Abstract
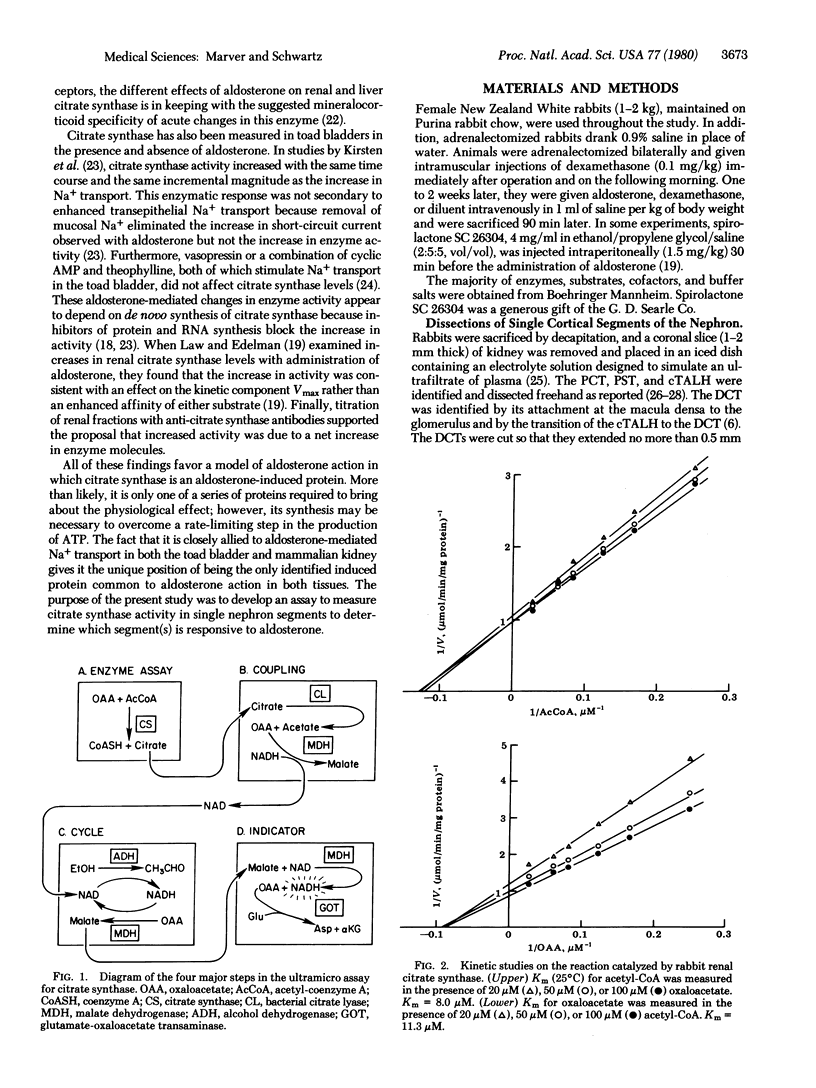
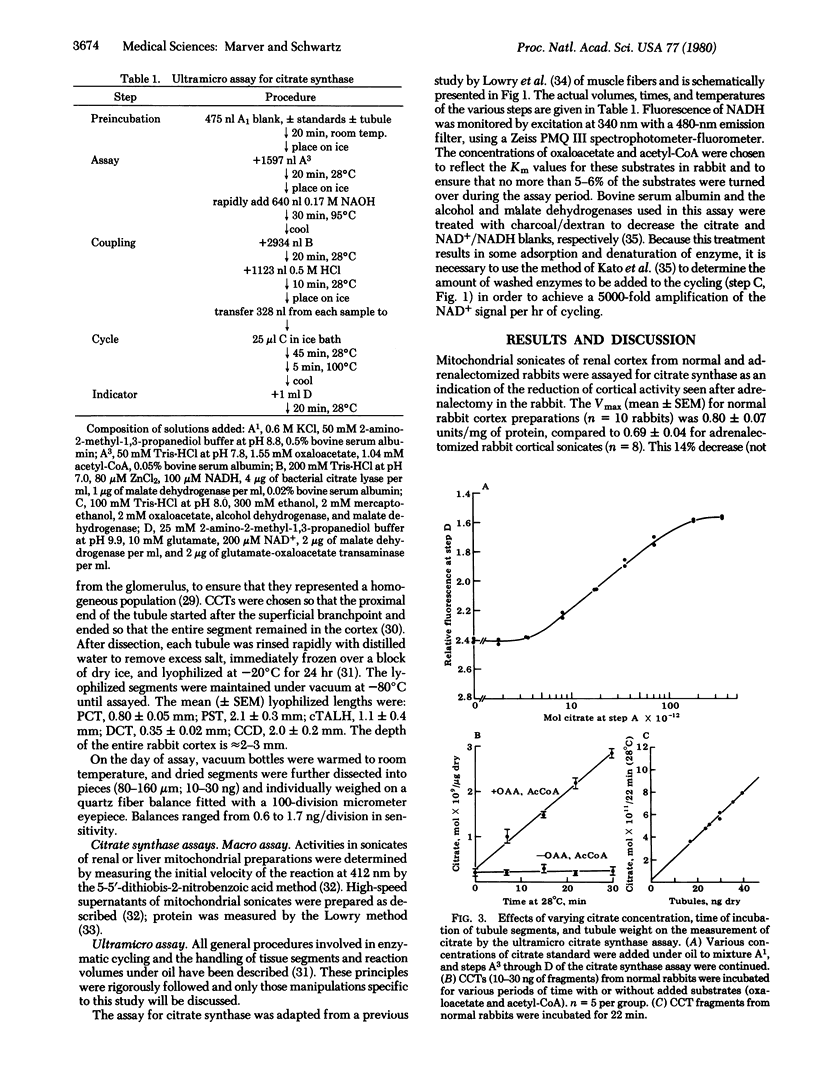
Previous evidence suggests that the activity of the mitochondrial enzyme citrate synthase [citrate oxaloacetate-lyase (pro-3S-CH2COO → acetyl-CoA), EC 4.1.3.7] is increased in target tissues upon acute administration of aldosterone. Therefore, an ultramicro assay was established to determine citrate synthase levels in isolated rabbit nephron segments as a means of localizing mineralocorticoid-responsive sites within the renal cortex. The relative citrate synthase activities in normal rabbit segments (per kg of dry tissue) correlated with the metabolic activity of the segments. The order was: distal convoluted tubule > proximal convoluted tubule > cortical thick ascending limb of Henle > cortical collecting duct > pars recta. When these segments were isolated from adrenalectomized rabbits, only the citrate synthase activity in the cortical collecting duct was significantly decreased compared to normal values (3.2 mol of citrate/kg dry wt per hr compared to 7.1; P < 0.001). Furthermore, enzyme activities in segments isolated from adrenalectomized rabbits 90 min after intravenous injection of aldosterone (10 μg/kg) were unchanged from normal or adrenalectomized rabbit tubule values for all segments except the cortical collecting duct. In this segment, aldosterone significantly increased citrate synthase activity compared to adrenalectomized rabbit values (8.1 mol/kg per hr compared to 3.2; P < 0.001), in contrast to the effect of dexamethasone at 10 μg/kg (4.4 mol/kg per hr compared to 3.2; P, NS). Spirolactone SC 26304 administered 30 min prior to injection of aldosterone inhibited the increase in collecting duct citrate synthase activity seen with aldosterone alone (3.4 mol/kg per hr compared to 8.1; P < 0.001). These findings suggest that the collecting duct is the primary target for aldosterone in the renal cortex.
Keywords: citrate synthase, enzymatic cycling, sodium transport, aldosterone, dexamethasone
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chignell C. F., Titus E. Effect of adrenal steroids on a Na+- and K+-requiring adenosine triphosphatase from rat kidney. J Biol Chem. 1966 Nov 10;241(21):5083–5089. [PubMed] [Google Scholar]
- Duval D., Funder J. W. The binding of tritiated aldosterone in the rat liver cytosol. Endocrinology. 1974 Feb;94(2):575–579. doi: 10.1210/endo-94-2-575. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. The roles of plasma binding and receptor specificity in the mineralocorticoid action of aldosterone. Endocrinology. 1973 Apr;92(4):994–1004. doi: 10.1210/endo-92-4-994. [DOI] [PubMed] [Google Scholar]
