Abstract
Background
Although the etiopathology of Autism Spectrum Disorder (ASD) is not clear there is increasing evidence that dysfunction in the immune system affects many children with ASD. Findings of immune dysfunction in ASD include increases in inflammatory cytokines, chemokines and microglial activity in brain tissue and CSF, as well as abnormal peripheral immune cell function.
Methods
Adhesion molecules, such as platelet endothelial adhesion molecule-1 (PECAM-1), intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), P-Selectin, and L-Selectin, function to facilitate leukocyte transendothelial migration. We assessed concentrations of soluble adhesion molecules, sPECAM-1, sICAM-1, sVCAM-1, sP-Selectin, and sL-Selectin in the plasma of 49 participants with ASD, and 31 typically developing controls of the same age, all of whom were enrolled as part of the Autism Phenome Project (APP). Behavioral assessment, the levels of soluble adhesion molecules, head circumference and MRI measurements of brain volume were compared in the same subjects.
Results
Levels of sPECAM-1 and sP-Selectin were significantly reduced in the ASD group compared to typically developing controls (p < 0.02). Soluble PECAM-1 levels were negatively associated with repetitive behavior and abnormal brain growth in children with ASD (p=0.03).
Conclusions
As adhesion molecules modulate the permeability and signaling at the blood brain barrier as well as leukocyte infiltration into the CNS, current data suggests a role for these molecules in the complex pathophysiology of ASD.
Keywords: Autism, PECAM-1, P-Selectin, CD31, CD62-P, Adhesion
Background
Autism spectrum disorder (ASD) is a collection of behaviorally defined neurodevelopmental conditions including autism, Asperger’s syndrome, and pervasive development disorder-not otherwise specified (PDD-NOS) (1). Currently, this disorder is estimated to affect 1 in 110 children in the U.S. (2). Symptoms of ASD generally appear in the first three years of life, and are characterized by repetitive behavior or interests, deficits in both verbal and non-verbal language, and impaired social interaction (1). To date, the etiology of ASD remains largely unknown, and is likely a complex combination of genetic and environmental factors. Recent literature has highlighted the impact of immune function on CNS development and neural function, and there is a growing body of evidence to suggests that atypical immune activity may play a role in the pathophysiology ASD (3–5).
Candidate immune genes and aberrant immune responses in individuals with ASD are consistently reported, reviewed in (6). Several lines of evidence point to ongoing neuroinflammation with marked activation of astroglia and microglia observed in individuals with ASD, as well as increased levels of several inflammatory cytokines and chemokines in the cerebral spinal fluid (CSF)(7). Further studies have demonstrated peripheral immune abnormalities in children with ASD including abnormal circulating levels of cytokines (8–10) and atypical levels of plasma immunoglobulin (11–13). Cellular immune dysfunction is also frequently reported in ASD, including abnormal T cell function (14–19), aberrant NK cell activation (20–22), and abnormal monocyte responses (23–25). Together, these data point to a role for cellular dysfunction and neuroinflammation in ASD and suggests that aberrant neuroimmune interactions may occur in the CNS (7, 8, 12, 13, 17, 21–23, 25–39).
To gain access to the CNS, immune cells must migrate across the blood-brain barrier (BBB), which separates circulating blood from the brain parenchyma. Adhesion molecules facilitate the process of leukocyte migration, and therefore, modulate the permeability of the BBB to immune cells (40). Platelet endothelial adhesion molecule-1 (PECAM-1), intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), P-Selectin, and L-Selectin facilitate leukocyte adhesion and transendothelial migration (41). These molecules are present in membrane bound form, and can also be found in circulating plasma under normal non-inflammatory conditions. However, atypical concentrations of these adhesion molecules in the blood and CSF have been repeatedly reported in neuroinflammatory diseases (42, 43).
