Abstract
Transplantation of one or two umbilical cord blood products is a useful alternative stem cell source. However, the limited number of stem cells in the infusion results in slow engraftment. In mouse models, administration of parathyroid hormone is an effective way to enhance the ability of limited numbers of hematopoietic stem cells to support hematopoiesis. In this study, patients received either a myeloablative or a reduced intensity double umbilical cord blood transplantation followed by parathyroid hormone at 100 μg daily for 28 days. Thirteen patients (median age 42 years) were enrolled. All patients engrafted; the median time to neutrophil and platelet engraftment >20x109 cells/L were 30 and 61 days respectively. The incidence of Grades II–IV acute GVHD was 38.5% at day 100. There were four deaths prior to Day 100, prompting early study closure. No patients receiving a myeloablative regimen relapsed. Overall survival at 6 months after transplantation was 62% and disease-free survival at 2 years was 39%. At the dose and schedule studied, there was no evidence that PTH influenced blood count recovery.
Keywords: Transplantation, Umbilical Cord Blood, Parathyroid Hormone
Introduction
The hematopoietic stem cell niche is critically important in the maintenance of hematopoiesis and osteolineage cells are an intrinsic component of the niche.1 In mouse models, the Scadden laboratory has shown that activation and stimulation of osteoblastic cells with parathyroid hormone (PTH) or the PTH-related protein (PTH-rP), through the PTH/PTHrP receptor (PPR), influences hematopoietic stem cell numbers and function to the extent that hematopoietic function can be restored when limiting numbers of stem cells are infused.2–4 This takes place at least in part through the Notch signaling pathway via the Notch ligand Jagged 1.5 Activation of the PPR on osteoblastic cells increases expression of the Notch ligand. 5
Based on promising data in the mouse models, we performed a Phase I study of PTH in patients with lymphoma or myeloma who had failed chemotherapy and/or growth factor mobilization.6 PTH was well tolerated up to doses of 100 μg daily and there was no dose limiting toxicity (defined as serum calcium level >11.5 mg/dl, an ionized calcium level > 1.5 mM, systolic blood pressure < 80 mm Hg, or phosphate < 1.0 mg/dl). Forty-seven percent of patients who had failed one prior stem cell mobilization had a successful mobilization (peripheral blood with > 5 CD34+ cells/μL) and 40% of patients who had failed two prior stem mobilizations had a successful stem cell mobilization.
Umbilical cord blood transplantation (UCBT) is a setting in which limiting numbers of hematopoietic stem cells are often used. Even with the use of two UCB units, engraftment and immune reconstitution can be slow, contributing to a high risk of infection and second malignancies.7–10 Various ex vivo expansion strategies have been undertaken to improve these results, but an improvement in survival has never been documented.11–13
Since PTH administration was able to facilitate hematopoietic reconstitution when limiting doses of hematopoietic stem cells were administered to mice, we hypothesized that a similar effect might be observed after UCBT. Thus, based on data demonstrating safety with PTH administration in our prior autologous stem cell transplantation trial, we performed a Phase II study of PTH following double UCB transplantation as an approach to enhance hematopoietic recovery in a clinical setting of low stem cell numbers. Although we could not reproduce the PTH dosing used in the murine experiments, we selected a dose of 100 μg daily, which was well tolerated in our Phase I study of mobilization, and could be given once daily as five subcutaneous injections. 14–15
PATIENTS AND METHODS
Patients
Patients aged 18 to 65 years with acute leukemia in first complete remission with high risk cytogenetics or second or subsequent remission, relapsed chemotherapy sensitive Hodgkin or non Hodgkin lymphoma, myelodysplasia, aplastic anemia, myelofibrosis, chronic myelogeneous leukemia in accelerated or second stable phase, advanced chronic lymphocytic leukemia, or multiple myeloma were eligible for this study. Patients with serum calcium >10.5 mg/dl or a phosphate level <1.6 mg/dl were excluded. Other transplantation eligibility criteria included ECOG performance status of 0, 1, or 2, creatinine < 2.0, bilirubin < 2.0, ejection fraction >50%, and DLCO ≥50% predicted. All subjects signed an informed consent form for this clinical trial approved by the Institutional Review Boards of both Dana Farber/Harvard Cancer Center and the University of Florida.
