Abstract
Leptin is an adipocyte-derived hormone that relays a satiety signal to the brain. The effect of leptin on the sympathetic nervous system is an important aspect in the regulation of energy homeostasis as well as several other physiological functions. The arcuate nucleus of the hypothalamus is considered a major site for the regulation of physiological processes by leptin. However, there is growing recognition that other hypothalamic and extra-hypothalamic brain nuclei are important for leptin regulation of physiological processes including sympathetic nerve traffic. The current review discusses the various hypothalamic and extra-hypothalamic nuclei that have been implicated in leptin-induced increase in regional sympathetic nerve activity. The continuous rise in the prevalence of obesity underscores the importance of understanding the underlying neural mechanisms regulating sympathetic traffic to different tissues to design effective strategies to reverse obesity and associated diseases.
Keywords: Leptin, Brain nuclei, Sympathetic nerve activity, Energy metabolism, Cardiovascular regulation
Introduction
Energy balance is a highly regulated phenomenon, and the importance of the autonomic nervous system in the regulation of energy homeostasis is well established. The brain receives afferent signals about the status of body energy reserves leading to autonomic, humoral and behavioral adaptations aimed at maintaining body weight homeostasis [1, 2]. The cloning of the ob gene and the identification of its encoded protein, leptin, defined a molecular signal linking the amount of body's energy reserves to the brain [3]. In addition, the characterization of leptin's sites of action in the brain has allowed tremendous progress in defining the brain circuits that regulate energy homeostasis and other physiological functions.
Leptin is a signal from adipose tissue that acts in the brain to complete the feedback loop that regulates appetite and energy expenditure. Leptin is secreted by adipocytes and circulates in the blood at concentrations proportional to fat mass content. Leptin action on its receptor present in the central nervous system inhibits food intake and increases energy expenditure through stimulation of sympathetic nerve activity (SNA). Leptin-triggered increase in regional sympathetic traffic promote thermogenesis in brown adipose tissue (BAT), lipolysis in white adipose tissue and increase metabolic activities in liver and skeletal muscle resulting in an increase in energy expenditure [4–6] (Fig. 1). In addition, the sympathoexcitation triggered by leptin promotes BAT-like remodeling of perigonadal white adipose tissue [7, 8].
Fig. 1.
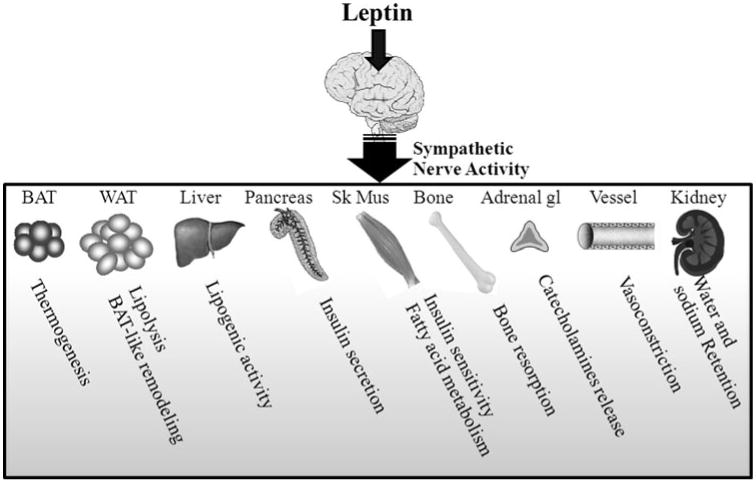
Several physiological processes are influenced by leptin through activation of the sympathetic nervous system. Leptin action in the brain triggers an increase in sympathetic nerve outflow to a variety of tissues involved in several physiological functions ranging from energy expenditure and metabolism to cardiovascular control. BAT brown adipose tissue, WAT white adipose tissue, Ske Mus skeletal muscle, Adrenal gl adrenal gland
Consistent with its pleiotropic effects, leptin increases sympathetic drive to tissues that are involved in the regulation of physiological functions other than energy homeostasis [9]. For instance, the centrally-mediated sympathoexcitatory effects of leptin appear to exert an influence on peripheral bone physiology [10]. Leptin also increase sympathetic drive to organs involved primarily in cardiovascular regulation such as the kidney with potential impact on energy metabolism. Leptin also induces arterial pressure elevation with important pathophysiological implications in obesity [11]. Indeed, recent work has highlighted a key role of leptin and hyperleptinemia in the adverse cardiovascular consequences of excessive adiposity. This is based on the findings that several animal models of obesity that have elevated arterial pressure exhibit selective leptin resistance, in that they have normal renal sympathetic and arterial pressure responses to leptin in spite of diminished beneficial anorectic and weight-reducing actions of leptin [11–14]. In support of the animal studies implicating hyperleptinemia in obesity-associated sympathetic overdrive are the findings that obese humans have elevated renal SNA [16] and the existence of a strong association between circulating leptin levels and renal sympathetic tone in human subjects of varying adiposity [17].
