Abstract
The early histological studies of organ allografts noted platelets attached to vascular endothelium. Platelets adhere to vessels before any morphological evidence of endothelial injury. Subsequently, in vitro and in vivo experiments have demonstrated that alloantibodies can induce exocytosis of von Willebrand factor and P-selectin from endothelial cells and attachment of platelets within minutes. Platelets also adhere to and stimulate leukocytes. These interactions are increased by complement activation. After attachment platelets degranulate, releasing preformed mediators. Some chemokines stored together in platelet granules can form heteromers with synergistic functions. Heteromers containing platelet factor 4 (PF4; CXCL4) are specific to platelets and provide insights to unique platelet functions and opportunities for therapeutic intervention.
Keywords: Platelets, antibody, monocyte, macrophage, chemokines
Introduction
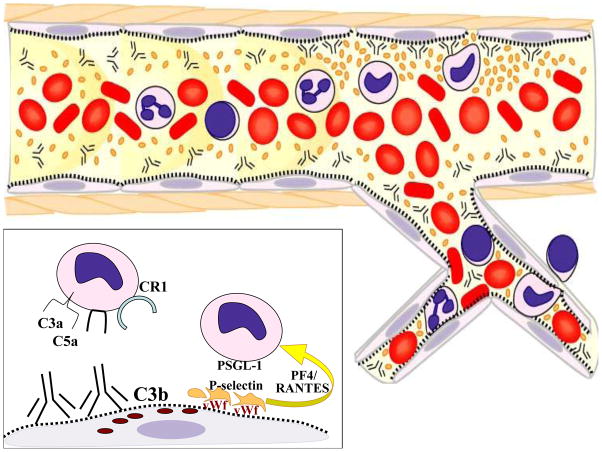
Platelets evolved as extremely responsive monitors of endothelial integrity. Within the laminar flow of blood vessels, larger leukocytes and erythrocytes have less contact with the vascular wall than platelets that remain adjacent to the endothelium because of their small size and discoid shape [1]. Platelets also outnumber leukocytes (150 to 450 million/ml vs 3 to 11 million/ml) and each platelet contains numerous granules with preformed mediators for rapid expression on the plasma membrane or release. These mediators include a wide range of immunologically active cytokines, chemokines, adhesion molecules and growth factors. In addition, upon activation platelets change shape increasing the surface area for expression of ligands and receptors. As a result, platelets are attuned to changes in vascular endothelial cells and can rapidly orchestrate responses. Alloantibodies and complement cause rapid responses in endothelial cells that are arresting to platelets, including the exocytosis of von Willebrand factor (vWf) and P-selectin from Weibel-Palade storage granules as well as exposure of subendothelial matrix components (Figure 1).
Figure 1.
The laminar flow of blood in arteries and arterioles causes platelets to be more concentrated adjacent to the vessel wall. As illustrated in the inset, alloantibodies cause exocytosis of von Willebrand factor (vWf) from storage granules in endothelial cells. Platelets bind to vWf and become activated. Activated platelets release chemokines from their alpha granules and express P-selectin on their membranes. Heteromers of PF4 and RANTES recruit monocytes, which are further activated by PSGL-1 receptors binding to P-selectin on platelets. Complement activation products provide additional chemoatraction (C3a and C5a) as well as ligands (C3b for CR1) to increase leukocyte localization to the endothelium.
There have been several recent reviews of platelets as mediators of inflammation and some have highlighted atherosclerosis and allograft rejection [2–7]. This review will concentrate on the inflammatory responses elicited by alloantibodies in organ transplants. Cells that are of particular relevance to both platelet function and antibody-mediated rejection, notably endothelial cells, neutrophils and monocytes, will be discussed in detail.
Evidence of Platelet Activation in Antibody-Mediated Rejection
Early histological studies of transplants undergoing severe rejection, particularly hyperacute rejection, described platelets amassed in capillaries [8]. Sharma and colleagues [9] published a detailed description of platelet accumulation in the first minutes of hyperacute rejection of renal allografts in dogs. In these studies, the recipient was sensitized by a series of skin grafts from the prospective kidney donor. The kinetics and location of platelet responses are instructive. Within one minute after circulation was restored to the transplanted kidney, platelet aggregates were observed in the arterioles before any endothelial injury was evident on electron microscopic preparations. In subsequent biopsies during the first 15 minutes after reperfusion, platelet aggregates accumulated in the peritubular capillaries. The platelets that were adjacent to the vascular endothelium were degranulated and some had pseudopods in contact with the endothelium or extending through the endothelial layer.