Several preceding studies have examined a variety of immune cell effectors in ASD, but few have examined molecules involved in facilitating the migration of immune cells into the CNS in young children with ASD and typically developing controls of the same age. The essential role of adhesion molecules in controlling the process of leukocyte migration across the BBB makes these molecules particularly interesting targets for study. Decreased levels of sPECAM-1, sVCAM-1, and sP-Selectin have been previously reported in high functioning adults with autism (44, 45), but the levels of these molecules have not been examined in children with ASD who are closer to the age of the onset of the disorder. To confirm the finding of lower levels of select adhesion molecules in ASD, and to determine if adhesion molecules were also reduced in children with ASD, sPECAM-1, sICAM-1, sVCAM-1, sP-Selectin, and sL-Selectin were quantified in peripheral blood plasma of children with ASD and typically developing controls as part of the Autism Phenome Project (APP).
Methods
Subjects and behavioral assessments
Eighty study participants aged between 2–4 years of age were recruited as part of the Autism Phenome Project (APP). The study protocol was approved by the Institutional Review Board for the UC Davis School of Medicine, and parents of each subject provided written informed consent for their child to participate. Participants consisted of 49 children with ASD, (median age 2.91 years; interquartile range 2.66–3.41 years; 42 males) and 31 typically developing (TD) controls children (median age 3.13 years; interquartile range 2.85–3.27; 20 males). Of the 49 children with ASD, 43 were diagnosed with autism and 6 with PDD-NOS. Diagnostic instruments included the Autism Diagnostic Observation Schedule – Generic (ADOS-G) (46) and the Autism Diagnostic Interview – Revised (ADI-R) (47). All diagnostic assessments were conducted or directly observed by trained, licensed clinical psychologists who specialize in autism and had been trained according to research standards for these tools.
Inclusion criteria for ASD were taken from the diagnostic definition of ASD in young children formulated and agreed upon by the Collaborative Programs of Excellence in Autism (1). Inclusion criteria for TD controls included developmental scores within two standard deviations of the mean on all subscales of the MSEL. Exclusion criteria for TD controls included a diagnosis of Mental Retardation, Pervasive Developmental Disorder or Specific Language Impairment, or any known developmental, neurological, or behavioral problems. TD children were screened and excluded for autism with the Social Communication Questionnaire (48) (scores > 11) (SCQ - Lifetime Edition). All participants were native English speakers, ambulatory, and had no suspected vision or hearing problems. The exclusion criteria for all subjects consisted of the presence of Fragile X or other serious neurological (e.g., seizures), psychiatric (e.g., bipolar disorder) or known medical conditions including autoimmune disease and inflammatory bowel diseases/celiac disease. All subjects were screened via parental interview for current and past physical illness. Children with known endocrine, cardiovascular, pulmonary, liver, or kidney disease were excluded from enrollment in the study. The ASD and TD children had similar vaccination histories with all except 2 children with ASD up-to date for the number and type of vaccinations. No differences were noted for time from last vaccine in the two groups.