The mechanisms underlying the selectivity in leptin resistance are not well understood, but could relate to the involvement of different neuronal populations in mediating the metabolic and cardiovascular effects of leptin. Alternatively, this could be due to the divergent intracellular signaling pathways engaged by the leptin receptor (ObRb) to differentially regulate the various physiological processes. This is supported by data using genetically modified mouse models with disruption of the various signaling pathways of ObRb [15]. Therefore, selectivity in leptin resistance may be due to the inability of ObRb to activate downstream signaling pathways mediating the beneficial effects on food intake and body weight (e.g. STAT3), but preservation of ObRb's ability to stimulate the intracellular pathways (e.g. PI3 kinase) that mediate the cardiovascular effects of leptin.
Thus, through its action on the sympathetic nervous system leptin is able to influence many physiological processes beyond energy homeostasis and body weight regulation (Fig. 1). In the past few years, significant advances have been made in defining the central neural pathways involved in leptin actions on the cardiovascular function and regional sympathetic nerve traffic. Various neuroanatomical sites have emerged as mediators of the cardiovascular and sympathetic effects induced by leptin.
Hypothalamic arcuate nucleus
Shortly after the discovery of leptin and its receptor, the hypothalamus was identified as a key site of leptin action. Indeed, the long signaling form of ObRb was found to be enriched in various hypothalamic nuclei. Within the hypothalamus, the arcuate nucleus (ARC) has emerged as a major site for the regulation of physiological processes by leptin (Fig. 2). This was based on the fact that the ARC contains the highest concentration of ObRb and is the most responsive brain nucleus to leptin in terms of activation of intracellular signaling pathways associated with ObRb [18, 19]. The relevance of leptin signaling in the ARC was further supported by lesioning studies, which demonstrated a lack of feeding response to leptin after the ARC had been destroyed. However, restoring the expression of ObRb in the ARC of leptin receptor-deficient Koletsky rats or in mice that have a leptin receptor null allele caused a modest decrease in food intake and body weight, but normalized the hyperglycemia [20–23]. Similarly, reactivation or deletion of ObRb in specific neuronal populations of the ARC had a modest effect on food intake and body weight, but a profound impact on glucose homeostasis [20, 24–26]. Together, these findings indicate that ARC neurons may be more important for mediating the effects of leptin on glucose balance than on energy homeostasis.
Fig. 2.
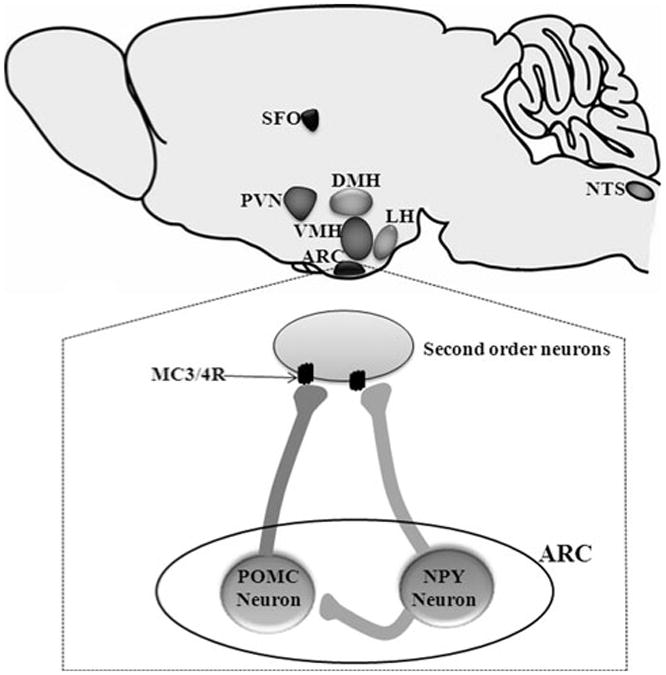
Schematic illustration of the various brain nuclei in which leptin receptor signaling has been shown to elicit an increase in sympathetic nerve activity. The insert shows the two neuronal populations within the arcuate nucleus and their projections to the second order neurons. In POMC neurons, leptin increases neuronal firing and POMC gene expression promoting the secretion of alpha-melanocyte stimulating hormone (α-MSH), an agonist of the melanocortin 3 and 4 receptors (MC3/4R) located in the second order neurons. Conversely, in NPY neurons, leptin inhibit neuronal firing rate and decrease the expression and secretion of AgRP (antagonist of the MC3/4R), promoting MC3/4R activation