Hobbs and Cliff [10] performed time-lapse cinematic microscopy of intravascular events in thin slices of allogeneic renal tissue that were implanted into observation chambers in rabbit ears. After these implants became revascularized, an orthotopic kidney was transplanted from the same donor, and the two transplants were found to reject simultaneously. Numerous platelets were observed to adhere to vascular endothelial cells within 2 days after transplantation. Some platelets detached from the endothelial cells and were replaced by leukocytes. Many platelets became attached to these adherent leukocytes and some platelets bridged between the leukocytes and endothelial cells. These rabbits were not deliberately sensitized, but the implanted renal slices required several days to establish revascularization in the ear chambers before the orthotopic renal allograft was performed. Therefore, it is not known whether alloantibodies mediated the platelet responses in this study. However, using a digital imaging camera to capture movements of fluorescently labeled platelets, we demonstrated that alloantibodies initiate rapid tethering of platelets to capillaries of skin allografts followed by leukocyte infiltrates [11]. For these experiments skin from ears of B10.A donors was transplanted to athymic Balb/c recipients. After 7 days when circulation was reestablished to the skin allografts, but the vessels were still of donor origin, fluorescently labeled Balb/c platelets were administered intravascularly followed by monoclonal antibodies to donor MHC antigens. Within 30 minutes of alloantibody administration, platelet rolling and tethering was evident. In the absence of complement activation, platelet flow returned to normal by 24 hours, but with complement activating antibodies, platelets returned to normal flow more gradually over 4 days. Repeated administration of alloantibodies caused repeated decreases in platelet flow. Although platelet flow decreased, most platelets did not fully arrest on the endothelium, but continued to roll and then returned to the circulation. Plasma samples from the systemic circulation contained platelets with evidence of activation including upregulation of P-selectin and membrane bound C3d. Immunohistology on skin grafts after passive transfer of alloantibodies confirmed that platelets were adherent to endothelial cells and that neutrophils infiltrated into the allografts. Depletion of platelets prior to administration of antibody greatly decreased neutrophil infiltrates indicating that platelets augment antibody induced leukocyte infiltrates. These interactions are not limited to skin allografts. In ongoing experiments, we have found that passively transferred alloantibodies rapidly induce complement deposition, platelet aggregates and monocyte adherence to capillary endothelium of cardiac and renal allografts in RAG−/− recipients [Kuo, H-H et al in preparation].
Complement deficient animals have been instructive in resolving the effects of different paths of the complement cascade on platelet activation in transplants. Rats deficient in C6, one of the complement components in the terminal complement cascade, allowed the effects of the membrane attack complex (MAC; C5b-C9) to be dissected from the effects of the preceding complement components. The absence of C6 did not alter rapid alloantibody production or C3d deposition in response to MHC class I incompatible cardiac allografts, but it did decrease the release of vWf from Weibel-Palade storage granules of endothelial cells and accumulation of intravascular platelet aggregates [12]. In C6 deficient recipients, vWf was largely retained in Weibel-Palade bodies of arterial endothelial cells even in sites of severe lymphocytic infiltrates. C6 deficient recipients also retained more vWf in capillary and venous endothelium than control recipients. These findings are not constrained to cardiac allografts because C6 deficiency was also associated with decreased vWf release and platelet aggregates in lung allografts [13].
These studies with C6 deficient animals do not indicate that endothelial cell lysis is necessary because many in vitro studies have demonstrated that endothelial cells express complement regulatory proteins such as CD59 that can control small amounts of C5b-C8 [14]. Small amounts of the complete MAC can be cleared by exocytosis or endocytosis [15]. As opposed to lysis, endothelial cells are activated by sublytic quantities of C5b-C9 or even C5b-C8, which assemble into transmembrane channels. These channels allow calcium flux and also interact with Gi-proteins that activate anti-apoptotic and proliferative pathways [16]. The immediate response to sublytic C5b-C9 or C5b-C8 is exocytosis of vWf and P-selectin [17]. In addition to releasing preformed mediators, endothelial cells activated by isolated terminal complement components in vitro synthesize tissue factor [18]. Tissue factor can expand the platelet responses by leading to thrombin production.
Transplants to B cell deficient recipients offer further evidence of the precipitous effects of alloantibodies on platelets. Cardiac transplants in B cell deficient mice elicit a vigorous cellular infiltrate but limited release of vWf or aggregation of platelets. Passive transfer of allantibodies to these mice results in complement deposition, vWf release and extensive platelet aggregates within 1 to 2 days [19, 20].
Over the years, several groups have reported that radiolabeled platelets localize to human renal transplants in the early stages of rejection [21, 22]. None of these studies subdivided rejections on the basis of antibody and cell-mediated diagnostic criteria. It is likely that platelets would localize to both types of rejection. A recent immunohistological study of human renal transplants reported that over half of the biopsies with C4d deposits had platelet aggregates that stained for CD61 in peritubular capillaries. However, this finding was not limited to antibody-mediated rejection; about one third of the biopsies with cellular rejection had intravascular platelet aggregates [23].
Taken together these studies indicate that platelets respond rapidly to endothelial disturbances in allografts, most notably to exocytosis of vWf and P-selectin. Antibodies alone, but even more effectively together with complement activation, cause endothelial cells to initiate platelet responses. Moreover, platelets attach to leukocytes and increase leukocyte interactions with endothelial cells.