Measurement of sPECAM-1, sICAM-1, sVCAM-1, P-Selectin, and L-Selectin
Peripheral blood plasma was collected in acid-citrate-dextrose Vacutainers (BD Biosciences, San Jose, CA) on the last day after behavioral assessments were performed. No participant presented with a cold, fever or other common illness, if such a condition occurred the blood draw were delayed until the child’s health status was stable for 48 hours. The plasma was collected immediately by centrifugation and stored in aliquots at 80 °C until the date of assay. Levels of sPECAM-1 were determined by Human sPECAM-1 enzyme-linked immunosorbant assay kit (ELISA) (Bender MedSystems, Vienna, Austria), with a sensitivity of 0.06 ng/ml, a mean intra-assay coefficient of variation of 1.7%, and a mean inter-assay coefficient of variation of 7.4%. Levels of sP-Selectin were determined sP-Selectin Quantikine® ELISA kits (R&D Systems, Minneapolis, MN) with sensitivities of 0.1ng/ml, a mean intra-assay coefficient of variation of 5.2%, and a mean inter-assay coefficient of variation of 8.9%. Levels of sICAM-1 were determined sICAM-1 Quantikine® ELISA kits (R&D Systems) with sensitivities of 0.6 ng/ml, a mean intra-assay coefficient of variation of 4.6%, and a mean inter-assay coefficient of variation of 5.5%. Levels of VCAM-1 were determined sICAM-1 Quantikine® ELISA kits (R&D Systems) with sensitivities of 0.5 ng/ml, a mean intra-assay coefficient of variation of 3.1%, and a mean inter-assay coefficient of variation of 7.0%. Levels of L-Selectin were determined sICAM-1 Quantikine® ELISA kits (R&D Systems) with sensitivities of 0.3 ng/ml a mean intra-assay coefficient of variation of 3.9%, and a mean inter-assay coefficient of variation of 9.2%. All standards and samples were assayed in duplicate. Two kits were used for each molecule measured, with ASD and typically developing control samples evenly distributed between the two kits. All assays were performed according to the protocols recommended by the manufacturers. Optical densities were determined on a Wallac Victor3 multilabel-plate reader (PerkinElmer, Boston, MA).
Statistical Analysis
As the data was non-parametrically distributed statistical analysis to compare levels of soluble adhesion between ASD and TD groups was conducted with Mann-Whitney U-test. Correlation analysis was performed with Spearman analysis. All analyses were two-tailed, and values of p<0.05 were considered statistically significant. Unadjusted P values are presented (49). All analyses were conducted with GraphPad Prism statistical software (GraphPad Software Inc., San Diego, CA).
Results
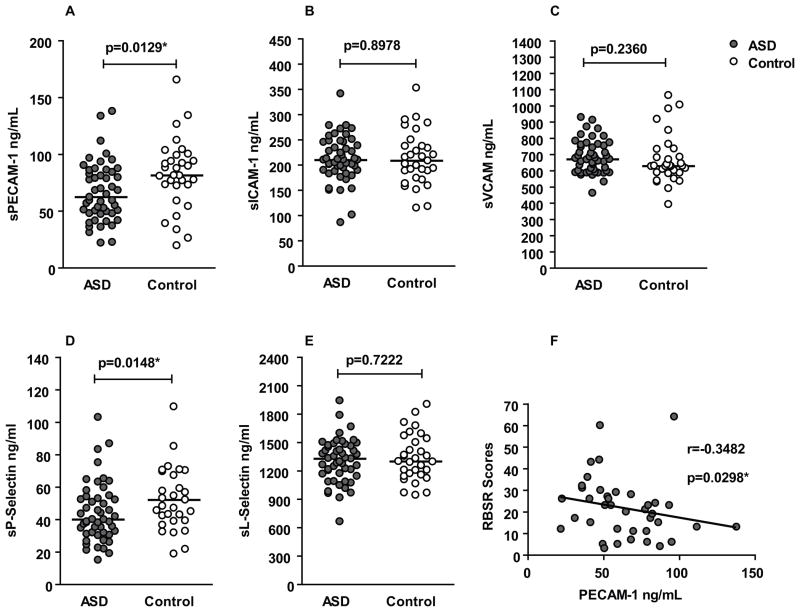
Median plasma levels of sPECAM-1 were reduced approximately 25% lower in the ASD group (median 62.4 ng/ml; interquartile range 48.0–85.1 ng/ml) compared with the TD control group (81.5 ng/ml; 72.4–99.2 ng/ml; p = 0.01) (Figure 1-A). Plasma levels of sP-Selectin were also significantly reduced, by approximately a 25%, in the ASD group (40.0 ng/ml; 31.4–53.5 ng/ml) in comparison with the TD control group (52.1 ng/ml; 39.3–70.2 ng/ml; p = 0.01) (Figure 1-D). There were no significant differences in plasma levels of sICAM-1, sVCAM-1, or sL-Selectin between the ASD participants and TD controls (Table 1). We did not observe any statistical significant differences in adhesion molecule levels based on sex or age in either group (data not shown).