Accumulating evidence also point to the importance of the ARC in mediating the sympathetic effects of leptin. Leptin injection into the ARC increases sympathetic traffic subserving BAT, hindlimb and kidney [27, 28]. In addition, the neurons of the ARC play a major role in mediating leptin-induced sympathetic activation to white adipose tissue, which promotes a BAT-like remodeling [7, 8]. Moreover, lesioning the ARC resulted in a blunted BAT SNA response to intravenous leptin [29]. Additional evidence pointing to the ARC as a critical nucleus in mediating leptin-induced increases in sympathetic traffic derives from the observation that specific deletion of the leptin receptor in this nucleus is sufficient to abolish leptin-induced renal and BAT sympathetic activation [30]. Interestingly, deletion of leptin receptor expression in the ARC also eliminated the elevated blood pressure associated with diet-induced obesity in mice [30]. Thus, leptin action in the ARC is critical in mediating the regional sympathetic nerve activation and cardiovascular effects of leptin.
Although the ARC contains several neuronal populations, two classes of neurons have been well characterized in terms of leptin action: proopiomelanocortin (POMC) neurons (which also express cocaine- and amphetamine-related transcripts (CART) that are activated by leptin); and neuropeptide Y (NPY) neurons (which also express agouti-related protein (AgRP) that are inhibited by leptin) [2, 27]. There is strong evidence that many of leptin's actions on the sympathetic traffic are mediated by stimulation of the melanocortin system (Fig. 2). The melanocortins are peptides that are processed from the polypeptide precursor, POMC. The POMC neurons are known to express the leptin receptor and leptin binding leads to stimulation of neuronal firing activity and increase the gene expression of POMC and CART genes. This results in higher secretion of alpha-melanocyte stimulating hormone (α-MSH), which in turn binds to a number of receptors of the melanocortin family located in second order neurons. Five melanocortin receptors (MC1R to MC5R) have been identified. The MC3R and MC4R are highly expressed in the central nervous system [31, 32]. The importance of the MC4R in energy balance was demonstrated by target disruption of the MC4R gene that induces hyperphagia and obesity in mice [33]. In addition, the involvement of the brain melanocortin receptors in regulating the sympathetic tone and arterial pressure was demonstrated in rodents and humans [34–38].
Mice lacking the leptin receptor only in POMC-expressing neurons had blunted arterial pressure response to leptin [39]. In addition, despite being overweight and exhibiting several metabolic alterations, which normally leads to arterial pressure increase, mice lacking the leptin receptor in POMC neurons remains normotensive [39]. Consistent with these findings, a critical role for MC4R in mediating leptin-induced increase in renal SNA as well as arterial pressure was demonstrated using pharmacological and genetic approaches [34–36]. Moreover, the MC3/4R appears essential in transducing the preserved renal SNA response to leptin in obesity [40]. Finally, the demonstration that blockade of the MC3/4R in the hypothalamic paraventricular nucleus abolished the renal sympathoexcitatory effect of leptin, support the idea that leptin activates the sympathetic nervous system by stimulating the release of α-MSH in the vicinity of the paraventricular nucleus neurons [41]. Thus, experimental data implicate the POMC-MC4R axis in mediating the cardiovascular and renal sympathetic effects of leptin.
Other hypothalamic nuclei
In addition to the ARC, other hypothalamic regions have been implicated in the sympathetic and cardiovascular effects of leptin. The ventromedial hypothalamus (VMH) has high concentrations of ObRb, albeit not as high as the ARC [18]. In addition, exogenous leptin treatment induces c-Fos protein expression and activates leptin receptor signaling in the VMH, indicating that leptin indeed acts in this nucleus [42, 43]. Furthermore, mice carrying a deletion of ObRb in SF1 neurons of the VMH exhibited higher body weight and adiposity associated with hyperinsulinemia, dyslipidemia and hepatic steatosis [44, 45]. These findings demonstrate the importance of the SF1 neurons of the VMH in mediating the metabolic effects of leptin.
