Abstract
We aimed to describe the cardiac magnetic resonance (CMR) findings and to determine prognostic variables in patients with a cardiomyopathy after treatment with anthracyclines. CMR imaging was performed in 91 patients (58% male, mean age of 43 ± 18 years and anthracycline dose of 276 ± 82 mg/m2) with reduced ejection fraction (EF) after anthracycline-based chemotherapy. Major adverse cardiovascular events (MACE) were defined as cardiovascular death, appropriate implantable cardioverter defibrillator therapy, and admission for decompensated heart failure. Patients presented a median of 88 months (IQR 37 to 138) after chemotherapy and were followed for 27 months (IQR 22 to 38). Late gadolinium enhancement was an uncommon finding (5 patients, 6%) despite a reduced EF (36 ± 8%). There was an inverse association between anthracycline dose and the indexed left ventricular (LV) mass by CMR (r = −0.67, p < 0.001). There were 52 adverse cardiac events (event rate of 22% per year). When patients were grouped according to the presence or absence of a MACE, indexed LV mass and glomerular filtration rate were lower and anthracycline dose was higher among patients that experienced an adverse event. In a multivariable model, indexed LV mass demonstrated the strongest association with MACE (HR = 0.89, chi-squared = 26, p < 0.001). In conclusion, scar by LGE-CMR imaging is infrequent in patients with an anthracycline-induced cardiomyopathy despite a reduced EF, the event rate in patients with an established anthracycline-induced cardiomyopathy is high, and indexed LV-mass by CMR is a predictor of adverse cardiovascular events.
Keywords: Anthracyclines, Doxorubicin, Cardiac Magnetic Resonance, Heart Failure
Anthracyclines are a key component of many chemotherapy regimens. Long term outcomes after anthracycline-based therapy are limited, in part, by cardiovascular morbidity and mortality.1 Over 50% of patients treated with anthracyclines will show cardiac dysfunction,2,3 with an associated 2-year survival of 40%.4 The risk factors for the development of anthracycline-cardiotoxicity are partially known;5 the predictors of adverse outcomes, once heart failure is established, are less clear. Cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) is a sensitive and reproducible technique for detection and quantification of myocardial fibrosis and function,6 and scar by LGE-CMR has been shown to be a strong predictor of outcomes in patients with a cardiomyopathy.7,8 However, data on the characteristic CMR findings, the presence and the extent of LGE specifically in patients with an anthracycline-cardiomyopathy are limited.9 Also, the predictors of subsequent adverse events in patients with anthracycline-cardiomyopathy are poorly defined. Therefore, we aimed to add to the data on the role of CMR in patients with a reduced ejection fraction (EF) after the administration of anthracycline chemotherapy.
Methods
We performed a retrospective, observational study of consecutive patients with a clinically-diagnosed anthracycline-cardiomyopathy who attended the Brigham and Women’s Hospital for a CMR that included gadolinium between 2003 and 2010. All patients were entered into a registry at the time of the CMR study. Patients were being referred for a CMR study for work-up of a cardiomyopathy. The diagnosis of anthracycline-cardiomyopathy was based on the following criteria: (1) lack of heart failure or reduced EF before treatment with anthracyclines; (2) exclusion of other causes of cardiomyopathy such as a history of significant coronary artery disease, myocardial infarction, excess alcohol, uncontrolled hypertension, or infiltrative cardiomyopathy; and (3) confirmation of depressed left ventricular (LV) EF after initiation of anthracyclines. Coronary artery disease was excluded by negative coronary angiography or negative stress testing with imaging. Ten patients aged < 30 years did not undergo either test. Patients with subendocardial or transmural LGE typical of an infarct in the territory of a coronary artery were excluded. After exclusion of patients with significant coronary artery disease, myocardial infarction, prior revascularization, 4 patients had unanticipated LGE, these patients were excluded. There were 2 cohorts. Cohort 1 consisted of patients with stage C heart failure (symptomatic reduction in EF). The 2nd cohort had stage B heart failure (asymptomatic reduction in EF) which was detected using routine surveillance post chemotherapy. The protocol was approved by the Partners Healthcare System Human Subjects Review Committee in the Brigham and Women’s Hospital.