Figure 1. Levels of soluble adhesion molecules in ASD and association with repetitive behavior.
(A) Median levels (black horizontal bar) of sPECAM-1 are significantly lower in ASD (grey dots) in comparison with typically developing controls (white dots). (B, C) There was no difference in the median levels of sICAM-1, sVCAM-1 or sL-Selectin between ASD participants and controls. (D) sP-Selectin levels are significantly lower in the ASD group as compared with controls. (E) There was no difference in sL-Selectin levels between the two groups. (F) Correlation between sPECAM-1 levels and RBSR scores in ASD subjects. RBSR scores correlate negatively with PECAM-1 levels.
Table 1. Descriptive statistics.
Descriptive statistics of study participants, with median and interquartile ranges of sPECAM-1, sICAM-1, sVCAM-1, sP-Selectin, and sL-Selectin levels and the statistical significance between groups presented.
| ASD (n=49) | Control (n=31) | P-Value | |
|---|---|---|---|
| Male/Female | 42 Male/7 Female | 20 Male/11 Female | |
| Age | 2.9 years (2.7–3.4 years) | 3.1 years (2.8–3.3 years) | |
| sPECAM-1* | 62.4 ng/ml (47.9–85.1 ng/ml) | 81.5 ng/ml (72.4–99.2 ng/ml) | 0.0129 |
| sICAM-1 | 210 ng/ml (188.5–245.5 ng/ml) | 208.5 ng/ml (174.5–237.4 ng/ml) | 0.8978 |
| sVCAM-1 | 670.2 ng/ml (595.9–756.5 ng/ml) | 628.9 ng/ml (586.3–732.1 ng/ml) | 0.2360 |
| sP-Selectin* | 40 ng/ml (31.4–53.5 ng/ml) | 52.1 ng/ml (39.3–70.2 ng/ml) | 0.0148 |
| sL-Selectin | 1328.00 ng/ml (1156–1465 ng/ml) | 1300.00 ng/ml (1157–1543 ng/ml) | 0.7222 |
We then examined whether there were associations between the levels of sPECAM-1 and sP-Selectin and clinical behavioral and medical variables among children with ASD. The Repetitive Behavior Scale-Revised (RBSR) is a standard clinical tool for measuring repetitive behavior in children. A negative association was observed between sPECAM-1 levels and scores on the RBSR (r = -0.35; p = 0.03) (Figure 1-F) such that, as sPECAM-1 levels decreased repetitive behaviors became more pronounced in the ASD group. There was no association in the TD group alone (p = 0.83). The potential relationships between PECAM-1 and P-selectin levels were also examined in ADOS and ADI-R scores, however no significant correlations were observed. Abnormal brain size and birth head circumference has previously been linked to sPECAM-1 levels in adults with high functioning autism disorder. In this study we found that birth head circumference was significantly associated with sPECAM-1 levels in TD children (r = 0.44; p = 0.04) but not in children with ASD (r = 0.17; p = 0.58).
Conclusions
For the first time, we show that levels of soluble sPECAM-1 and sP-Selectin, two molecules which mediate leukocyte migration, are significantly decreased in young children with ASD compared to typically developing controls of the same age. This finding is consistent with previous reports of decreased levels of both sPECAM-1 and sP-Selectin in adults with high functioning autism (44, 45). In addition, we observed significant associations between PECAM-1 levels and higher repetitive behavior scores in children with ASD. Repetitive, restricted, and stereotyped behavior is one of the core features of ASD, and this data suggests a potential relationship between PECAM-1 levels, and the severity of repetitive behaviors. Moreover, head circumference was associated with increased sPECAM-1 levels in typically developing children but not children with ASD, suggesting that sPECAM-1 may play a role in normal brain growth, and that this relationship is dysregulated in ASD.