Treatment Plan
Patients under age 50 were eligible to receive a myeloablative conditioning (MAC) regimen of cyclophosphamide 1800 mg/m2/day on days –5 and –4 (total dose 3600 mg/m2), fludarabine 25 mg/m2/day on days –6, -5, -4 (total dose 75 mg/m2) and total body radiation 1400 cGy in 7 divided fractions (days –3, -2, -1, 0). The study was later amended to include a reduced intensity conditioning(RIC) regimen for patients up to age 65 which consisted of fludarabine 30mg/m2/day on days –8 to -3 (total dose 180mg/m2), melphalan 100 mg/m2 on day –2, and rabbit antithymocyte globulin (ATG) 1.5 mg/kg/day on days –7-5,-3,-1 (total dose 6.0 mg/kg) as described previously.9,10 All subjects received commercial human PTH 1–34 (teriparatide) 100 μg given as 5 subcutaneous injections once daily (each dosing pen contains 20 μg per injection) for Days 1–28 of the study or until neutrophil engraftment (absolute neutrophil count (ANC) > 0.5×109 cells/L). Patients received filgrastim from day + 5 after UCB transplantation until neutrophil engraftment. Graft vs host disease (GVHD) prophylaxis was tacrolimus (starting dose 0.05 mg/kg orally or intravenously), adjusted to maintain serum trough level of 5–10 ng/ml and mycophenolate mofetil (MMF) at a dose of 15 mg/kg intravenously starting on day –3 prior to transplantation. In the absence of GVHD, MMF was tapered after day +60 with the goal of discontinuation by day +100 after transplantation and tacrolimus was tapered after day +100 with the goal of discontinuation by 6–9 months after transplantation.
Umbilical Cord Blood Units
UCB units were obtained from national and international UCB banks. All patients received two UCB units. UCB units were required to be a human leukocyte antigen (HLA) 4/6 A, B, DR match with the patient and with each other and achieve a minimal cell dose of >3.7×107 nucleated cells (NC)/kg combined pre-cryopreservation. Typing for HLA-A and -B was performed at molecular intermediate resolution and for HLA-DR at the allele level. Each individual UCB unit cell dose was at least > 1.5×107 NC/kg pre-cryopreservation. On Day 0, UCB were thawed according to the methods of Rubinstein and sequentially infused within 2–5 hours of each other. 16 The UCB unit with the higher total nucleated cells /kg pre-cryopreservation was infused first.
Definitions of Toxicity and Response
Calcium levels, phosphate level, ionized calcium, and albumin were monitored thrice weekly to determine any additional toxicity from the parathyroid hormone. Neutrophil engraftment was defined as the first of 3 consecutive days with neutrophil recovery to at least 0.5×109 cells/L. Platelet engraftment was defined as the first day of an unsupported platelet count >20×109 cells/L (no platelet transfusion within 7 days). Early graft failure was defined as the absence of neutrophil engraftment by day + 42 after transplantation. Late graft failure was defined as the presence of neutrophil engraftment by day +42, followed by decline of neutrophil count to less than 0.5×109 cells/L after day +42 and loss of donor chimerism. GVHD was graded according to the consensus criteria.17 Donor chimerism was determined from peripheral blood at weeks 2, 4, 8 and 12 and at months 6, 12, and 24. Chimerism assays were performed by short tandem repeat analysis.9
Statistics
The primary objective was to evaluate the time to neutrophil engraftment among patients receiving parathyroid hormone following double unrelated UCBT. The target sample size of 40 had power to allow us to observe a 30% reduction in the median time to neutrophil engraftment using a historical median time of 23 days in our institution, with 80% and one-sided significance level of 10%. The incidence of neutrophil, platelet engraftment, acute GVHD, chronic GVHD and relapse were calculated using the cumulative incidence function, treating death as the competing risk.18 The probability of overall survival (OS) was calculated using the Kaplan-Meier estimator.19 Death from any cause after transplantation was considered the event with patients censored at the last date of follow-up. Analyses were performed using the SAS System (Cary, NC) version 9.2 and R version 2.8.1.