Platelets as a dynamic source of immune modulators
In spite of their small size and lack of nucleus, platelets have a dynamic range of responses of relevance to transplants. These anucleate cells form as buds on pseudopodia of megakaryocytes that extend into the blood vessels in the bone marrow [24]. Large numbers of platelets (about 1011 per day) are sheared off the megakaryocytes into the circulation of adult humans. During inflammatory responses, platelet production can be increased; a response referred to as reactive thrombocytosis. Proteomic arrays have identified over 300 mediators that platelets can secrete [25, 26]. Most of these mediators are loaded into cytoplasmic granules during platelet formation. Platelets contain three morphological types of secretory granules with different contents: alpha-granules, dense granules and lysosomes. The 40 to 80 alpha-granules in each platelet contain the majority of immunological mediators: adhesion molecules, cytokines, chemokines, and growth factors. Additional mediators can be imbibed from plasma and others can be synthesized by splicing mRNA transcripts or produced through the cyclooxygenase pathway [7, 27]. In order to deliver these mediators to sites of endothelial disturbance, platelets have membrane receptors for adhesion molecules and subendothelial matrix constituents.
Antibody induced endothelial responses of consequence to platelets
Actual disruption of vascular endothelial integrity as occurs in hyperacute rejection exposes subendothelial matrix constituents that initiate thrombus formation. However, platelet adhesion occurs in allografts before there is evidence of endothelial injury on an electron microscopic level [9]. Exocytosis of Weibel-Palade storage granules is the most consistent immediate response of endothelial cells to alloantibodies that is associated with platelet adherence and aggregation [11, 13, 19, 20]. Although in vitro and in vivo experiments indicate that antibodies to MHC class I antigens can elicit release of vWf in the absence of complement activation [11, 28], complement does augment vWf release. More prolonged platelet adherence resulted in skin allografts to RAG−/− mice following passive transfer of complement activating than non complement activating antibodies [11], and less vWf release was found in cardiac allografts to C6 deficient than C6 sufficient recipients [12]. Experiments with platelets under flow conditions indicate that the interaction between vWF and platelet membrane glycoprotein Ib (GPIb) in the GP Ib-IX-V complex provides the strongest binding [29]. In addition to vWf, the GP Ib-IX-V complex binds to P-selectin. P-selectin is stored together with vWF in Weibel-Palade granules and is brought to the surface with vWf and may serve as an anchor for vWf [30]. Following these first interactions, GPIIb/IIIa undergoes a receptor conformational change on the platelet membrane that permits binding to ICAM-1.
The interaction of activated platelets with endothelial cells causes further activation of both cells. In vitro incubation of activated platelets that express CD40L with human endothelial cells causes the endothelial cells to upregulate E-selectin, VCAM-1 and ICAM-1 as well as IL-8 and MCP-1 within 4 hours [31]. In addition to receptor-ligand interactions, IL-1β synthesized by activated platelets can cause expression of ICAM-1 and secretion of IL-6 and MCP-1 [32, 33].
Human platelets also express FcγRIIA (CD32) and C1qR. The interaction of these with alloantibodies on endothelium has not been studied. However, soluble immune complexes from lupus patients can activate platelets through FcγRIIA to upregulate CD40L (CD154) and P-selectin [34].
Platelet interactions with complement
Platelets express receptors that bind the collagen-like tails of soluble C1q and this inhibits platelet interactions with collagen, but clusters of C1q as would be present on vessels in antibody-mediated rejection, can initiate platelet aggregation [35]. Although platelets only express receptors for C1q, other components of complement bind to platelets and alter their function.
Sims and Wiedmer [36] studied the effects of sublytic amounts of C5b-C9 on platelets. They found that lysis does not occur when less than 1,000 C5b-C9 complexes form on the platelet membrane. Instead platelets are activated to degranulate and to shed microparticles. This scenario could occur when antibody activates complement on endothelial cells and platelets are in the vicinity.
The interaction of platelets with C3 and C3 split products is less straightforward. Platelets do not express receptors for C3 and yet C3 is frequently associated with activated platelets. Del Conde and colleagues [37] reported that activated platelets could initiate the complete complement cascade through to the formation of C5b-C9. In these experiments, C3b was found to bind to P-selectin on the surface of platelets. Hamad and colleagues [38] confirmed that C3 binds to activated platelets, but in their experiments activation of C3 by cleavage was not required and C3 was not bound to P-selectin. Of greater relevance to antibody-mediated rejection, C3 on platelets was demonstrated to promote binding to complement receptor 1 (CR1; CD35) which is expressed on lymphocytes as well as neutrophils and monocytes. Therefore, C3 on the membranes of activated platelets would be expected to enhance platelet-leukocyte interactions.
Another interaction between platelets and C3 was recently reported, in which C3b on bacteria bound to platelets through GPIb [39]. This interaction promoted localization of opsonized bacteria to a subpopulation of dendritic cells in the spleen. The interaction between platelet GPIb and C3b could also facilitate platelet attachment to complement deposits in allografts. Moreover, if microparticles are shed from transplants with C3b attached, platelets could transport them to dendritic cells in the spleen.