- Gross J. B., Imai M., Kokko J. P. A functional comparison of the cortical collecting tubule and the distal convoluted tubule. J Clin Invest. 1975 Jun;55(6):1284–1294. doi: 10.1172/JCI108048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. B., Kokko J. P. Effects of aldosterone and potassium-sparing diuretics on electrical potential differences across the distal nephron. J Clin Invest. 1977 Jan;59(1):82–89. doi: 10.1172/JCI108625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. H., Cortas N., Walser M. Aldosterone action and sodium- and potassium-activated adenosine triphosphatase in toad bladder. J Clin Invest. 1973 Jan;52(1):185–189. doi: 10.1172/JCI107163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy J. O., Oscai L. B., Don I. J., Molé P. A. Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1368–1373. doi: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- Imai M. The connecting tubule: a functional subdivision of the rabbit distal nephron segments. Kidney Int. 1979 Apr;15(4):346–356. doi: 10.1038/ki.1979.46. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R., Kokko J. P. Intrinsic differences in various segments of the proximal convoluted tubule. J Clin Invest. 1976 Apr;57(4):818–825. doi: 10.1172/JCI108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P. L. The role of aldosterone in the regulation of (Na + + K + )-ATPase in rat kidney. J Steroid Biochem. 1972 Feb;3(2):181–191. doi: 10.1016/0022-4731(72)90049-0. [DOI] [PubMed] [Google Scholar]
- Kato T., Berger S. J., Carter J. A., Lowry O. H. An enzymatic cycling method for nicotinamide-adenine dinucleotide with malic and alcohol dehydrogenases. Anal Biochem. 1973 May;53(1):86–97. doi: 10.1016/0003-2697(73)90409-0. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Doucet A., Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979 Aug;237(2):F114–F120. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Imai M., Seldin D. W., Kukko J. P. Characteristics of salt and water transport in superficial and juxtamedullary straight segments of proximal tubules. J Clin Invest. 1975 Jun;55(6):1269–1277. doi: 10.1172/JCI108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne R., Kirsten R. Der Einfluss von Aldosteron auf die Aktivität mitochondrialer und cytoplasmatischer Enzyme in der Rattenniere. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;300(4):244–254. [PubMed] [Google Scholar]
- Kirsten E., Kirsten R., Leaf A., Sharp G. W. Increased activity of enzymes of the tricarboxylic acid cycle in response to aldosterone in the toad bladder. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;300(4):213–225. doi: 10.1007/BF00364295. [DOI] [PubMed] [Google Scholar]
- Kirsten E., Kirsten R., Sharp G. W. Effects of sodium transport stimulating substances on enzyme activities in the toad bladder. Pflugers Arch. 1970;316(1):26–33. doi: 10.1007/BF00587894. [DOI] [PubMed] [Google Scholar]
- Kirsten R., Kirsten E. Redox state of pyridine nucleotides in renal response to aldosterone. Am J Physiol. 1972 Jul;223(1):229–235. doi: 10.1152/ajplegacy.1972.223.1.229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law P. Y., Edelman I. S. Induction of citrate synthase by aldosterone in the rat kidney. J Membr Biol. 1978 Jun 22;41(1):41–64. doi: 10.1007/BF01873339. [DOI] [PubMed] [Google Scholar]