Immune Reconstitution Studies
T cell receptor excision circles (TREC) analysis was performed according to previously described protocol. 20 DNA was isolated from PBMCs using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA). Quantitation of signal-joint TCR excision circle (sjTREC) DNA was performed by Taqman real-time PCR (RT-PCR) using an AB 7000 qPCR machine from Applied Biosystems. Quantitation of the sjTREC copy number for each patient sample was performed as described previously using a standard curve prepared with 10-fold dilutions of a plasmid containing the sjTREC sequence (kindly provided by Dr. Daniel Douek, NIAID).21
RESULTS
Patient Characteristics
Patient and UCB characteristics are outlined in Table 1. Thirteen patients were enrolled before the trial was halted due to early deaths, thought unrelated to PTH. The median age was 42 years (range, 25–67). Nine patients received the MAC regimen and four patients received the RIC regimen. A majority of patients (77%) were a 4/6 HLA A, B, DR match with both UCB units. The median infused (post thaw) TNC/kg dose for the combined units was 4.2×107/kg (range 3.4–6.6). The median infused (post thaw) CD34+ cell dose/kg for the combined units was 2.1×105/kg(range 0.4–9.0).
Table 1.
Baseline Patient and Unit Characteristics
| 13 Patients Transplanted | |
|---|---|
| Race: | |
| White | 10 (76.9%) |
| Asian | 1 (7.7%) |
| Other | 2 (15.4%) |
| Ethnicity: Hispanic | 4 (30.8%) |
| Age at Transplantation (years): Median (Range) | 39 (25–67) |
| Primary Disease | |
| MDS | 2(15.4%) |
| Aplastic Anemia | 2(15.4%) |
| Hodgkin’s Disease | 1(7.7%) |
| Chronic lymphocytic leukemia | 1(7.7%) |
| Acute myeloid leukemia | 5(38.5%) |
| Acute lymphoblastic leukemia | 2(15.4%) |
| ECOG Performance Status at Transplantation | |
| 0- | 4(30.8%) |
| 1- | 8(61.5%) |
| 2- | 1(7.7%) |
| HLA Match: Recipient to Unit 1 and Unit 2 | |
| 4/6, 4/6 | 10 (76.9%) |
| 4/6, 5/6 | 1 (7.7%) |
| 5/6, 5/6 | 2 (15.4%) |
| HLA Match: Unit 1 to Unit 2 | |
| 4/6 | 10(76.9%) |
| 5/6 | 3(23.1%) |
| Combined Post-thaw Cell Doses | |
| TNC dose×107/kg | 4.2 (3.4–6.6) |
| CD34 dose×105/kg | 2.1(0.4–9.0) |
| Conditioning Regimen | |
| Myeloablative | 9 (69.2%) |
| Reduced Intensity | 4 (30.8%) |
Notes: N and (%) or median (minimum-maximum) are displayed. ECOG=Eastern Cooperative Oncology Group, TNC=Total nucleated cell
Toxicity
The trial was halted due to excess early mortality after four patients expired prior to day 100, triggering review by the Data Safety Monitoring Board. Three stopping rules were in place. The first stopping rule, which required closure for graft failure, was not reached. All patients who survived engrafted by Day 42. The second rule, also not reached, called for early study closure if 3 or more patients developed Grades III–IV GVHD; there were 2 patients with Grade III–IV GVHD. The third stopping rule mandated a review after four patients died of transplant-related mortality (TRM) within the first 100 days after transplantation. The causes of death were polymicrobial sepsis (day +24), hepatic venoocclusive disease (day +35), RSV and aspergillus pneumonia (day +25) and polymicrobial sepsis, liver and respiratory failure (day +59). Autopsy in this last case revealed pneumonia, venoocclusive disease of the liver, and GVHD. Three of the four early deaths were among patients who had received the MAC regimen. Importantly, none of the deaths were thought likely due to PTH, but given the early stopping rule and after discussion with the Data Safety Monitoring Board, the study was closed to accrual after 13 patients were treated. Two additional patients were consented but had not started treatment and did not receive PTH. Additional toxicities included four patients with a syndrome of fever, rash and diarrhea within the first two weeks after transplantation, thought consistent with an immune reaction, as described by other centers. 22 One patient was treated with topical steroids; three of the four patients had complete resolution of symptoms and one patient had progression of rash to acute GVHD. No hypercalcemia or other toxicities felt to be related to PTH were reported.