All CMR images were acquired on either a 1.5 (n=25) or 3.0-T (n=66) system (Signa CV/i, General Electric Healthcare, Wisconsin, USA; Tim Trio, Siemens, Erlangen, Germany, respectively). The basic CMR protocol consisted of imaging for LV function and LV mass as previously desrcibed.10 For the calculation of LV mass and EF, the endocardial and epicardial borders of the LV were manually traced on successive short-axis cine images at end-diastole and systole. The papillary muscles were excluded in the LV mass calculation. The LV mass by CMR was derived by the summation of discs method by multiplying myocardial muscle volume by 1.05 g/cm3. To assess the contribution of cardiac edema, we performed a T2-weighted inversion recovery prepared fast spin echo sequence using three short axis slices of 12 mm thickness at the base, mid, and apex and a single long axis-slice in a 4 chamber view.11 Qualitatively, the sequence was considered abnormal if there were patchy areas of high T2 signal intensity indicating focal or regional edema. Quantitatively, the sequence was considered abnormal if the average signal intensity of the myocardium, normalized to skeletal muscle, was ≥ 2.11 Results were considered abnormal if positive using either qualitative or quantitative methods. Images were analyzed with specialized software (Mass Research, Leiden University Medical Center, Netherlands) by researchers blinded to clinical outcome. All patients underwent an LGE imaging protocol as previously described.10
Left ventricular mass by echocardiography was derived from the 2-D measurements of intraventricular septal thickness, posterior wall thickness and LV internal dimensions in diastole as recommended by the American Society of Echocardiography.12 Left ventricular EF was measured using the biplane method of discs. Pulmonary artery systolic pressure was estimated from the regurgitant TR velocity plus an estimate of right atrial pressure derived from the inferior vena cava.
Patients were also followed at 3- to 6-month intervals via clinic visits performed as part of routine care. The duration of follow-up was determined from the CMR study date. The outcome of interest was a composite of cardiovascular death, appropriate implantable cardioverter defibrillator (ICD) therapy (ICD discharge or anti-tachycardia pacing), and admission for decompensated heart failure. We ascertained mortality by the Social Security Death Index and confirmed using chart review and, if required, discussions with the primary health care providers. For patients who experienced an adverse cardiac outcome, time to that first event was used. For patients who did not experience an adverse cardiac outcome, censoring occurred on the last date of clinical follow-up.
Continuous data are presented as mean ± SD and compared using an unpaired Student t-test or Mann-Whitney non-parametric test as appropriate. Nominal data are presented as number and percentages and were compared with a Chi-square test for a trend. To test a correlation between LV mass index and anthracycline dose, a Spearman’s rank correlation coefficient was used. The difference in LV mass measurement between methods was calculated for 50 randomly selected subjects. To test the inter- and intra-observer variability of the CMR derived LV mass and CMR-derived LV end-diastolic volume (EDV), another 15 randomly selected patients were selected. Bland-Altman plots were performed to determine the 95% limits of agreement between methods. The hazard ratio was calculated for the combined outcome using a Cox regression model. Considering all the significant variables in from the univariate analysis, we sought the best-overall multivariable models for the composite end-point, by stepwise-forward selection with a probability to enter set and leave at p = 0.01. To test whether, prior heart failure admissions, was associated with subsequent MACE, we performed a second model that included prior heart failure hospitalization to univariable analysis. Receiver operator characteristic (ROC) curves were constructed to determine optimal cut-off of CMR-derived LV mass index, LVEF, anthracycline dose and glomerular filtration rate (GFR) to predict adverse cardiovascular events. Event curves were determined according to the Kaplan-Meier method and comparisons of cumulative event rates were performed by the log-rank test. A two-tailed p value of < 0.05 was deemed significant for all other analyses and SAS was used for statistical analysis (SAS Institute Inc, Cary).
Results
We identified 91 patients, 53 men and 38 women, with a mean age of patients at initiation of chemotherapy of 43 ± 18 years, a mean anthracycline dose of 276 ± 82 mg/m2, and presenting 88 months (IQR 37 to 138) after chemotherapy. Overall, 82% were on either an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker (Table 1). Estimated GFR was lower among patients with adverse events and the dose of anthracyclines administered was higher, otherwise non-imaging parameters were similar among groups.
Table 1. Baseline Characteristics Grouped and by Major Adverse Cardiac Events (MACE).