- Lowry C. V., Kimmey J. S., Felder S., Chi M. M., Kaiser K. K., Passonneau P. N., Kirk K. A., Lowry O. H. Enzyme patterns in single human muscle fibers. J Biol Chem. 1978 Nov 25;253(22):8269–8277. [PubMed] [Google Scholar]
- Marver D. Aldosterone receptors in rabbit renal cortex and red medulla. Endocrinology. 1980 Feb;106(2):611–618. doi: 10.1210/endo-106-2-611. [DOI] [PubMed] [Google Scholar]
- Marver D., Stewart J., Funder J. W., Feldman D., Edelman I. S. Renal aldosterone receptors: studies with (3H)aldosterone and the anti-mineralocorticoid (3H)spirolactone (SC-26304). Proc Natl Acad Sci U S A. 1974 Apr;71(4):1431–1435. doi: 10.1073/pnas.71.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T., Srere P. A. Purification of rat heart and rat liver citrate synthases. Physical, kinetic, and immunological studies. J Biol Chem. 1971 May 25;246(10):3217–3223. [PubMed] [Google Scholar]
- Mukherjee A., Srere P. A., Frenkel E. P. Studies of the mechanism by which hepatic citrate synthase activity increases in vitamin B12 deprivation. J Biol Chem. 1976 Apr 10;251(7):2155–2160. [PubMed] [Google Scholar]
- Schmidt U., Schmid J., Schmid H., Dubach U. C. Sodium- and potassium-activated ATPase. A possible target of aldosterone. J Clin Invest. 1975 Mar;55(3):655–660. doi: 10.1172/JCI107973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Burg M. B. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol. 1978 Dec;235(6):F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- Srere P. A. An eclectic view of metabolic regulation: control of citrate synthase activity. Adv Enzyme Regul. 1970;9:221–233. doi: 10.1016/s0065-2571(71)80046-8. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibitions of the medullary portion. J Clin Invest. 1979 Aug;64(2):495–502. doi: 10.1172/JCI109487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B., Kokko J. P. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest. 1977 Jun;59(6):1099–1104. doi: 10.1172/JCI108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B., Tisher C. C., Kokko J. P. Structural-functional heterogeneity along the rabbit collecting tubule. Kidney Int. 1978 Dec;14(6):585–593. doi: 10.1038/ki.1978.167. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Behn C., Schoormans W., Hansen L. Effect of aldosterone on sodium and potassium transport in the kidney. J Steroid Biochem. 1972 Feb;3(2):151–159. doi: 10.1016/0022-4731(72)90045-3. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Stolte H., Brecht J. P., Hierholzer K. Mikropunktionsuntersuchungen über den Einfluss von Aldosteron, Cortison und Dexamethason auf die renale Natriumresorption adrenalektomierter Ratten. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(4):316–333. [PubMed] [Google Scholar]