Using circulating catecholamines as a read out of sympathetic activity, Satoh et al. [46] found that microinjection of leptin into the VMH increased plasma catecholamines. Later studies using direct SNA recording demonstrated that leptin injection into the VMH caused renal and lumbar sympathetic nerve activation [28, 47, 48]. The role of the VMH in the cardiovascular effects of leptin has also been investigated, with contradicting results. Tanida et al. [48] demonstrated that direct injection of leptin into the VMH did not affect arterial pressure. On the other hand, studies by two other groups demonstrated that leptin injection into the VMH increased heart rate and arterial pressure [28, 47]. The reasons for such discrepancy in the cardiovascular effects caused by leptin into the VMH are not clear, but may be due to methodological differences between the various studies.
The dorsomedial hypothalamus (DMH) is another hypothalamic nucleus that has been involved as a site of leptin action on sympathetic nerve traffic and cardiovascular function. Activation of neurons of the DMH with bicuculline (a GABA receptor antagonist) evoked an increase in arterial pressure, heart rate and renal sympathetic drive implicating the DMH in the control of sympathetic tone and cardiovascular regulation [49]. Interestingly, leptin injection into the DMH elicited a significant increase in arterial pressure and heart rate without significantly altering renal SNA [47]. However, other studies have implicated the ObRb expressing neurons of the DMH in the regulation of sympathetic drive to BAT. For instance, during acute cold exposure c-Fos expression is induced in ObRb expressing neurons of the DMH [50]. In addition, leptin injection into the DMH raised inter-scapular BAT temperature, which is thought to be sympathetically-mediated [51]. Finally, retrograde labeling studies identifying infected leptin receptor expressing neurons in the DMH following the viral inoculation of BAT provide a neuroanatomical basis for the control of BAT SNA by leptin action in this nucleus [50]. However, direct evidence that leptin action in the DMH causes BAT sympathetic activation is lacking.
Based on lesioning and electrical stimulation studies, the lateral hypothalamus (LH) has long been known as a “feeding center” [2]. This has raised the possibility that the LH may be a major site of leptin action. Consistent with such notion, the leptin receptor was found to be expressed in a subset of LH neurons, and leptin microinjection into this region decreased food intake and caused weight loss in rats [52]. In addition, deletion of ObRb in the neurotensin expressing neurons of the LH caused obesity in mice [53]. In the LH, leptin appears to act on a discrete set of neurons, presumably those expressing neurotensin, which in turn can modulate the function of neighboring melanin-concentrating hormone and orexin-containing neurons via an integrated system [52]. The connectivity of the LH with the autonomic and sympathetic centers has prompted the investigation of a potential involvement of this nucleus in the sympathetic and cardiovascular effects of leptin. Microinjection of leptin into the LH evoked lumbar sympathetic activation with no increase in arterial pressure [28]. The potential effects of leptin action in the LH on the sympathetic activity to beds other than the hindlimb deserve further investigation.
The presence of leptin receptors in the hypothalamic paraventricular nucleus (PVN), which provide major descending pathways to parasympathetic and sympathetic preganglionic neurons raised the possibility that leptin action in this nucleus may be critical for sympathetic and cardiovascular regulation. However, silencing the leptin receptor signaling in the PVN using antisense oligonucleotide against ObRb mRNA did not alter arterial pressure, heart rate or sympathetic vasomotor tone [46], which argues against a meaningful role for the leptin action in the PVN in the tonic regulation of sympathetic and cardiovascular functions. The sympathetic and cardiovascular responses elicited by direct leptin microinjection into the PVN were also examined, though with contrasting results. Indeed, direct injection of leptin into the PVN was found to cause either no change in arterial pressure or renal SNA [47], to increase lumbar SNA but not arterial pressure [28], or to increase arterial pressure as well as sympathetic vasomotor tone [54]. Therefore, the relevance of PVN leptin receptors for sympathetic and cardiovascular regulation remains unclear.
Extra-hypothalamic regions
Several brain nuclei outside the hypothalamus have been implicated in mediating the sympathetic and cardiovascular effects of leptin. In the brainstem, the nucleus tractus solitarii (NTS) expresses ObRb, and exogenous peripheral leptin administration increased c-Fos immunoreactivity and activated leptin receptor signaling (e.g. Stat3 phosphorylation) in this nucleus [55]. In addition, leptin injection into the NTS led to a significant decrease in food intake and body weight validating leptin action in this nucleus [56].