Engraftment of Neutrophils and Platelets
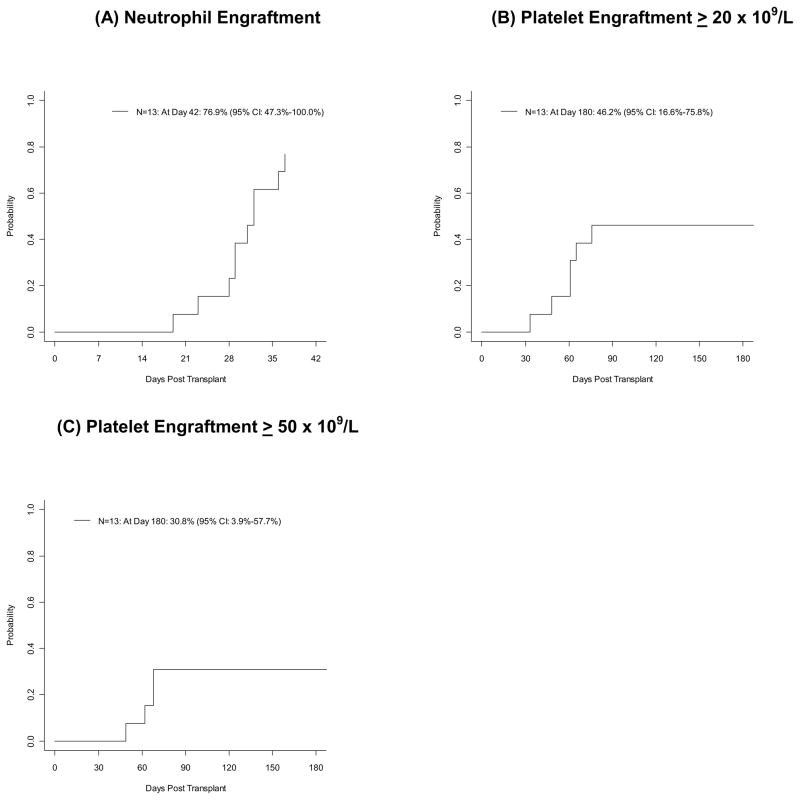
Patient outcomes are illustrated in Table 2. Engraftment of neutrophils to at least 0.5×109 cells/L occurred at a median of 30 days after UCBT, with a range of 19 to 37 days. The incidence at day 42 was 76.9% (95% CI, 47.3%–100.0%), as shown in Figure 1. There were no cases of graft failure among the patients who survived to Day +42. The three patients who died prior to day +42 had not yet engrafted neutrophils. Analysis of donor/host engraftment in the peripheral blood showed the first unit infused to be the predominant dominating unit in 50% of patients by day 14 and 67% of patients at day 100. Engraftment to platelet count >20×109 cells/L occurred at a median of 61 days with a range of 33 to 76 days and an incidence at day 180 of 46.2% (95% CI, 17%–76%). Engraftment to platelet count >50×109 cells/L occurred among 4 patients at days 49, 62, 68, and 68 with a cumulative incidence at day 180 of 30.8% (95% CI, 4%–58%).
Table 2.