| Variable | Cohort | MACE | p Value | |
|---|---|---|---|---|
|
(n=91) |
Yes (n=52) |
No (n=39) |
||
| Age at start of chemotherapy (yrs) | 43 (18) | 43 (19) | 44 (15) | 0.76 |
| Male, | 53 (58%) | 27 (52%) | 22 (56%) | 0.13 |
| Chest radiation, | 30 (33%) | 20 (38%) | 10 (26%) | 0.07 |
| Anthracycline Dose (mg/m2) | 276 (82) | 296 (76) | 246 (85) | 0.005 |
| Chemotherapy to clinical presentation (months, median, IQR) |
88 (37, 138) | 86 (38, 145) | 90 (26,141) | 0.53 |
| Clinical presentation to CMR study (months, median, IQR) |
3 (1, 5) | 3 (1,5) | 3 (1, 5) | 0.89 |
| Heart failure stage C | 75 (82%) | 45 (87%) | 30 (77%) | 0.86 |
| Prior Admission for heart failure | 14 (15%) | 10 (19%) | 4 (10%) | 0.38 |
| Diabetes Mellitus | 11 (12%) | 7 (13%) | 4 (10%) | 1.00 |
| Hypertension | 35 (38%) | 21 40%) | 14 (36%) | 0.64 |
| Atrial fibrillation | 20 (22%) | 11 (21%) | 9 (23%) | 1.00 |
| New York Heart Association Functional Class: | ||||
| I | 16 (18%) | 9 (17%) | 7 (18%) | 0.76 |
| II | 60 (66%) | 35 (67%) | 25 (64%) | 0.56 |
| III | 15 (16%) | 10 (19%) | 5 (13%) | 0.30 |
| Body Mass Index (kg/m2) | 28 (5) | 27 (4) | 29 (5) | 0.18 |
| Systolic blood pressure (mmHg) | 116 (16) | 115 (17) | 117(12) | 0.43 |
| Diastolic blood pressure (mmHg) | 72 (12) | 71 (12) | 73 (12) | 0.4 |
| Heart rate (beats/min) | 73 (14) | 74 (15) | 72 (13) | 0.37 |
| Medications | ||||
| ACE/ARB | 75 (82%) | 46 (88%) | 29 (74%) | 0.40 |
| Beta-blocker | 75 (82%) | 42 (81%) | 33 (84%) | 0.77 |
| Spironolactone | 21 (22%) | 13 (25%) | 8 (21%) | 0.44 |
| Diuretics | 52 (57%) | 31 (60%) | 21 (54%) | 0.83 |
| Antiarrythmic | 17 (19%) | 11 (21%) | 6 (15%) | 0.58 |
| Warfarin | 23 (25%) | 14 (27%) | 9 (23%) | 0.63 |
| Aspirin | 21 (23%) | 11 (21%) | 10 (26%) | 0.83 |
| Statin | 29 (32%) | 17 (33%) | 12 (31%) | 0.65 |
| QRS duration (ms) | 96 (16) | 97 (17) | 95 (16) | 0.47 |
| QTc duration (ms) | 454 (28) | 458 (28) | 447 (24) | 0.07 |
| Glomerular filtration rate (ml/min/1.73m2) |
78 (24) | 75 (23) | 81 (25) | 0.0003 |
All data are mean (SD) unless otherwise indicated; ACE/ARB = angiotensin converting enzyme inhibitor/ angiotensin receptor blocker; Glomerular filtration rate using the Modification of Diet in Renal Disease formula;
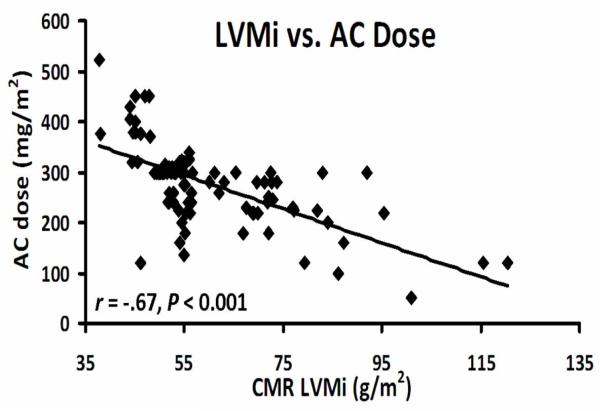
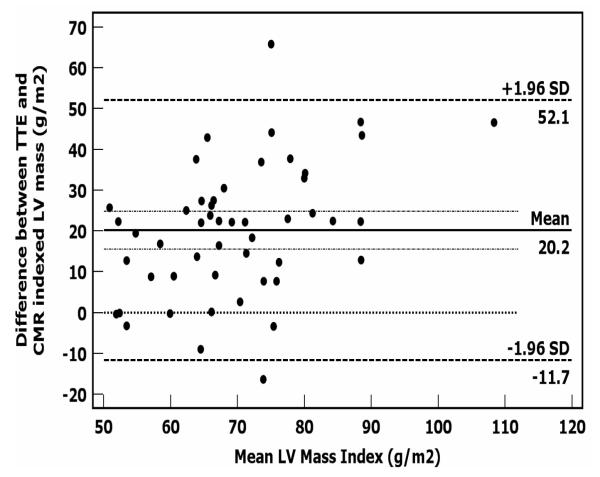
The mean LVEF by echocardiography was 35 ± 8% (Table 2). Indexed LV mass by CMR was lower among groups that received higher doses of chemotherapy (Table 2). Otherwise, imaging characteristics were similar between groups. There was an inverse association between CMR-derived indexed LV mass index and the dose of anthracyclines (r = −0.67, p < 0.001, Figure 1). There was a mean difference between TTE-derived LV mass index and CMR-derived LV mass index of 20 g/m2 (Figure 2). We found an absolute mean difference in CMR-derived LV mass of 5 ± 2 grams within an observer and of 6.4 ± 2 grams for between observers. We found an average difference of 8 ± 3 mls for intra-observer and 11 ± 3 mls for inter-observer variability for CMR-derived LVEDV (data not shown).One patient in the entire cohort had qualitative and quantitative evidence of myocardial edema (qualitative edema and a global relative signal intensity of 2.4 ± 0.2). The ratio of signal intensity of myocardium to skeletal muscle was normal,11 and similar between groups with and without an adverse event (Table 2). Late gadolinium enhancement was an infrequent finding occurring in 5 (6%) of patients. The distribution of LGE was either mid-myocardial (n=2), RV insertion point (n=1), and epicardial (n=2).