Platelet interactions with neutrophils and monocytes
Neutrophils, monocytes and macrophages are characteristically found in biopsies of transplants with acute antibody-mediated rejection [40, 41]. In transplants, platelets quickly engage leukocytes and increase leukocyte interactions with vascular endothelium [10]. Platelets interact with leukocytes through several mechanisms including release of chemokines, shedding of microparticles and direct cell contact. In models of arterial injury, expression of P-selectin by platelets is the primary mechanism of initiating monocyte recruitment and activation [42–45]. We have found that P-selectin expressing platelets are associated with monocytes in the vessels of cardiac and renal transplants within an hour of administering alloantibody to RAG−/− recipients (Figure 2). P-selectin on platelets binds to PSGL-1 on monocytes and signals through NFκB to cause secretion of MCP-1, TNFα, IL-8 and IL-1β [42, 43]. Of the many chemokines in platelet alpha-granules, RANTES (CCL5) is integral to monocyte recruitment [44, 45]. When compared to the two platelet specific chemokines, PF4 and neutrophil-activating protein-2 (NAP-2), RANTES was over twice as effective at binding to inflamed endothelium and arresting monocytes [46]. RANTES can be released directly by platelets or from microparticles shed from platelets, and then associates with the glycosaminoglycans on the surface on the endothelial cells. Platelet derived RANTES has a distinctive property of forming heteromers with PF4 in the alpha-granules [47, 48]. These heteromers have enhanced chemokine functions that are thought to be due to increased binding of RANTES to leukocytes. The combined stimulation through P-selectin and RANTES-PF4 heteromers polarizes monocytes towards a proinflammatory function [6]. In contrast, macrophages stimulated with recombinant PF4 alone express a transcriptome distinct from either inflammatory or alternatively activated macrophages [49]. PF4 also prevents monocytes from undergoing spontaneous apoptosis during 72 hours of culture and induces differentiation to macrophages as evidenced by morphological and functional changes including expression of the co-stimulatory molecule CD86 and secretion of TNFα [50]. After activation, macrophages have been noted to phagocytize platelets and this process further inhibits apoptosis and enhances secretion of TNFα, IL-6 and IL-23 [51, 52].
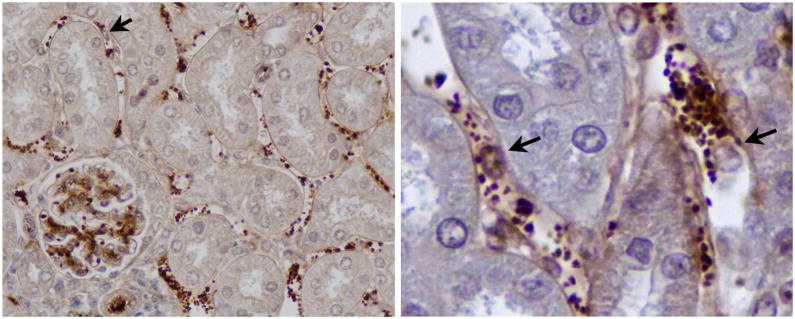
Figure 2.
Passive transfer of alloantibodies to C57BL/6 RAG−/− recipients of B10.A renal transplants causes release of vWf and extensive platelet accumulation in peritubular capillaries and glomeruli within 1 hour. Platelets (stained brown for vWf) interact extensively with endothelial cells and leukocytes (arrows). Leukocytes with adherent platelets are better appreciated on the enlarged image on right.
In time-lapse cinematic microscopic studies of renal implants, Hobbs and Cliff [10] observed platelets attach to leukocytes that were adherent to vessel walls: “Up to 10 platelets may be seen adhering to one leukocyte, producing a pincushion or porcupine effect.” Such platelet-leukocyte conjugates are found in the circulation in inflammatory diseases. In addition, platelet microparticles form conjugates with leukocytes. Formation of these conjugates depends on P-selectin binding to PSGL-1. Platelets stimulate upregulation of integrins (CD11/CD18) on monocytes and promote migration into tissues [53]. In recent studies, it has been suggested that platelet microparticles may be transported into tissues by “piggy-backing” on neutrophils or monocytes [54]. More recently, evidence has been presented that platelets themselves may migrate into tissues under the influence of stromal derived factor-1 [55, 56]. The fact that platelets are capable of extensive shape change and motility is well documented. It is estimated that actin constitutes about a fifth of the total protein in platelets [57]. Platelets are also known to express several chemokine receptors including CCR1, CCR3, CCR4, and CXCR4 [58]. The concept of platelet migration into tissues has significant implications for transplantation because it provides a mechanism for platelets to enhance interactions among macrophages, dendritic cells and T cells in the interstitium of transplanted organs.