Patient Outcomes
| Patient ID | Conditoning Regimen | Days to ANC>500 | Days to Platelet>20,000 | Days to Platelet>50,000 | Maximum Acute GVHD Grade by Day 100 | Chronic GVHD(grade) | Days to Relapse | Days to Death | Primary Cause of Death | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 000001 | MA | 24 | Polymicrobial sepsis | Died 24 days post- transplant | ||||||
| 000002 | MA | 19 | 48 | 62 | II | Yes (Limited) | Alive 720 days post- transplant | |||
| 000003 | MA | 37 | 76 | Alive 727 days post- transplant | ||||||
| 000004 | MA | 29 | II | Yes (Limited) | 355 | Pneumonia JC Virus |
Died 355 days post- transplant | |||
| 000005 | MA | 23 | 65 | IV | 243 | Pulmonary Hemorrhage, Interstitial pneumonia-Acute bronchopneumonia | Died 243 days post- transplant | |||
| 000006 | MA | I | 35 | Veno Occlusive Disease | Died 35 days post- transplant | |||||
| 000007 | RIC | 32 | 214 | 246 | Recurrence Disease | Died 246 days post- transplant | ||||
| 000008 | RIC | 25 | RSV Pneumonia Aspergillus Pneumonia |
Died 25 days post-transplan | ||||||
| 000009 | MA | 32 | III | 59 | Polymicrobial sepsis Liver Failure Respiratory Failure |
Died 59 days post- transplant | ||||
| 000010 | RIC | 29 | 101 | 133 | Recurrence Disease | Died 133 days post- transplant | ||||
| 000011 | MA | 31 | 61 | 68 | I | Yes (Limited) | Alive 733 days post- transplant | |||
| 000012 | RIC | 28 | 33 | 49 | Yes (Limited) | 751 | Alive 751 days post- transplant | |||
| 000014 | MA | 36 | 61 | 68 | II | Yes (Limited) | Alive 768 days post- transplant |
Notes: ANC=Absolute neutrophil count, GVHD=Graft-versus-host disease, MA=Myeloablative, RIC=Reduced Intensity
Figure 1.
Neutrophil and Platelet Engraftment.
Graft versus Host Disease and Relapse
The incidence of Grades II–IV and Grades III–IV acute GVHD was 38.5% (95% CI, 10.3%–66.7%) at day 100 and 15.4% (95% CI, 0.0%–36.2%) at day 100 respectively. Three patients had Grade II acute GVHD and 2 patients had Grades III–IV acute GVHD. Five patients developed chronic GVHD, all limited, with an incidence of 38.5% (95% CI, 8.1%–68.9%) at 2-years. Three patients (all of whom received RIC conditioning) relapsed at days 101, 214, and 751. The incidence of relapse at 2-years was 15.4% (95% CI, 0.0%–36.2%). There were no relapses in the patients who received a MAC regimen.
Overall and Disease-Free Survival
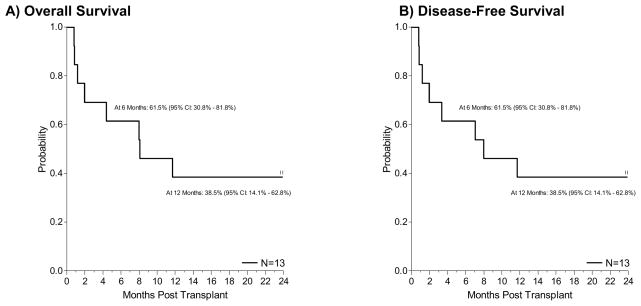
With a median follow up among the survivors of 733 days (range, 720–768), the overall survival probability at 6 months and 2 years was 61.5% (95% CI: 30.8–81.8) and 38.5% (95% CI: 14.1–62.8), respectively (Figure 2A). Disease free survival probability at 6 months and 2 years was 61.5% (95% CI: 30.8–81.8) and 38.5% (95% CI: 14.1–62.8), respectively (Figure 2B). Eight patients have died; four patients died prior to Day +100 as described above. Four patients died after Day +100, two of relapsed disease, one of diffuse alveolar hemorrhage and one of central nervous system infection with JC virus. Disease-free survival probability at 2-years was 25.0% (95% CI: 0.9–66.5) for the RIC patients and 44.4% (95% CI: 13.6–71.9) for the MAC patients.
Figure 2.
Overall Survival and Disease-Free Survival.