Table 2. Baseline Characteristics Grouped and by Major Adverse Cardiac Events (MACE).
| Variable | Cohort | MACE | p Value | |
|---|---|---|---|---|
| (n=91) | Yes (n=52) |
No (n=39) |
||
| Echocardiography: | ||||
| Left ventricular ejection fraction (%) | 35 (8) | 36 (9) | 35 (7) | 0.61 |
| Left ventricular internal dimensions in diastole (mm) | 49 (6) | 49 (5) | 49 (7) | 0.95 |
| Estimated pulmonary artery systolic pressure (mmHg) | 33 (10) | 33 (10) | 33 (9) | 0.98 |
| Left ventricular mass index (gm/m2) | 78 (18) | 78 (18) | 78 (17) | 0.97 |
| Cardiac Magnetic Resonance | ||||
| Left ventricular end diastolic volume (ml) | 181 (48) | 186 (44) | 173 (52) | 0.23 |
| Indexed left ventricular end diastolic volume (ml/m ) | 92 (22) | 100 (23) | 89 (18) | 0.31 |
| Left ventricular end systolic volume (ml) | 116 (34) | 118 35) | 112 (32) | 0.42 |
| Indexed left ventricular end systolic volume (ml/m ) | 59 (17) | 61 (22) | 53 (14) | 0.16 |
| Left ventricular ejection fraction (%) | 36 (8) | 37 (7) | 35 (7) | 0.23 |
| Left ventricular mass index (g/m2) | 60 (16) | 51 (5) | 71 (12) | <0.0001 |
| Right ventricular end diastolic volume (ml) | 154 (41) | 155 (41) | 154 (42) | 0.73 |
| Indexed right ventricular end diastolic (ml/m2) | 78 (18) | 80 (20) | 77 (16) | 0.41 |
| Right ventricular end systolic volume (ml) | 84 (33) | 77 (32) | 82 (29) | 0.18 |
| Indexed right ventricular end systolic volume (ml/m2) | 43 (15) | 45 (15) | 41 (14) | 0.21 |
| Right ventricular ejection fraction (%) | 46 (12) | 44 (11) | 47 (11) | 0.25 |
| Relative T2 Signal Intensity | 1.6 (0.2) | 1.6 (0.2) | 1.5 (0.2) | 0.46 |
| Late gadolinium enhancement | 5 (6) | 3 (6) | 2 (5) | 0.88 |
| Volume of late gadolinium enhancement (% of mass) | 7 (4) | 9 (5) | 4 (1) | 0.27 |
All values are mean (SD); Volume of LGE = volume of late gadolinium enhancement as a percentage of total LV volume using the 2 SD method in patients who were identified as having LGE
Figure 1.
Association of LV mass derived by CMR with baseline anthracycline dose (r = −0.67, P < 0.001).
Figure 2.
Bland-Altman plots showing the 95% limits of agreement between 3D-CMR-derived LV mass and ASE cube LV mass calculation.