Recent literature demonstrates that immune cells migrate into the CNS under normal non-inflammatory conditions, and that this process is required for normal neurodevelopment and cognitive function (50). Immune deficient mice display cognitive impairments and reconstitution of the mice with T cells by adoptive transfer improves behavior (51–53). In particular IL-4 producing TH2 cells are important for cognitive function (54). Moreover, immune cell activity during an inflammatory episode in the CNS can play a protective effect against further damage and be beneficial, and depletion of immune cells results in exacerbation of neuroinflammation (55–58). In ASD, behavioral improvements have been observed in individuals with fever (59, 60), likely due in part to increased immune cell activity and interactions of immune cells with the blood brain barrier (BBB) and CNS. To further extend these observations, it has recently been shown that increased activation of T cells with a TH2 profile is associated with improved expressive language, fine motor skills and visual reception as determined by Mullens assessments (9); while abnormal circulating lymphocyte numbers and phenotypes are directly associated with better executive function in ASD (61). These findings suggest an intimate relationship between immune cells, and CNS function with a close controlled trafficking of lymphocytes key to the development of typical behaviors.
PECAM-1 and P-Selectin both facilitate leukocyte migration across the endothelial barrier, including the BBB. P-Selectin is constitutively expressed in platelets and endothelial cells, but only transported to the cell surface in response to inflammatory signals, such as the presence of histamine or tumor necrosis factor-alpha in response to injury or infection (62, 63). P-Selectin binds its receptor, P-Selectin Glycoprotein Ligand-1 (PSGL-1) with high affinity, which is expressed on virtually all leukocytes (64). Large amounts of soluble P-Selectin are reportedly produced by shedding from the surface of endothelial cells and platelets (65, 66). PECAM-1 is also expressed on endothelial cells, and is heavily enriched within the intercellular junctions of vasculature. PECAM-1 is also expressed in the majority of leukocytes and platelets, including granulocytes, myeloid lineage cells, and lymphocytes (67). PECAM-1, binds itself in a homophilic manner facilitating adhesion transendothelial migration of leukocytes (68). Soluble PECAM-1 exists in a 90kd cleaved form, and a 120kd form produced by mRNA alternative splicing, both of which are present at approximately equal levels in plasma (69). Although the primary source of soluble PECAM-1 under homeostatic conditions in humans has not been conclusively determined the literature suggests a sizeable source of soluble PECAM-1 is from endothelial cells (69, 70).
PECAM-1 and P-Selectin are important for normal transendothelial migration, and pre-exposure of leukocytes to anti-PECAM-1 blocking antibodies prevents transendothelial migration of leukocytes in-vitro (68, 71). Similarly, in an animal model of neuroinflammation, intravenous injection of anti-PECAM-1 antibodies markedly reduces T cell infiltration into the CNS (72). Like PECAM-1, P-Selectin is essential for facilitating immune cell migration into the CNS. In-vivo administration of blocking antibodies specific for P-Selectin, or its ligand, PSGL-1, result in greatly reduced T cell infiltration in animal models (73, 74). The data from our current study suggest a link between sPECAM-1 levels and normal brain growth and cognition in TD children but that is aberrant in children with ASD. Decreased sPECAM-1 was also associated with increased repetitive behaviors. This data suggests that reduced levels of either PECAM-1 or P-Selectin may result in reduced immune cell-endothelial cell interactions and possible reduced access to the CNS. Fewer immune-endothelial cell interactions could effect a number of physiological processes that impact neurodevelopment and or behavior (7, 26–28, 38, 39, 51, 52, 68, 71, 72, 75–81).