Immune Reconstitution
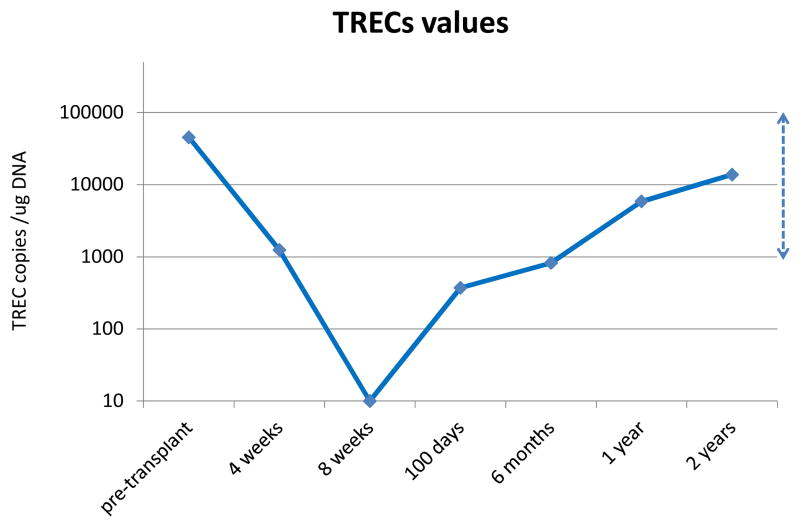
Immune reconstitution studies were performed in seven patients. To examine immune reconstitution, we evaluated recovery of thymic function by assessing T cell receptor excision circles. TRECs are useful for evaluating the de novo production of T cells originating from newly engrafted stem cells. Prior to transplantation, median TREC values were within normal range for this age group, at 52,475 copies/μg. After transplantation, median TREC values fell substantially but were below the limit of detection only at 8 weeks after transplantation (Figure 3). Average TREC values reached the lower limit of normal range at six months (819 copies/μg), returning to the normal range around 1 year after transplantation. This return of TREC levels is in contrast to prior work from our center in double UCBT patients receiving the same RIC regimen but without PTH which demonstrated TREC levels of 0 at 100 days and 74.9 copies/μg at 6 months.21 The rapid kinetics of TREC reconstitution in the PTH patients was not observed in our prior studies. CD4 counts at Day 100 were also higher in the PTH treated patients than in our historical control population, 142 cells/ul vs 86 cells/ul.
Figure 3.
T cell receptor excision circles (TREC) levels. TREC counts per microgram PBMC DNA were determined by quantitative PCR. The line connects the average values of the individual time points. The limit of detection of the TREC assay was 10 copies / ug DNA. The dashed line to the far right shows range of values for 10 normal controls of similar ages.
DISCUSSION
We undertook this study because delayed engraftment and poor immune reconstitution remain significant causes of mortality after double UCB transplantation in adults. The optimal strategy to enhance the kinetics of myeloid recovery and immune reconstitution remain unclear; however, preclinical data suggested that PTH might enhance hematopoietic recovery in the setting of limited stem cell dose by stimulating an in vivo increase in the number of HSC as a result of increased levels of osteoblastic expression of the Notch ligand Jagged 1 and thereby activation of Notch1 signalling.2 The Seattle group has successfully used immobilized Notch ligands for the ex vivo expansion of UCB progenitor cells to enhance neutrophil recovery following a MAC regimen. 12 In our study, an early stopping rule was met based on early TRM and accrual was stopped early. No clear pattern of toxicity was apparent, as the patients died of common transplantation complications, and there was no evidence that PTH contributed to the early TRM. The decision to halt the study was also supported by no indication of positive PTH effect on engraftment in the first 13 patients.