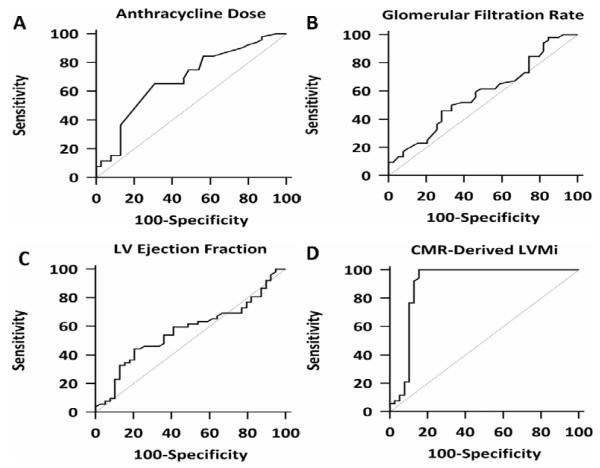
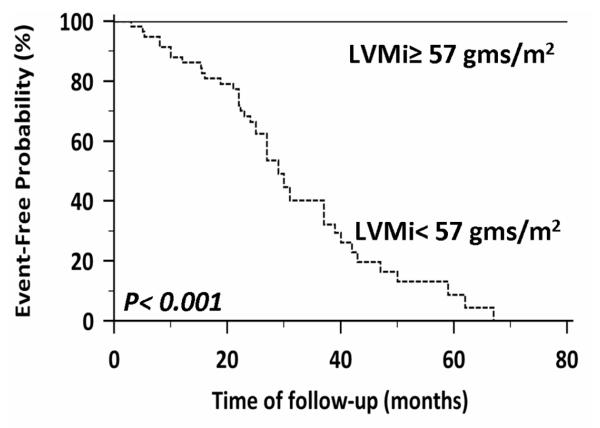
There were 52 events during follow-up (5 cardiovascular deaths, 8 appropriate ICD therapies, and 39 admissions for decompensated heart failure, 22% event rate per year) during a median follow-up period of 27 months (IQR, 22-38 months). Fifty patients underwent implantation of an implantable cardioverter defibrillator. By univariate analysis, the dose of anthracycline, glomerular filtration rate (GFR), and CMR-derived indexed LV mass were predictors of major adverse cardiovascular events (Table 3). Of these, indexed LV mass by CMR had the strongest unadjusted association with the adverse outcomes (HR = 0.85, chi-squared = 26, p < 0.0001) (Table 3). In a multivariable analysis using predictors developed from the univariate model, CMR-derived indexed LV mass demonstrated the strongest adjusted association with MACE. In a second analysis that included prior heart failure hospitalization, indexed LV mass still provided the strongest adjusted association with the outcome of interest (HR = 0.87, chi-squared = 23, p < 0.0001). Variables are also separated according to the median CMR-derived LV mass and are presented (Table 4). Receiver operator curve analyses, testing the ability of anthracycline dose, GFR, CMR-derived LVEF, and CMR-derived indexed LV mass to predict MACE revealed an area under the curve (AUC) of 0.90 for CMR-derived indexed LV mass and that a CMR-derived indexed LV mass of < 57 grams/m2 had a sensitivity of 100% and a specificity of 85% for prediction of MACE (Figure 3). As LV mass is lower in women, the entire analysis was also performed in the cohort separated by gender. When groups were separated by gender, LV mass index was significantly reduced in males and females who had an adverse event (Indexed LV mass 72 ± 11 vs. 51 ± 4 grams/m2, women with MACE vs. women without MACE, p <0.001; indexed LV mass 73 ± 20 vs. 51 ± 4 grams/m2, men with MACE vs. men without MACE, p <0.001). In ROC analysis confined to women, an indexed LV mass of < 56 grams/m2 provided the strongest combination of sensitivity and specificity for prediction of MACE, sensitivity 100 (95% CI 86-100) and specificity of 92% (95% CI 64 – 99). In men, we found that an indexed LV mass of < 57 grams/m2 provided the strongest combination of sensitivity (95% CI 87-100) and specificity (81% CI 61-94). Kaplan-Meier curves showing event-free survival are shown, after stratifying the entire cohort according to an indexed LV mass of < 57 grams/m2. Patients with an indexed LV mass of < 57 grams/m2 developed more long-term adverse outcomes than patients with an indexed LV mass of ≥ 57 grams/m2 (Figure 4).
Table 3. Baseline Characteristics Grouped and by Major Adverse Cardiac Events (MACE).