In conclusion, adhesion molecules, sPECAM-1 and sP-Selectin, play a crucial role in regulating immune cell access to the CNS. Data for the current studies shows that there are decreased sPECAM-1 and sP-Selectin levels in young children with ASD. These reduced levels were associated with increased repetitive behaviors, abnormal brain growth and impaired cognition. These findings are novel and further investigations aimed at determining the interactions between immune cells and behavior in children with ASD are needed. Although we have demonstrated that the lower PECAM-1 levels previously reported in adults is also present in children between the ages of 2–5 years, little is known about possible fluctuations of these levels during development. The results from this study warrant follow-up in a replication study to measure soluble PECAM-1 levels longitudinally. In addition to further human studies, animal models of deficient PECAM-1 production can also be utilized. The B6.129P2-Pecam1tm1Mak mouse is virtually devoid of PECAM-1, and has been utilized in a number of studies to demonstrate the immunological and vascular impact of PECAM-1 deficiency (82, 83). However, to our knowledge, neurodevelopment and behavior have not been thoroughly examined in the B6.129P2-Pecam1tm1Mak mouse. Examination of this mouse model, and heterozygotes of this mouse line, may be a promising tool to examine the relationship between low PECAM-1 levels, immune cell neuroendothelial interactions and behavior. Such future studies may elucidate the physiological relevance of low sPECAM-1 levels in ASD described herein.
Acknowledgments
This study was funded by the National Institute of Neurological Disorders and Stroke R21HD065269, Autism Speaks Foundation, the Jane Botsford Johnson Foundation, National Alliance for Research on Schizophrenia and Depression, and, the Peter Emch Foundation. We would like to thank the staff of both the UC Davis M.I.N.D. Institute and the APP study for their technical support. The commitment of the families who took part in these studies, at both the M.I.N.D Institute and as part of the APP study, is also gratefully acknowledged.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.APA. Diagnostic and Statistical Manual of Mental Disorders. 4. Arlington, VA: American Psychiatric Publishing, Inc; 2000. Text Revision ed. [Google Scholar]
- 2.MMWR. Prevalence of Autism Spectrum Disorders --- Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- 3.Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 4.Enstrom AM, Van de Water JA, Ashwood P. Autoimmunity in autism. Curr Opin Investig Drugs. 2009;10:463–473. [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Careaga M, Van de Water J, Ashwood P. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 8.Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, et al. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204:149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croonenberghs J, Wauters A, Devreese K, Verkerk R, Scharpe S, Bosmans E, et al. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med. 2002;32:1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- 12.Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, et al. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1:275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, et al. Increased IgG4 levels in children with autism disorder. Brain Behav Immun. 2009;23:389–395. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren RP, Yonk LJ, Burger RA, Cole P, Odell JD, Warren WL, et al. Deficiency of suppressor-inducer (CD4+CD45RA+) T cells in autism. Immunol Invest. 1990;19:245–251. doi: 10.3109/08820139009041839. [DOI] [PubMed] [Google Scholar]
- 15.Yonk LJ, Warren RP, Burger RA, Cole P, Odell JD, Warren WL, et al. CD4+ helper T cell depression in autism. Immunol Lett. 1990;25:341–345. doi: 10.1016/0165-2478(90)90205-5. [DOI] [PubMed] [Google Scholar]
- 16.Denney DR, Frei BW, Gaffney GR. Lymphocyte subsets and interleukin-2 receptors in autistic children. J Autism Dev Disord. 1996;26:87–97. doi: 10.1007/BF02276236. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Aggarwal S, Rashanravan B, Lee T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol. 1998;85:106–109. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 18.Ashwood P, Anthony A, Pellicer AA, Torrente F, Walker-Smith JA, Wakefield AJ. Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. J Clin Immunol. 2003;23:504–517. doi: 10.1023/b:joci.0000010427.05143.bb. [DOI] [PubMed] [Google Scholar]