Given the well-known importance of the NTS as key regulator of the sympathetic tone and cardiovascular system, a potential role for this nucleus was considered [55]. Strikingly, leptin injection into the commissural and medial subnuclei of the NTS at the level of the area postrema evoked a significant increase in renal SNA and arterial pressure with no significant change in BAT SNA [55]. The responses induced by leptin injection into the NTS were dependent on functional leptin receptor, as no increase in renal SNA or arterial pressure was detected in Zucker rats that carry a mutation in the leptin receptor [55]. Of note, leptin injection into the rostral NTS failed to elicit any sympathetic or cardiovascular responses, which is consistent with data demonstrating that leptin receptor signaling within the NTS is restricted to the caudal region of this nucleus [55, 57, 58].
The subfornical organ (SFO) is another extra-hypothalamic nucleus, which was recently implicated in the regulation of arterial pressure by leptin. Leptin action in the SFO has been shown to activate the same neurons as amylin suggesting a possible role for leptin action in this nucleus in the regulation of food intake and energy expenditure [59]. In addition, direct injection of leptin into the SFO of rats caused a significant decrease in arterial pressure as compared to control injections [59]. Based on these findings the authors argue that the role of leptin receptor signaling in the SFO is to maintain normal arterial pressure by counteracting the pro-hypertensive effects induced by leptin action in other brain nuclei. Therefore, the blunted depressor response to leptin microinjection into the SFO in the obese animals was suggested to promote the development of hypertension [59]. Whether the autonomic nervous system is involved in the hypotensive effects of leptin action in the SFO and whether the effects induced by leptin receptor signaling in the SFO are restricted to arterial pressure remains to be determined.
Concluding remarks
The sympathetic nervous system is an important regulatory mechanism of several physiological functions including energy balance and cardiovascular homeostasis [60]. The ability of leptin to modulate sympathetic nerve traffic subserving various tissues impacting several physiological processes offer a plausible explanation for the importance of this hormone in coordinating homeostasis. Several brain nuclei have been implicated in the regional sympathetic nerve responses induced by leptin. Such observations combined with other evidences are consistent with the notion that leptin action in the brain involves a distributed neuronal network. However, given the multiple brain nuclei that can mediate leptin-induced activation of the sympathetic nerve traffic it is unclear why deleting the leptin receptor from one nucleus (e.g., the ARC) abolishes the regional sympathetic responses evoked by leptin [24]. Also, the conclusions derived from studies that rely mainly on brain site-specific microinjection of leptin need to be modulated given that such approach does not mimic the normal way by which leptin gets into those nuclei. Indeed, it is well documented that circulating leptin crosses the blood–brain barrier through a specific and saturable transport system [9]. Thus, despite the recent advances our understanding of the central nervous system circuits involved in the regulation of the sympathetic nervous system activity by leptin remain far from complete. Defining the neuronal circuits underlying the regional sympathetic nerve responses induced by leptin will be essential for the development of safe and effective therapies for the treatment of obesity and associated diseases.
Acknowledgments
The authors' work is supported by grants from NIH (HL084207 and HL014388) and American Diabetes Association (1-11-BS-127). SMH is supported by Postdoctoral Fellowship Award from The American Heart Association (12POST9410009).
Footnotes
Conflict of interest: None.
Contributor Information
Shannon. M. Harlan, Department of Pharmacology, University of Iowa Carver College of Medicine, 3135C MERF, Iowa City, IA 52242, USA
Kamal Rahmouni, Email: kamal-rahmouni@uiowa.edu, Department of Pharmacology, University of Iowa Carver College of Medicine, 3135C MERF, Iowa City, IA 52242, USA; Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, IA, USA.