| Variable | Hazard Ratio |
Confidence Interval |
Chi-Square (x2) |
p Value |
|---|---|---|---|---|
| Age of chemotherapy | 0.00 | 0.97-1.02 | 0.001 | 0.97 |
| Anthracycline dose | 1.00 | 1.00-1.00 | 7.7 | 0.005 |
| Male | 0.68 | 0.28-1.69 | 0.69 | 0.40 |
| Stage B heart failure | 0.68 | 0.16-2.97 | 0.27 | 0.60 |
| Stage C heart failure | 1.48 | 0.38-6.43 | 0.27 | 0.60 |
| Glomerular filtration rate | 0.97 | 0.96-0.99 | 8.48 | 0.0003 |
| Echocardiography: | ||||
| Left ventricular ejection fraction | 0.98 | 0.95-1.02 | 1.11 | 0.29 |
| Estimated pulmonary artery systolic pressure |
1.01 | 0.98-1.05 | 0.58 | 0.44 |
| Indexed left ventricular mass by echocardiography |
0.99 | 0.98-1.01 | 0.02 | 0.86 |
| Cardiac Magnetic Resonance: | ||||
| Left ventricular end diastolic volume | 0.99 | 0.99-1.02 | 0.03 | 0.86 |
| Left ventricular end systolic volume | 1.01 | 0.99-1.02 | 1.73 | 0.52 |
| Left ventricular ejection fraction | 0.98 | 0.95-1.03 | 0.53 | 0.46 |
| Indexed left ventricular mass by CMR | 0.85 | 0.80-0.90 | 26.2 | <0.0001 |
| Right ventricular end diastolic volume | 1.03 | 0.98-1.07 | 0.62 | 0.44 |
| Right ventricular end systolic volume | 1.01 | 0.99-1.02 | 1.83 | 0.18 |
| Right ventricular ejection fraction | 0.97 | 0.92-1.01 | 1.11 | 0.25 |
Table 4. Characteristics by Cardiac Magnetic Resonance (CMR)-derived Median Left Ventricular Mass Index (LVMi).
| Variable | CMR-LVMi (<55 mg/m2) (n=45) |
CMR-LVMi (>55 mg/m2) (n=46) |
p Value |
|---|---|---|---|
| Age of chemotherapy (yrs) | 42 (18) | 43 (17) | 0.69 |
| Male | 24 (45%) | 29 (55%) | 0.39 |
| Chest radiation | 19 (63%) | 11 (37%) | 0.07 |
| Anthracycline dose (mg/m ) | 317 (76) | 235 (63) | <0.0001 |
| Major adverse cardiac events | 40 (77%) | 12 (23%) | <0.0001 |
| Chemo to heart failure (months, mean, mths) | 91 (81) | 105 (90) | 0.43 |
| Heart failure stage C | 39 (52%) | 35 (48%) | 0.28 |
| Diabetes Mellitus | 6 (55%) | 5 (45%) | 0.75 |
| Hypertension | 18 (51%) | 17 (49%) | 0.83 |
| Atrial fibrillation | 10 (50%) | 10 (50%) | 1.00 |
| Systolic blood Pressure (mmHg) | 116 (15) | 115 (17) | 0.51 |
| Diastolic blood Pressure (mmHg) | 73 (13) | 70 (11) | 0.21 |
| Heart rate (beats/min) | 71 (13) | 74 (13) | 0.37 |
| Medication | |||
| ACE/ARB | 38 (51%) | 37 (49%) | 0.78 |
| Beta-blocker | 38 (51%) | 37 (49%) | 0.76 |
| Spironolactone | 13 (62%) | 8 (38%) | 0.12 |
| QRS duration (ms) | 96 (14) | 96 (18) | 0.99 |
| Glomerular filtration rate (ml/min/1.73m ) | 72 (23) | 84 (23) | 0.02 |
| Echocardiography: | |||
| Estimated pulmonary artery systolic pressure (mmHg) | 33 (10) | 33 (10) | 0.95 |
| Left ventricular mass index by echo (gm/m2) | 80 (19) | 76 (16) | 0.33 |
| Cardiac Magnetic Resonance | |||
| Indexed left ventricular end diastolic volume (ml/m2) | 97 (21) | 88 (21) | 0.05 |
| Indexed left ventricular end systolic volume (ml/m ) | 63 (18) | 55 (14) | 0.04 |
| Left ventricular ejection fraction (%) | 36 (8) | 36 (7) | 0.62 |
| Indexed right ventricular end diastolic volume (ml/m2) | 80 (20) | 77 16) | 0.38 |
| Indexed right ventricular end systolic volume (ml/m ) | 44 (15) | 42 (15) | 0.56 |
| Right ventricular ejection fraction (%) | 45 (9) | 46 (10) | 0.68 |
| Relative T2 signal intensity | 1.4 (0.2) | 1.5 (0.2) | 0.36 |
| Late gadolinium enhancement | 3 (7%) | 2 (4%) | 0.56 |
| Late gadolinium enhancement volume | 8.9 (4.6) | 4.4 (1.1) | 0.22 |
All data are mean (SD) unless otherwise indicated
Figure 3.
Receiver operator curve comparison of anthracycline dose, CMR-derived LVEF and CMR-derived indexed LV mass. Analysis of anthracycline dose revealed an area under the curve (AUC) of 0.68 (95% CI, 0.58-0.78) (A), analysis of GFR revealed an AUC of 0.58 (95% CI, 0.47-0.68) (B), analysis of LV EF revealed an AUC of 0.57 (95% CI, 0.46-0.68) (C0, and analysis of CMR-derived indexed LV mass revealed an AUC of 0.90 (95% CI, 0.82-0.95) (D).