- 19.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren RP, Foster A, Margaretten NC. Reduced natural killer cell activity in autism. J Am Acad Child Adolesc Psychiatry. 1987;26:333–335. doi: 10.1097/00004583-198705000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Vojdani A, Mumper E, Granpeesheh D, Mielke L, Traver D, Bock K, et al. Low natural killer cell cytotoxic activity in autism: the role of glutathione, IL-2 and IL-15. J Neuroimmunol. 2008;205:148–154. doi: 10.1016/j.jneuroim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Enstrom AM, Lit L, Onore CE, Gregg JP, Hansen RL, Pessah IN, et al. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun. 2009;23:124–133. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- 24.Sweeten TL, Posey DJ, McDougle CJ. High blood monocyte counts and neopterin levels in children with autistic disorder. Am J Psychiatry. 2003;160:1691–1693. doi: 10.1176/appi.ajp.160.9.1691. [DOI] [PubMed] [Google Scholar]
- 25.Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66:143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- 31.Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, et al. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Croen LA, Goines P, Braunschweig D, Yolken R, Yoshida CK, Grether JK, et al. Brain-derived neurotrophic factor and autism: maternal and infant peripheral blood levels in the Early Markers for Autism (EMA) Study. Autism Res. 2008;1:130–137. doi: 10.1002/aur.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grigorenko EL, Han SS, Yrigollen CM, Leng L, Mizue Y, Anderson GM, et al. Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics. 2008;122:e438–445. doi: 10.1542/peds.2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45:1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- 35.Jyonouchi H, Geng L, Ruby A, Reddy C, Zimmerman-Bier B. Evaluation of an association between gastrointestinal symptoms and cytokine production against common dietary proteins in children with autism spectrum disorders. J Pediatr. 2005;146:605–610. doi: 10.1016/j.jpeds.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Onore C, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen R, Van de Water J, et al. Decreased cellular IL-23 but not IL-17 production in children with autism spectrum disorders. J Neuroimmunol. 2009;216:126–129. doi: 10.1016/j.jneuroim.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmerman AW, Jyonouchi H, Comi AM, Connors SL, Milstien S, Varsou A, et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;33:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 42.Sharief MK, Noori MA, Ciardi M, Cirelli A, Thompson EJ. Increased levels of circulating ICAM-1 in serum and cerebrospinal fluid of patients with active multiple sclerosis. Correlation with TNF-alpha and blood-brain barrier damage. J Neuroimmunol. 1993;43:15–21. doi: 10.1016/0165-5728(93)90070-f. [DOI] [PubMed] [Google Scholar]
- 43.Losy J, Niezgoda A, Wender M. Increased serum levels of soluble PECAM-1 in multiple sclerosis patients with brain gadolinium-enhancing lesions. J Neuroimmunol. 1999;99:169–172. doi: 10.1016/s0165-5728(99)00092-2. [DOI] [PubMed] [Google Scholar]
- 44.Tsuchiya KJ, Hashimoto K, Iwata Y, Tsujii M, Sekine Y, Sugihara G, et al. Decreased serum levels of platelet-endothelial adhesion molecule (PECAM-1) in subjects with high-functioning autism: a negative correlation with head circumference at birth. Biol Psychiatry. 2007;62:1056–1058. doi: 10.1016/j.biopsych.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Iwata Y, Tsuchiya KJ, Mikawa S, Nakamura K, Takai Y, Suda S, et al. Serum levels of P-selectin in men with high-functioning autism. Br J Psychiatry. 2008;193:338–339. doi: 10.1192/bjp.bp.107.043497. [DOI] [PubMed] [Google Scholar]
- 46.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 47.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 48.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 49.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 50.Schwartz M, Kipnis J. A conceptual revolution in the relationships between the brain and immunity. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 53.Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziv Y, Finkelstein A, Geffen Y, Kipnis J, Smirnov I, Shpilman S, et al. A novel immune-based therapy for stroke induces neuroprotection and supports neurogenesis. Stroke. 2007;38:774–782. doi: 10.1161/01.STR.0000255784.27298.23. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz M, Moalem G, Leibowitz-Amit R, Cohen IR. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22:295–299. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- 57.Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiu IM, Chen A, Zheng Y, Kosaras B, Tsiftsoglou SA, Vartanian TK, et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci U S A. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curran LK, Newschaffer CJ, Lee LC, Crawford SO, Johnston MV, Zimmerman AW. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics. 2007;120:e1386–1392. doi: 10.1542/peds.2007-0360. [DOI] [PubMed] [Google Scholar]