Under in vitro conditions, more activated platelets are bound to monocytes than neutrophils [59]. This preferential binding may contribute to the observation that macrophages often are more prevalent than neutrophils in antibody-mediated rejection, [41, 60, 61]. Macrophages are also much more numerous than neutrophils in experimental cardiac and renal transplants following passive transfer of alloantibodies to RAG−/− recipients (Kuo, H-H et al, in preparation).
Platelet interactions with lymphocytes
Antibody-mediated rejection often has some mixture of cellular rejection. Similarly, platelet interactions are not limited to neutrophils and monocytes [5]. Platelets express both CD40 and CD40L, which are integral to interactions with lymphocytes. When T cells are activated through CD3 and CD28, their production of IFNγ is enhanced by platelets [62]. This interaction is dependent on platelets binding to T cells and is blocked by antibodies to P-selectin, CD40L and GPIIb/IIIa. Platelets and T-cells can engage in a feedback loop in which platelets secrete RANTES to recruit T cells, and then activated T cells stimulate platelets through CD40-CD40L interactions to secrete RANTES thereby recruiting more T cells to the site of interaction [63, 64]. The relevance of platelet derived CD40L has been demonstrated by Kirk and colleagues [65]. Platelets are the major source of soluble circulating CD40L, and the authors demonstrated that purified CD40L trimers can induce rejection of cardiac allografts in CD40L knockout mice.
Platelets may sustain T cell recruitment to allografts through additional mechanisms including the secretion of glutamate. Activated platelets secrete glutamate, which can feed back in an autocrine loop through their glutamate receptors to increase thromboxane production [66]. Treatment with glutamate receptor antagonists or platelet depleting antibodies increases survival of skin grafts on athymic mice that are reconstituted with limited numbers of T cells [67]. These treatments delay CD4 and CD8 T cell infiltration of the skin grafts, and decrease thromboxane, PF4 and IFNγ levels in the plasma.
Platelets can also supplement low levels of T cell help for antibody production in models of viral infection. In studies of antibody responses to adenovirus, Elzey and co-workers [68] demonstrated that passive transfer of CD40L expressing platelets could provide help in CD40L deficient mice for isotype switching to IgG antibody production. This may be relevant to possible local alloantibody production in tertiary lymphoid structures that form in some clinical transplants [69, 70].
Inhibition of platelet interactions
Although no therapeutic interventions specifically targeted to platelets are administered to transplant recipients currently, aspirin has been demonstrated to have beneficial effects on renal allograft outcomes [71]. Knowledge of the major mechanisms of platelet interactions with endothelial cells and leukocytes may provide new approaches to modulate inflammation.
Monoclonal antibodies and soluble inhibitors for P-selectin and RANTES have been used to inhibit platelet-leukocyte interactions experimentally [48]. However, these inhibitors do not just limit platelet functions. The most promising approach is to focus on platelet specific mediators. In terms of transplantation, inhibition of the RANTES-PF4 heteromer would have many advantages. Koenen and colleagues [47] have developed small molecules designed to prevent RANTES-PF4 heteromer formation. Human and mouse peptides have been demonstrated to inhibit RANTES mediated adhesion of monocytes to endothelial cells in vitro. They have applied one such inhibitor (MKEY) to prevent acute lung injury in mice that was induced by 3 different stimuli: aerosolized LPS, sepsis or acid [48]. This strategy prevented neutrophil infiltration and edema in the lungs to a similar degree as blocking antibodies to either PF4 or RANTES, but it did not interfere with general neutrophil functions such as phagocytosis or killing of bacteria.
Summary
The increasing data linking platelets with vascular inflammatory responses has obvious implications for allograft rejection. The functional versatility of platelets makes it likely that platelets modify many aspects of both humoral and cellular rejection. The interactions of platelets with endothelial cells and monocytes are particularly relevant to the pathological findings in antibody-mediated rejection.
Acknowledgments
WMB and HHK are supported by P01AI087586 from the National Institutes of Health (NIH); CNM is supported by R01HL093179, R01HL093179-02S109, and R01HL094547 from the NIH.