Studies in mouse models contribute to the understanding of the importance of the osteolineage cells in the regulatory stem cell niche; increasing osteoblastic cell activity by PPR stimulation affects hematopoietic stem cell number and function.23 Jung and colleagues demonstrated that PTH increases bone marrow stromal derived factor-1 (SDF-1) expression, possibly leading to increased homing of stem cells.24 However, in this limited sample size, the neutrophil engraftment did not appear to be enhanced, and in fact, may even have been delayed, compared to our historical control of 23 days. This lack of effect may have been secondary to a lack of biologic activity of the PTH, the small sample size, or inability to administer equivalent doses to humans that were effective in mice.25 Conversion of the dose and type of hormone used in the mouse to the human is difficult due to different sensitivity of mice to PTH. Mice and rats can tolerate bigger doses of PTH than humans can, and these high peptide doses produce in mice and rats dose-dependent improvements in bone mass and bone strength which are greater than the benefits produced by exogeneous or endogeneous PTH in humans.26,27 We chose a dose of 100 μg daily based on safety data from a Phase I study on mobilization as well as a desire to limit the number of injections to 5 daily injections (each dosing pen contains 20 μg per injection).6 However, the study was hampered by no prior Phase I data for PTH dose escalation after double UCB transplantation. The FDA approved dose of PTH is 20 μg daily; the large osteoporosis studies in men and postmenopausal women have used doses of 20–40 μg daily of PTH 1–34.28–30 Side effects in these studies have included elevated calcium level, headaches, joint pain, muscle aches, and fatigue. The dose for the current study of 100 μg might be too low to obtain the effect seen in mice with respect to enhanced engraftment, and it is unclear that the appropriate dose (and delivery) was identified in the setting of an UCB transplantation. In addition, the kinetics of the effect of PTH may be different in the mouse, and a meaningful change in the microenvironment may only be achieved after a longer interval of exposure to PTH.
Immune reconstitution remains a major limitation of UCB transplantation. T-cell receptor rearrangement excision circle (TREC) analysis was performed to evaluate the de novo production of T cells originating from newly engrafted cells. We compared the results in this study with TREC analysis performed in our historical control double UCB patients treated with fludarabine, melphalan, and ATG without PTH. Previous work from our group in double UCBT patients treated without PTH indicated median TREC levels of 0 at 100 days post UCBT and 117 copies/μg at 6 months after UCBT. Patients who achieved TREC levels of 2000 or more copies/μg DNA had improved survival. 21 Although the number of patients in our study is small and included patients treated with both MAC and RIC regimens, more rapid TREC reconstitution was observed compared to our historical controls: an average of 365 copies/μg DNA at 100 days in the PTH group compared to 0 in the historical control group, and 819 copies/μg in the PTH group compared to 74.9 copies/μg in the group without PTH at 6 months. These results are encouraging as higher TREC levels have been correlated with lower TRM and improved survival. However, in our study, there were a number of opportunistic infections including JC virus, respiratory syncytial virus (RSV), and aspergillus. There were two patients with CMV infection and one patient with EBV infection post transplant, which is similar to our historical control population. 9,10 CD4 counts at Day 100 were higher in the PTH treated patients than in our historical control population. 9 The small sample size of this study might have prevented appreciation of any clinical benefit of improved TREC levels or higher CD4 counts.
There are concerns about administration of PTH to humans with malignancies because of an increased incidence of osteosarcoma in Fischer rats receiving high doses of PTH. Intermittent administration of PTH may induce chromosome and DNA breaks in osteoblasts, but has never been associated with human cancer.30 In this study, with limited follow-up, there was no evidence of secondary malignancies. In this group of heavily pretreated patients, there were only three relapses, all of which occurred in patients who received a reduced intensity conditioning regimen with ATG, and it is possible that the use of ATG may have contributed to the relapses after RIC transplantation.31 There were no relapses in the patients treated with the MAC regimen. This low incidence of relapse in our small cohort is encouraging, but the number of patients is too small to definitively establish the safety of PTH therapy after transplantation. In addition, there may be a competing risk with early TRM since some of the patients receiving a MAC regimen died within the first 100 days, and did not live long enough to relapse. Further studies to address the potential effect of PTH on the leukemia stem cell will involve patients with acute myeloid leukemia and chronic myeloid leukemia undergoing standard, non transplantation treatment. While we were unable to observe an improvement in hematopoiesis in this trial, it is possible that different approaches to stimulation of osteoblastic cells will be more effective, and may warrant further investigation.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (U54 HL081030) and the Jock and Bunny Adams Research and Education Endowment.
The authors acknowledge the contributions of Dr. Gregor Adams, Dr. Henry Kronenberg, Dr. Robert Neer, and Dr. David Scadden for their helpful discussions and comments.