Figure 4.
Receiver operator curve analysis revealed that a CMR-derived LVMi of < 57 grams/m2 had a sensitivity of 100% and a specificity of 85% for prediction of adverse cardiac events. Kaplan Meier curves displaying event-free probability according to an LVMi of ≥ or < 57 grams/m2.
Discussion
The present study demonstrated several findings in a cohort of patients with an anthracycline-cardiomyopathy. The first of the findings of this study were that despite a reduced EF, myocardial scar by LGE was an infrequent finding. Second, cardiac edema using T2 imaging was an uncommon finding. Third, there was an inverse correlation between anthracycline dose and LV mass. Fourth, the cardiovascular event rate in follow-up of patients with an anthracycline-cardiomyopathy was high. Fifth, CMR-derived LV mass helped further stratify risk in patients with an anthracycline cardiomyopathy and provided further prognostic information in addition to that provided by standard variables.
Limited clinical CMR data exists in patients previously treated with anthracyclines.9 However, the findings of an inverse relationship between anthracycline dose and CMR-derived LV mass are complementary and additive to work using other imaging modalities.2 Lipshultz et al performed serial echocardiography on children receiving anthracyclines. They found that LV mass was normal immediately after anthracycline therapy but decreased over time and became significant reduced approximately 6 to 9 years after diagnosis.13 The decrease in LV mass was seen in all dose ranges but was greater in children receiving a higher dose. We add complementary data in an adult population and show a dose-dependent decrease in CMR-derived indexed LV mass. Cardiac magnetic resonance is the gold standard non-invasive test for measurement of LV mass. The reproducibility of CMR-derived LV mass compares favourably with other imaging modalities with an error for measurement of LV mass using echocardiography of 8-14%,14 while using CMR the error is 6%.15
Cardiac magnetic resonance with LGE is the imaging method of choice to detect myocardial scar. However, the focal myocardial enhancement seen with LGE in conditions such as anthracycline-cardiomyopathy is far less prominent than would be suggested by pathological examination.16-18 The limitation of current CMR methods for detection of diffuse myocardial fibrosis is the lack of a normal reference myocardium as LGE relies on focal contrast enhancement relative to remote normal areas of the same heart. In ischemic cardiomyopathy, LGE is found in close to 100%, in patients with non-ischemic cardiomyopathy, LGE is found in about 35%,19 and we found LGE in about 6% of patients with an anthracycline-cardiomyopathy. However, pathologically, myocardial fibrosis is generally ubiquitous and present throughout the entire ventricle.17,18 This suggests that LGE underestimates both the presence and the extent of myocardial fibrosis in anthracycline-cardiomyopathy. Alternative CMR methods are currently in research to address these limitations. These include mapping of T1 relaxation times,20,21 and measuring the signal intensity of gadolinium uptake in the myocardium.9,22
Modification of the renin-angiotensin-aldosterone system and the sympathetic nervous system improves outcomes in broad groups of populations with class B and C heart failure.23 However, data on modification of these pathways after treatment with anthracyclines suggest that identification of a high-risk subgroup would be of value.24,25 Repeated non-invasive estimates of EF, are usually within the normal range, 24despite clear pathological evidence of myocyte damage.26 This suggests that cardiotoxicity occurs at much lower doses than appreciated by a decline in LVEF.26 Several serum biomarkers have also been tested with promising results,27 however, clinical applicability is limited by the rigorous sampling protocol required and there are some conflicting data.27 As a result, there is need for a robust biomarker to predict adverse clinical events in patients receiving chemotherapy. While our data shows that measurement of LV mass provides an additive biomarker to sub-stratify patients with established an anthracycline-cardiomyopathy, what is needed is a biomarker for early detection of cardiotoxicity prior to the development of LV dysfunction.
This study has to be interpreted within the context of the design format. Patients were being referred for a clinically-indicated CMR study for work-up of a cardiomyopathy. Not all patients with a reduced EF undergo a CMR study within our institution and this referral is most strongly related to provider preference. Reduced LV mass by CMR was able to define a population at higher risk of events, however, clinical management was not altered on the basis of the result, thus it is unclear, as yet, the utility of identification of a higher risk group by LV mass late after cardiotoxicity. Echocardiographic LV mass was derived using the cube method derived from 2D measurements; the area-length method from 2D echocardiography or 3D echocardiography may have provided additive data. Furthermore, diastolic data or novel echocardiographic indices were not measured and similarly may have provided additive insight.28
Despite a reduced EF, LGE was an uncommon finding in patients with an anthracycline-cardiomyopathy. The event rate in patients with an established anthracycline-cardiomyopathy is high, and LV mass using CMR imaging may help further stratify patients with an established anthracycline-cardiomyopathy. Further work is needed to determine whether, like with echocardiography, that LV mass using CMR is reduced prior the development of reduced EF and whether intervention based on CMR-derived LV mass is beneficial.