Abbreviations
- MHC
Major Histocompatibility Complex
- vWf
von Willebrand factor
- PSGL-1
P-selectin glycoprotein ligand-1
- RANTES
Regulated on Activation, Normal T Cell Expressed and Secreted
- PF4; CXCL4
Platelet Factor 4
- NAP-2
neutrophil-activating protein-2
- C1qR
C1q receptor
- FcγR
Fc gamma receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woldhuis B, Tangelder GJ, Slaaf DW, Reneman RS. Concentration profile of blood platelets differs in arterioles and venules. Am J Physiol. 1992;262(4 Pt 2):H1217. doi: 10.1152/ajpheart.1992.262.4.H1217. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin WM, 3rd, Kuo HH, Morrell CN. Platelets: versatile modifiers of innate and adaptive immune responses to transplants. Curr Opin Organ Transplant. 2011 doi: 10.1097/MOT.0b013e3283425365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk AD, Morrell CN, Baldwin WM., 3rd Platelets influence vascularized organ transplants from start to finish. Am J Transplant. 2009;9(1):14. doi: 10.1111/j.1600-6143.2008.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenen RR, Weber C. Platelet-derived chemokines in vascular remodeling and atherosclerosis. Semin Thromb Hemost. 2010;36(2):163. doi: 10.1055/s-0030-1251500. [DOI] [PubMed] [Google Scholar]
- 5.Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol. 2008;83(5):1069. doi: 10.1189/jlb.0907615. [DOI] [PubMed] [Google Scholar]
- 6.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85(2):195. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 7.Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2011;34(1):5. doi: 10.1007/s00281-011-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowenhaupt R, Nathan P. Platelet accumulation observed by electron microscopy in the early phase of renal allotransplant rejection. Nature. 1968;220(5169):822. doi: 10.1038/220822b0. [DOI] [PubMed] [Google Scholar]
- 9.Sharma HM, Moore S, Merrick HW, Smith MR. Platelets in early hyperacute allograft rejection in kidneys and their modification by sulfinpyrazone (Anturan) therapy. An experimental study. Am J Pathol. 1972;66(3):445. [PMC free article] [PubMed] [Google Scholar]
- 10.Hobbs JB, Cliff WJ. A study of allograft kidney rejection occurring simultaneously in whole organ and ear chamber grafts in the rabbit. J Exp Med. 1973;137(3):776. doi: 10.1084/jem.137.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrell CN, Murata K, Swaim AM, Mason E, Martin TV, Thompson LE, Ballard M, Fox-Talbot K, Wasowska B, Baldwin WM., 3rd In vivo platelet-endothelial cell interactions in response to major histocompatibility complex alloantibody. Circ Res. 2008;102(7):777. doi: 10.1161/CIRCRESAHA.107.170332. [DOI] [PubMed] [Google Scholar]
- 12.Ota H, Fox-Talbot K, Hu W, Qian Z, Sanfilippo F, Hruban RH, Baldwin WM., 3rd Terminal complement components mediate release of von Willebrand factor and adhesion of platelets in arteries of allografts. Transplantation. 2005;79(3):276. doi: 10.1097/01.tp.0000146195.76904.d3. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM., III Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol. 2002;169:4620. doi: 10.4049/jimmunol.169.8.4620. [DOI] [PubMed] [Google Scholar]
- 14.Lachmann PJ. The control of homologous lysis. Immunol Today. 1991;12(9):312. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- 15.Morgan BP. Effects of the membrane attack complex of complement on nucleated cells. Curr Top Microbiol Immunol. 1992;178:115. doi: 10.1007/978-3-642-77014-2_8. [DOI] [PubMed] [Google Scholar]
- 16.Rus HG, Niculescu FI, Shin ML. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol Rev. 2001;180:49. doi: 10.1034/j.1600-065x.2001.1800104.x. [DOI] [PubMed] [Google Scholar]
- 17.Hattori R, Hamilton KK, McEver RP, Sims PJ. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989;264(15):9053. [PubMed] [Google Scholar]
- 18.Tedesco F, Pausa M, Nardon E, Introna M, Mantovani A, Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185(9):1619. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasowska BA, Qian Z, Cangello DL, Behrens E, Van Tran K, Layton JL, Sanfilippo F, Baldwin WM., III Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71:727. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 20.Rahimi S, Qian Z, Layton J, Fox-Talbot K, Baldwin WM, Wasowska BA. Non-complement- and complement-activating antibodies synergize to cause rejection of cardiac allografts. Am J Transplant. 2004;4(3):326. doi: 10.1111/j.1600-6143.2004.00334.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith N, Chandler S, Hawker RJ, Hawker LM, Barnes AD. Indium-labelled autologous platelets as diagnostic aid after renal transplantation. Lancet. 1979;2(8154):1241. doi: 10.1016/s0140-6736(79)92358-4. [DOI] [PubMed] [Google Scholar]
- 22.Collier BD, Adams MB, Kauffman HM, Trembath L, Hoffmann RG, Tisdale PL, Rao SA, Hellman RS, Isitman AT. Accurate diagnosis of renal transplant rejection by indium-111 platelet imaging despite postoperative cyclosporin therapy. Clin Nucl Med. 1988;13(8):606. doi: 10.1097/00003072-198808000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Meehan SM, Limsrichamrern S, Manaligod JR, Junsanto T, Josephson MA, Thistlethwaite JR, Haas M. Platelets and capillary injury in acute humoral rejection of renal allografts. Hum Pathol. 2003;34(6):533. doi: 10.1016/s0046-8177(03)00189-8. [DOI] [PubMed] [Google Scholar]