Footnotes
Financial Disclosure Statement: The authors have no financial conflicts to disclose.
ClinicalTrials.gov number: NCT00393380
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang J, Niu C, Huang H, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 2.Calvi LM, Adams GB, Welbrecht KW, et al. Osteoblastic cells regulate the hematopoietic stem cell niche. Nature. 2003;425:841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 3.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87:518–24. [PubMed] [Google Scholar]
- 4.Taichman RS, Emerson SJ. Human osteoblasts support the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–82. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karanu FN, Murdoch B, Gallacher C, et al. The notch ligand Jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192:1365–72. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballen KK, Shpall EJ, Avigan D, et al. Phase I trial of parathyroid hormone to facilitate stem cell mobilization. Biol Blood Marrow Transplant. 2007;13:838–843. doi: 10.1016/j.bbmt.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Ballen KK, Cutler C, Yeap BY, et al. Donor Derived Second Hematologic Malignancies after Cord Blood Transplantation. Biol Blood Marrow Transplant. 2010;16:1025–31. doi: 10.1016/j.bbmt.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahu X, Rialand F, Touzeau C, et al. Infectious complications after unrelated umbilical cord blood transplantation in adult patients with hematologic malignancies. Biol Blood Marrow Transplant. 2009;15:1531–37. doi: 10.1016/j.bbmt.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–9. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutler C, Stevenson K, Kim HT, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46:659–67. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–78. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–36. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler CS, Shoemaker D, Ballen KK, et al. FT1050 (16, 16 dimethylprostaglandin E2–enhanced umbilical cord blood) accelerates engraftment after reduced intensity conditioning and double umbilical cord blood transplantation. Blood. 2011 Nov 18;118(21):653a (abstract). [Google Scholar]
- 14.Barker JN, Weisdorf D, Defor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–7. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 15.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–22. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–28. [PubMed] [Google Scholar]
- 18.Gooley T, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Statist Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan E, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998 Dec 17;396 (6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 21.Brown JA, Stevenson K, Kim HT, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood. 2010;115(20):4111–4119. doi: 10.1182/blood-2009-09-244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumura T, Narimatsu H, Kami M, et al. Cytomegalovirus infections following umbilical cord blood transplantation using reduced intensity conditioning regimens for adult patients. Biol Blood Marrow Transplant. 2007;13 (5):577–83. doi: 10.1016/j.bbmt.2006.12.454. [DOI] [PubMed] [Google Scholar]
- 23.Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche. Nature Biotechnology. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 24.Jung Y, Wang J, Schneider A, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Calvi LM, Bromberg O, Rhee Y, et al. Osteoblastic expansion induced by parathyroid hormone receptor signaling in murine osteocytes is not sufficient to increase hematopoietic stem cells. Blood. 2012;119 (11):2489–2499. doi: 10.1182/blood-2011-06-360933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu EW, Neer RM, Lee H, et al. Time-dependent changes in skeletal responses to teriparatide: escalating vs constant dose teriparatide (PTH 1–34) in osteoporotic women. Bone. 2011 Apr 1;48(4):713–9. doi: 10.1016/j.bone.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitlak BH, Burdette-Miller P, Schoenfeld D, Neer RM. Sequential effects of chronic human PT (1–84) treatment of estrogen-deficiency osteopenia in the rat. J Bone Miner Res. 1996;11(4):430–9. doi: 10.1002/jbmr.5650110403. [DOI] [PubMed] [Google Scholar]
- 28.Finkelstein JS, Hayes A, Hunzelman L, et al. The effect of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 29.Black DM, Greensplan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination with postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 30.Alves De Oliveira EC, Szeinfeld VL, Pereira da Silva N, et al. Intermittent PTH 1–34 causes DNA and chromosomal breaks in osteoblasts and nonosteoblastic cells. Calc Tissue Int. 2010;87:424–36. doi: 10.1007/s00223-010-9396-6. [DOI] [PubMed] [Google Scholar]
- 31.Soiffer RJ, LeRademacher J, Ho V, et al. Impact of immune modulation with anti-T cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]