinsic and progressive disease process.
Although multiple brain regions display progressive changes in GM, the most severe loss occurs in the frontal lobes. The frontal lobes are the last brain region to mature in human beings, and this maturational process also occurs during adolescence and early adulthood. It is noteworthy that we find progressive brain change after onset in both frontal lobes and in the thalamus, because prefrontal cortex receives major input from the thalamus (45,46). In schizophrenia, these two closely interconnected regions, which are already decreased in size in first-episode patients, continue to decrease further after onset.
We have also found that progressive brain changes affect WM even more pervasively than GM. This finding adds to the growing evidence that schizophrenia is a disease of abnormal structural connectivity and is both a WM and GM disorder (12,44 – 46). These WM findings cannot be directly explained by aberrant neurodevelopmental processes, such as overpruning, pathologically extended pruning, or diminished neuroplasticity, because these mechanisms affect GM. In fact, during normal adolescent brain development, WM is expanding while GM is declining; this maturational process is particularly prominent in the frontal lobes (32–34). One possible explanation for a decline of WM after the clinical onset of schizophrenia is that normal processes are simply diminished, e.g., WM expansion is reduced, because of an impairment in myelination and factors that affect it, such as the function of oligodendroglia and the genes that regulate myelination (e.g., NRG1, ERBB3, ERBB4) (47–51).
This study has a variety of limitations. We were not able to obtain scans for each subject at all time points, making the analysis of progression over time more complex and potentially less definitive. Control subjects and patients were not perfectly matched; the control subjects were slightly older and included a larger proportion of female subjects, adding further complexity to the analysis of progression. In the analysis of the pattern of change over time, the sample sizes of both patients and control subjects become progressively smaller over time, potentially reducing the power to detect changes at later time points. The surveillance period is limited to the first 12 to 15 years after onset or an age range of the 20s to early 40s; this does not permit us to determine if progression again accelerates in patients during later life and leads to more rapid later deterioration. The amount of change over time (when calculated in a linear fashion as percent change per year) is less than that reported in many studies; as indicated in Table 2 and Figure 2, this type of linear calculation oversimplifies the measurements; on the other hand, very few other studies have measurements on such large samples at so many time points. Finally, none of the analyses has been corrected for multiple comparisons. In general, a priori directional hypotheses had been made for the brain measure analyses, thereby lessening the need for Bonferroni corrections. The analyses examining the relationship between brain measures and clinical variables are regarded as exploratory and require replication in subsequent studies.
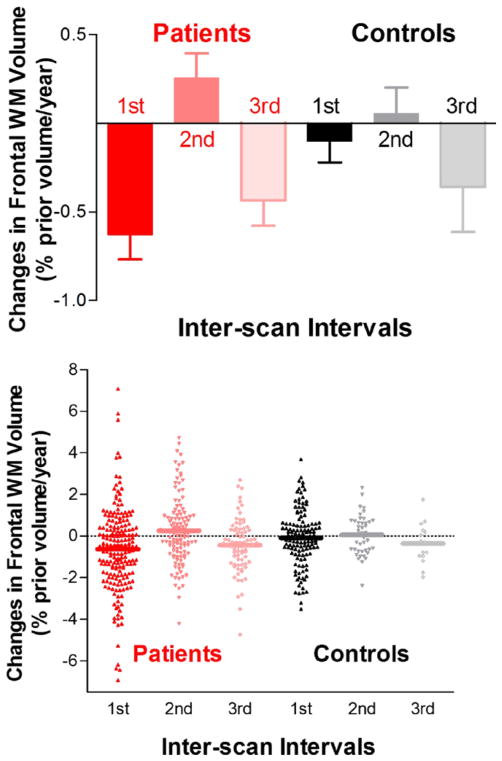
Figure 2.
Frontal white matter (WM) volume reductions in schizophrenia patients were most pronounced early in the course of schizophrenia. Frontal WM reductions in schizophrenia patients differed significantly from healthy volunteers during the first interscan interval but not during subsequent intervals.