- 24.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE, Jr, Shivdasani RA, von Andrian UH. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 25.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103(6):2096. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 26.Senzel L, Gnatenko DV, Bahou WF. The platelet proteome. Curr Opin Hematol. 2009;16(5):329. doi: 10.1097/MOH.0b013e32832e9dc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122(3):379. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, Wasowska BA, Baldwin WM, III, Pober JS, Lowenstein CJ. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci U S A. 2007;104(4):1301. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggeri ZM. Platelet adhesion under flow. Microcirculation. 2009;16(1):58. doi: 10.1080/10739680802651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padilla A, Moake JL, Bernardo A, Ball C, Wang Y, Arya M, Nolasco L, Turner N, Berndt MC, Anvari B, Lopez JA, Dong JF. P-selectin anchors newly released ultralarge von Willebrand factor multimers to the endothelial cell surface. Blood. 2004;103(6):2150. doi: 10.1182/blood-2003-08-2956. [DOI] [PubMed] [Google Scholar]
- 31.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 32.Gawaz M, Brand K, Dickfeld T, Pogatsa-Murray G, Page S, Bogner C, Koch W, Schomig A, Neumann F. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis. 2000;148(1):75. doi: 10.1016/s0021-9150(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 33.Hawrylowicz CM, Howells GL, Feldmann M. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J Exp Med. 1991;174(4):785. doi: 10.1084/jem.174.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duffau P, Seneschal J, Nicco C, Richez C, Lazaro E, Douchet I, Bordes C, Viallard JF, Goulvestre C, Pellegrin JL, Weil B, Moreau JF, Batteux F, Blanco P. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010;2(47):47ra63. doi: 10.1126/scitranslmed.3001001. [DOI] [PubMed] [Google Scholar]
- 35.Cazenave JP, Assimeh SN, Painter RH, Pachham MA, Mustard JF. Clq inhibition of the interaction of collagen with human platelets. J Immunol. 1976;116(1):162. [PubMed] [Google Scholar]
- 36.Sims PJ, Wiedmer T. The response of human platelets to activated components of the complement system. Immunol Today. 1991;12(9):338. doi: 10.1016/0167-5699(91)90012-I. [DOI] [PubMed] [Google Scholar]
- 37.Del Conde I, Cruz MA, Zhang H, Lopez JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201(6):871. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamad OA, Nilsson PH, Wouters D, Lambris JD, Ekdahl KN, Nilsson B. Complement component C3 binds to activated normal platelets without preceding proteolytic activation and promotes binding to complement receptor 1. J Immunol. 2010;184(5):2686. doi: 10.4049/jimmunol.0902810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verschoor A, Neuenhahn M, Navarini AA, Graef P, Plaumann A, Seidlmeier A, Nieswandt B, Massberg S, Zinkernagel RM, Hengartner H, Busch DH. A platelet-mediated system for shuttling blood-borne bacteria to CD8alpha+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat Immunol. 2011;12(12):1194. doi: 10.1038/ni.2140. [DOI] [PubMed] [Google Scholar]
- 40.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria - an addition to the banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3(6):708. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 41.Berry GJ, Angelini A, Burke MM, Bruneval P, Fishbein MC, Hammond E, Miller D, Neil D, Revelo MP, Rodriguez ER, Stewart S, Tan CD, Winters GL, Kobashigawa J, Mehra MR. The ISHLT working formulation for pathologic diagnosis of antibody-mediated rejection in heart transplantation: Evolution and current status (2005–2011) J Heart Lung Transplant. 2011;30(6):601. doi: 10.1016/j.healun.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95(5):2297. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97(6):1525. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106(12):1523. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 45.Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25(7):1512. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- 46.Baltus T, von Hundelshausen P, Mause SF, Buhre W, Rossaint R, Weber C. Differential and additive effects of platelet-derived chemokines on monocyte arrest on inflamed endothelium under flow conditions. J Leukoc Biol. 2005;78(2):435. doi: 10.1189/jlb.0305141. [DOI] [PubMed] [Google Scholar]
- 47.Koenen RR, von Hundelshausen P, Nesmelova IV, Zernecke A, Liehn EA, Sarabi A, Kramp BK, Piccinini AM, Paludan SR, Kowalska MA, Kungl AJ, Hackeng TM, Mayo KH, Weber C. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009;15(1):97. doi: 10.1038/nm.1898. [DOI] [PubMed] [Google Scholar]
- 48.Grommes J, Alard JE, Drechsler M, Wantha S, Morgelin M, Kuebler WM, Jacobs M, von Hundelshausen P, Markart P, Wygrecka M, Preissner KT, Hackeng TM, Koenen RR, Weber C, Soehnlein O. Disruption of Platelet-derived Chemokine Heteromers Prevents Neutrophil Extravasation in Acute Lung Injury. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201108-1533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gleissner CA, Shaked I, Little KM, Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184(9):4810. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheuerer B, Ernst M, Durrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, Flad HD, Petersen F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95(4):1158. [PubMed] [Google Scholar]