Acknowledgments
Funding: This work was supported in part by an American Heart Association Fellow to Faculty Grant (12FTF12060588, TGN), a National Institute of Health T32 Training Grant (T32HL09430101A1, TGN), a National Institutes of Health Career Development Grant (K08HL097031-02)0, JM), and project grants (R01HL090634-01A1, MJH; R01HL091157, RYK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The Authors report that they have no relationships relevant to the contents of this manuscript to disclose.
References
- 1.Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, Skinner R, Stevens MC, Hawkins MM. Long-term cause-specific mortality among survivors of childhood cancer. Jama. 2010;304:172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 3.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. Jama. 1991;266:1672–1677. [PubMed] [Google Scholar]
- 4.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 5.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 6.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 7.Bruder O, Schneider S, Nothnagel D, Pilz G, Lombardi M, Sinha A, Wagner A, Dill T, Frank H, van Rossum A, Schwitter J, Nagel E, Senges J, Sabin G, Sechtem U, Mahrholdt H. Acute adverse reactions to gadolinium-based contrast agents in CMR: multicenter experience with 17,767 patients from the EuroCMR Registry. JACC Cardiovasc Imaging. 2011;4:1171–1176. doi: 10.1016/j.jcmg.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 8.O’Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Wassmuth R, Lentzsch S, Erdbruegger U, Schulz-Menger J, Doerken B, Dietz R, Friedrich MG. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J. 2001;141:1007–1013. doi: 10.1067/mhj.2001.115436. [DOI] [PubMed] [Google Scholar]
- 10.Kwong RY, Sattar H, Wu H, Vorobiof G, Gandla V, Steel K, Siu S, Brown KA. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118:1011–1020. doi: 10.1161/CIRCULATIONAHA.107.727826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Jerosch-Herold M, Jacobs DR, Jr., Shahar E, Folsom AR. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:565–572. doi: 10.1016/j.jacc.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Missouris CG, Forbat SM, Singer DR, Markandu ND, Underwood R, MacGregor GA. Echocardiography overestimates left ventricular mass: a comparative study with magnetic resonance imaging in patients with hypertension. J Hypertens. 1996;14:1005–1010. [PubMed] [Google Scholar]
- 16.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 17.Bernaba BN, Chan JB, Lai CK, Fishbein MC. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc Pathol. 2010;19:308–311. doi: 10.1016/j.carpath.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Unverferth DV, Fetters JK, Unverferth BJ, Leier CV, Magorien RD, Arn AR, Baker PB. Human myocardial histologic characteristics in congestive heart failure. Circulation. 1983;68:1194–1200. doi: 10.1161/01.cir.68.6.1194. [DOI] [PubMed] [Google Scholar]
- 19.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 20.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 22.Lightfoot JC, D’Agostino RB, Jr., Hamilton CA, Jordan J, Torti FM, Kock ND, Jordan J, Workman S, Hundley WG. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3:550–558. doi: 10.1161/CIRCIMAGING.109.918540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 24.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, Grenier D, Jassal DS. The Utility of Cardiac Biomarkers, Tissue Velocity and Strain Imaging, and Cardiac Magnetic Resonance Imaging in Predicting Early Left Ventricular Dysfunction in Patients With Human Epidermal Growth Factor Receptor II-Positive Breast Cancer Treated With Adjuvant Trastuzumab Therapy. J Am Coll Cardiol. 2011;57:2263–2270. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 25.Georgakopoulos P, Roussou P, Matsakas E, Karavidas A, Anagnostopoulos N, Marinakis T, Galanopoulos A, Georgiakodis F, Zimeras S, Kyriakidis M, Ahimastos A. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol. 2010;85:894–896. doi: 10.1002/ajh.21840. [DOI] [PubMed] [Google Scholar]
- 26.Ewer MS, Ali MK, Mackay B, Wallace S, Valdivieso M, Legha SS, Benjamin RS, Haynie TP. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J Clin Oncol. 1984;2:112–117. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 27.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 28.Neilan TG, Jassal DS, Perez-Sanz TM, Raher MJ, Pradhan AD, Buys ES, Ichinose F, Bayne DB, Halpern EF, Weyman AE, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur Heart J. 2006;27:1868–1875. doi: 10.1093/eurheartj/ehl013. [DOI] [PubMed] [Google Scholar]