Our findings have multiple clinical implications. First, schizophrenia is phenotypically diverse at the neural level; progressive brain change occurs only in a subset of patients. This subset may represent a biologically distinct subgroup of schizophrenia patients that could be designated as having neuroprogressive schizophrenia and that may have a distinct pathophysiology and molecular and cell biology. Basing phenotype definition on measures of progressive brain change may offer a more powerful strategy for identifying genetic mechanisms than symptom patterns or DSM diagnoses. It is also important to note that the majority of patients are not losing brain tissue at an abnormally rapid rate, a finding that offers good news. Second, progressive brain tissue loss is correlated most closely with cognitive performance; its relationship with symptom dimensions or remission is more modest. These clinical associations provide some additional support for defining neuroprogressive schizophrenia as a biologically meaningful subtype but also suggest that symptom measures may provide a relatively weak approach for phenotype definition. Third, the losses are greatest during the early stages of the illness, and they may occur as a consequence of impaired neuroplasticity or structural or functional connectivity in the brain as a whole but particularly the frontal lobes and the thalamus. This suggests a need to continue the ongoing search for treatments that might be particularly effective during early phases and that might enhance connectivity, neuroplasticity, and cognition. Early use of cognitive rehabilitative strategies may be a promising choice, while we also continue to search for pharmaceutical treatments that will directly target neurodevelopmental mechanisms.
Supplementary Material
Acknowledgments
This article was written with support from the following Grants: Mental Health Clinical Research Center: Neurobiology and Phenomenology of the Major Psychoses (MH43271); Phenomenology and the Classification of Schizophrenia (5R01MH031593); Magnetic Resonance Imaging in the Major Psychoses (5R01MH040856); Training in the Neurobiology of Schizophrenia and Evaluation with diffusion tensor imaging (Magnotta K Award); and Brain research: Analysis of Images, Networks, and Systems Morphology and Image Analysis (5R01NS050568). Dr. Ho received funding support from National Institutes of Health (MH68380) and National Association for Research on Schizophrenia and Depression.
Footnotes
Supplementary material cited in this article is available online.
Drs. Andreasen and Ho report receiving research funding from Ortho-McNeill Janssen Scientific Affairs, LLC. Mr. Pierson reports that he is owner of Brain Image Analysis, LLC. Drs. Nopoulos and Magnotta and Mr. Ziebell report no biomedical financial interests or potential conflicts of interest.
References
- 1.Murray CJL, Lopez AD Harvard School of Public Health, World Health Organization, World Bank. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: Harvard School of Public Health, Harvard University Press; 1996. [Google Scholar]
- 2.Andreasen NC. Pieces of the schizophrenia puzzle fall into place. Neuron. 1996;16:697–700. doi: 10.1016/s0896-6273(00)80090-2. [DOI] [PubMed] [Google Scholar]
- 3.Kraepelin E, Barclay RM, Robertson GM. Dementia Praecox and Paraphrenia. Edinburgh: E&S Livingstone; 1919. [Google Scholar]
- 4.Andreasen N, Nasrallah HA, Dunn V, Olson SC, Grove WM, Ehrhardt JC, et al. Structural abnormalities in the frontal system in schizophrenia. A magnetic resonance imaging study. Arch Gen Psychiatry. 1986;43:136–144. doi: 10.1001/archpsyc.1986.01800020042006. [DOI] [PubMed] [Google Scholar]
- 5.Castle DJ, Murray RM. The neurodevelopmental basis of sex differences in schizophrenia. Psychol Med. 1991;21:565–575. doi: 10.1017/s0033291700022194. [DOI] [PubMed] [Google Scholar]
- 6.Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S. Infants at risk for schizophrenia: Sequelae of a genetic neurointegrative defect. A review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry. 1992;49:221–235. doi: 10.1001/archpsyc.1992.01820030053007. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 8.Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 10.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: An anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nopoulos P, Torres I, Flaum M, Andreasen NC, Ehrhardt JC, Yuh WT. Brain morphology in first-episode schizophrenia. Am J Psychiatry. 1995;152:1721–1723. doi: 10.1176/ajp.152.12.1721. [DOI] [PubMed] [Google Scholar]
- 12.Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: A longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 13.Lim KO, Tew W, Kushner M, Chow K, Matsumoto B, DeLisi LE. Cortical gray matter volume deficit in patients with first-episode schizophrenia. Am J Psychiatry. 1996;153:1548–1553. doi: 10.1176/ajp.153.12.1548. [DOI] [PubMed] [Google Scholar]