- 51.Scull CM, Hays WD, Fischer TH. Macrophage pro-inflammatory cytokine secretion is enhanced following interaction with autologous platelets. J Inflamm (Lond) 2010;7:53. doi: 10.1186/1476-9255-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lang D, Dohle F, Terstesse M, Bangen P, August C, Pauels HG, Heidenreich S. Down-regulation of monocyte apoptosis by phagocytosis of platelets: involvement of a caspase-9, caspase-3, and heat shock protein 70-dependent pathway. J Immunol. 2002;168(12):6152. doi: 10.4049/jimmunol.168.12.6152. [DOI] [PubMed] [Google Scholar]
- 53.da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol. 2006;79(3):499. doi: 10.1189/jlb.0605318. [DOI] [PubMed] [Google Scholar]
- 54.Zimmerman GA, Weyrich AS. Arsonists in rheumatoid arthritis. Science. 2010;327(5965):528. doi: 10.1126/science.1185869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraemer BF, Borst O, Gehring EM, Schoenberger T, Urban B, Ninci E, Seizer P, Schmidt C, Bigalke B, Koch M, Martinovic I, Daub K, Merz T, Schwanitz L, Stellos K, Fiesel F, Schaller M, Lang F, Gawaz M, Lindemann S. PI3 kinase-dependent stimulation of platelet migration by stromal cell-derived factor 1 (SDF-1) J Mol Med (Berl) 2010;88(12):1277. doi: 10.1007/s00109-010-0680-8. [DOI] [PubMed] [Google Scholar]
- 56.Kraemer BF, Schmidt C, Urban B, Bigalke B, Schwanitz L, Koch M, Seizer P, Schaller M, Gawaz M, Lindemann S. High shear flow induces migration of adherent human platelets. Platelets. 2011;22(6):415. doi: 10.3109/09537104.2011.556277. [DOI] [PubMed] [Google Scholar]
- 57.Hartwig JH. The platelet: form and function. Semin Hematol. 2006;43(1 Suppl 1):S94. doi: 10.1053/j.seminhematol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Clemetson KJ, Clemetson JM, Proudfoot AE, Power CA, Baggiolini M, Wells TN. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood. 2000;96(13):4046. [PubMed] [Google Scholar]
- 59.Rinder HM, Bonan JL, Rinder CS, Ault KA, Smith BR. Dynamics of leukocyte-platelet adhesion in whole blood. Blood. 1991;78(7):1730. [PubMed] [Google Scholar]
- 60.Magil AB, Tinckam K. Monocytes and peritubular capillary C4d deposition in acute renal allograft rejection. Kidney Int. 2003;63(5):1888. doi: 10.1046/j.1523-1755.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 61.Fishbein MC, Kobashigawa J. Biopsy-negative cardiac transplant rejection: etiology, diagnosis, and therapy. Curr Opin Cardiol. 2004;19(2):166. doi: 10.1097/00001573-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 62.Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H, Hansson GK, Li N. Platelets regulate CD4 T-cell differentiation via multiple chemokines in humans. Thromb Haemost. 2011;106(2):353. doi: 10.1160/TH11-01-0020. [DOI] [PubMed] [Google Scholar]
- 63.Danese S, de la Motte C, Sturm A, Vogel JD, West GA, Strong SA, Katz JA, Fiocchi C. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003;124(5):1249. doi: 10.1016/s0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 64.Danese S, Fiocchi C. Platelet activation and the CD40/CD40 ligand pathway: mechanisms and implications for human disease. Crit Rev Immunol. 2005;25(2):103. doi: 10.1615/critrevimmunol.v25.i2.20. [DOI] [PubMed] [Google Scholar]
- 65.Xu H, Zhang X, Mannon RB, Kirk AD. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J Clin Invest. 2006;116(3):769. doi: 10.1172/JCI27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrell CN, Sun H, Ikeda M, Beique JC, Swaim AM, Mason E, Martin TV, Thompson LE, Gozen O, Ampagoomian D, Sprengel R, Rothstein J, Faraday N, Huganir R, Lowenstein CJ. Glutamate mediates platelet activation through the AMPA receptor. J Exp Med. 2008;205(3):575. doi: 10.1084/jem.20071474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swaim AM, Field DJ, Fox-Talbot K, Baldwin WMI, Morrell CN. Platelets contribute to allograft rejection through glutamate receptor signaling. J Immunol. 2010 doi: 10.4049/jimmunol.1000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, Stein CS, Nieswandt B, Wang Y, Davidson BL, Ratliff TL. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19(1):9. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 69.Zarkhin V, Li L, Sarwal M. “To B or not to B?” B-cells and graft rejection. Transplantation. 2008;85(12):1705. doi: 10.1097/TP.0b013e318177793e. [DOI] [PubMed] [Google Scholar]
- 70.Baldwin WM, 3rd, Halushka MK, Valujskikh A, Fairchild RL. B cells in cardiac transplants: From clinical questions to experimental models. Semin Immunol. 2011 doi: 10.1016/j.smim.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grotz W, Siebig S, Olschewski M, Strey CW, Peter K. Low-dose aspirin therapy is associated with improved allograft function and prolonged allograft survival after kidney transplantation. Transplantation. 2004;77(12):1848. doi: 10.1097/01.tp.0000129407.31494.45. [DOI] [PubMed] [Google Scholar]