- 60.Torres AR. Is fever suppression involved in the etiology of autism and neurodevelopmental disorders? BMC Pediatr. 2003;3:9. doi: 10.1186/1471-2431-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han YMY, Leung WWM, Wong CK, Lam JMK, Cheung MC, Chan AS. Lymphocyte subset alterations related to executive function deficits and repetitive stereotyped behavior in autism. Res Autism Spect Dis. 2011;5:486–494. [Google Scholar]
- 62.Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- 64.McEver RP, Cummings RD. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:485–491. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 66.Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, et al. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci U S A. 1996;93:11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 68.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldberger A, Middleton KA, Oliver JA, Paddock C, Yan HC, DeLisser HM, et al. Biosynthesis and processing of the cell adhesion molecule PECAM-1 includes production of a soluble form. J Biol Chem. 1994;269:17183–17191. [PubMed] [Google Scholar]
- 70.Ilan N, Mohsenin A, Cheung L, Madri JA. PECAM-1 shedding during apoptosis generates a membrane-anchored truncated molecule with unique signaling characteristics. FASEB J. 2001;15:362–372. doi: 10.1096/fj.00-0372com. [DOI] [PubMed] [Google Scholar]
- 71.Liao F, Ali J, Greene T, Muller WA. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J Exp Med. 1997;185:1349–1357. doi: 10.1084/jem.185.7.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qing Z, Sandor M, Radvany Z, Sewell D, Falus A, Potthoff D, et al. Inhibition of antigen-specific T cell trafficking into the central nervous system via blocking PECAM1/CD31 molecule. J Neuropathol Exp Neurol. 2001;60:798–807. doi: 10.1093/jnen/60.8.798. [DOI] [PubMed] [Google Scholar]
- 73.Piccio L, Rossi B, Scarpini E, Laudanna C, Giagulli C, Issekutz AC, et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. J Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- 74.Kisucka J, Chauhan AK, Zhao BQ, Patten IS, Yesilaltay A, Krieger M, et al. Elevated levels of soluble P-selectin in mice alter blood-brain barrier function, exacerbate stroke, and promote atherosclerosis. Blood. 2009;113:6015–6022. doi: 10.1182/blood-2008-10-186650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DeLisser HM, Newman PJ, Albelda SM. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994;15:490–495. doi: 10.1016/0167-5699(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 76.Schimmenti LA, Yan HC, Madri JA, Albelda SM. Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J Cell Physiol. 1992;153:417–428. doi: 10.1002/jcp.1041530222. [DOI] [PubMed] [Google Scholar]
- 77.Reinke EK, Lee J, Zozulya A, Karman J, Muller WA, Sandor M, et al. Short-term sPECAM-Fc treatment ameliorates EAE while chronic use hastens onset of symptoms. J Neuroimmunol. 2007;186:86–93. doi: 10.1016/j.jneuroim.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, et al. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, et al. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:3499–3510. [PubMed] [Google Scholar]
- 80.Wispelwey B, Lesse AJ, Hansen EJ, Scheld WM. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest. 1988;82:1339–1346. doi: 10.1172/JCI113736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilkinson R, Lyons AB, Roberts D, Wong MX, Bartley PA, Jackson DE. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) acts as a regulator of B-cell development, B-cell antigen receptor (BCR)-mediated activation, and autoimmune disease. Blood. 2002;100:184–193. doi: 10.1182/blood-2002-01-0027. [DOI] [PubMed] [Google Scholar]
- 82.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, et al. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1). CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- 83.Mahooti S, Graesser D, Patil S, Newman P, Duncan G, Mak T, et al. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol. 2000;157:75–81. doi: 10.1016/S0002-9440(10)64519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]