- 14.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arango C, Moreno C, Martínez S, Parellada M, Desco M, Moreno D, et al. Longitudinal brain changes in early-onset psychosis. Schizophr Bull. 2008;34:341–353. doi: 10.1093/schbul/sbm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degreef G, Ashtari M, Wu HW, Borenstein M, Geisler S, Lieberman J. Follow up MRI study in first episode schizophrenia. Schizophr Res. 1991;5:204–206. doi: 10.1016/0920-9964(91)90075-3. [DOI] [PubMed] [Google Scholar]
- 17.DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Res. 2004;130:57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 18.DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: A study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 19.Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 21.Whitford TJ, Grieve SM, Farrow TF, Gomes L, Brennan J, Harris AW, et al. Progressive grey matter atrophy over the first 2–3 years of illness in first-episode schizophrenia: A tensor-based morphometry study. Neuroimage. 2006;32:511–519. doi: 10.1016/j.neuroimage.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, et al. Brain volume changes in first-episode schizophrenia: A 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 23.Ho BC, Andreasen NC, Dawson JD, Wassink TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatry. 2007;164:1890–1899. doi: 10.1176/appi.ajp.2007.05111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreasen NC, Cizadlo T, Harris G, Swayze V, 2nd, O’Leary DS, Cohen G, et al. Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci. 1993;5:121–130. doi: 10.1176/jnp.5.2.121. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW, 2nd, Flashman LA, et al. Automatic atlas-based volume estimation of human brain regions from MR images. J Comput Assist Tomogr. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in MRI: A three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 27.Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, et al. Quantitative in vivo measurement of gyrification in the human brain: Changes associated with aging. Cereb Cortex. 1999;9:151–160. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- 28.Magnotta VA, Heckel D, Andreasen NC, Cizadlo T, Corson PW, Ehrhardt JC, Yuh WT. Measurement of brain structures with artificial neural networks: Two- and three-dimensional applications. Radiology. 1999;211:781–790. doi: 10.1148/radiology.211.3.r99ma07781. [DOI] [PubMed] [Google Scholar]
- 29.Pierson R, Johnson H, Harris G, Keefe H, Paulsen JS, Andreasen NC, Magnotta VA. Fully automated analysis using BRAINS: Auto-Workup. Neuroimage. 2011;54:328–336. doi: 10.1016/j.neuroimage.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 31.O’Leary DS, Flaum M, Kesler ML, Flashman LA, Arndt S, Andreasen NC. Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. J Neuropsychiatry Clin Neurosci. 2000;12:4–15. doi: 10.1176/jnp.12.1.4. [DOI] [PubMed] [Google Scholar]
- 32.Andreasen NC, Carpenter WT, Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 33.Selemon LD, Kleinman JE, Herman MM, Goldman-Rakic PS. Smaller frontal gray matter volume in postmortem schizophrenic brains. Am J Psychiatry. 2002;159:1983–1991. doi: 10.1176/appi.ajp.159.12.1983. [DOI] [PubMed] [Google Scholar]
- 34.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819–820. [DOI] [PubMed] [Google Scholar]
- 35.Giedd JN, Jeffries NO, Blumenthal J, Castellanos FX, Vaituzis AC, Fernandez T, et al. Childhood-onset schizophrenia: Progressive brain changes during adolescence. Biol Psychiatry. 1999;46:892–898. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 36.Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agartz I, Sedvall GC, Terenius L, Kulle B, Frigessi A, Hall H, Jönsson EG. BDNF gene variants and brain morphology in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:513–523. doi: 10.1002/ajmg.b.30338. [DOI] [PubMed] [Google Scholar]
- 39.Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 40.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: A comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 41.Konopaske GT, Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology. 2007;32:1216–1223. doi: 10.1038/sj.npp.1301233. [DOI] [PubMed] [Google Scholar]
- 42.Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 44.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: A longitudinal study of first episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. 4. New York: Raven; 2008. [Google Scholar]
- 46.Andreasen NC, Arndt S, Swayze V, 2nd, Cizadlo T, Flaum M, O’Leary D, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 47.Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- 48.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, et al. White matter changes in schizophrenia: Evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 49.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- 50.Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia—primary factor in pathology or epiphenomenon? Schizophr Bull. 2008;34:72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: Genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.