References
- 1.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 2.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 4.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 5.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschop MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117(11):3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warne JP, Alemi F, Reed AS, Varonin JM, Chan H, Piper ML, Mullin ME, Myers MG, Jr, Corvera CU, Xu AW. Impairment of central leptin-mediated PI3 K signaling manifested as hepatic steatosis independent of hyperphagia and obesity. Cell Metab. 2011;14(6):791–803. doi: 10.1016/j.cmet.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Bruning JC. Enhanced leptin-stimulated Pi3 k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6(6):431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12(1):78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahmouni K, Haynes WG, Mark AL. Cardiovascular and sympathetic effects of leptin. Curr Hypertens Rep. 2002;4(2):119–125. doi: 10.1007/s11906-002-0036-z. [DOI] [PubMed] [Google Scholar]
- 10.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 11.Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118(4):1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51(2):439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 13.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55(4):862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 14.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54(7):2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 15.Harlan SM, Morgan DA, Dellsperger DJ, Myers MG, Jr, Mark AL, Rahmouni K. Cardiovascular and sympathetic effects of disrupting tyrosine 985 of the leptin receptor. Hypertension. 2011;57(3):627–632. doi: 10.1161/HYPERTENSIONAHA.110.166538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96(10):3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 17.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41(5):1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 18.Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC, Palmiter RD, Schwartz MW. Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes. 1998;47(4):538–543. doi: 10.2337/diabetes.47.4.538. [DOI] [PubMed] [Google Scholar]
- 19.Myers MG., Jr Outstanding Scientific Achievement Award Lecture 2010: deconstructing leptin: from signals to circuits. Diabetes. 2010;59(11):2708–2714. doi: 10.2337/db10-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1(1):63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K, Yoshimasa Y, Nishi S, Hosoda K, Nakao K. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci Lett. 1997;224(3):149–152. doi: 10.1016/S0304-3940(97)00163-8. [DOI] [PubMed] [Google Scholar]
- 22.Morton GJ, Niswender KD, Rhodes CJ, Myers MG, Jr, Blevins JE, Baskin DG, Schwartz MW. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fa(k)/fa(k)) rats. E Endocrinology. 2003;144(5):2016–2024. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- 23.Dawson R, Pelleymounter MA, Millard WJ, Liu S, Eppler B. Attenuation of leptin-mediated effects by monosodium glutamate-induced arcuate nucleus damage. Am J Physiol. 1997;273(1Pt1):E202–6. doi: 10.1152/ajpendo.1997.273.1.E202. [DOI] [PubMed] [Google Scholar]
- 24.Berglund ED, Vianna CR, Donato J, Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122(3):1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjorbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9(6):537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2(6):411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49(3):647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 28.Montanaro MS, Allen AM, Oldfield BJ. Structural and functional evidence supporting a role for leptin in central neural pathways influencing blood pressure in rats. Exp Physiol. 2005;90(5):689–696. doi: 10.1113/expphysiol.2005.030775. [DOI] [PubMed] [Google Scholar]
- 29.Haynes W, Morgan D, Walsh S. Sympathetic activation to leptin is mediated by the hypothalamus. J Hypertens. 1998;16:S11. [Google Scholar]
- 30.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the Leptin Receptor in the Hypothalamic Arcuate Nucleus Abrogates Leptin-Induced Sympathetic Activation. Circ Res. 2011;108(7):808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24(1):155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 32.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci U S A. 1993;90(19):8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46(2):326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 34.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23(14):5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo JJ, da Silva AA, Tallam LS, Hall JE. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension. 2004;43(2):370–375. doi: 10.1161/01.HYP.0000111836.54204.93. [DOI] [PubMed] [Google Scholar]
- 36.Kuo JJ, Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension. 2003;41(3Pt2):768–774. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- 37.Sayk F, Heutling D, Dodt C, Iwen KA, Wellhoner JP, Scherag S, Hinney A, Hebebrand J, Lehnert H. Sympathetic function in human carriers of melanocortin-4 receptor gene mutations. J Clin Endocrinol Metab. 2010;95(4):1998–2002. doi: 10.1210/jc.2009-2297. [DOI] [PubMed] [Google Scholar]
- 38.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360(1):44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 39.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57(5):918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1730–R1736. doi: 10.1152/ajpregu.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang ZH, Felder RB. Melanocortin receptors mediate the excitatory effects of blood-borne murine leptin on hypothalamic paraventricular neurons in rat. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R303–R310. doi: 10.1152/ajpregu.00504.2003. [DOI] [PubMed] [Google Scholar]
- 42.Hubschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W. Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci. 2001;21(7):2413–2424. doi: 10.1523/JNEUROSCI.21-07-02413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138(2):839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 44.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149(5):2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 46.Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y, Nakao K. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48(9):1787–1793. doi: 10.2337/diabetes.48.9.1787. [DOI] [PubMed] [Google Scholar]
- 47.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42(4):488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 48.Tanida M, Nagai K, Kaneko H. Activation of the renal sympathetic nerve by leptin microinjection into the ventromedial hypothalamus in rats. In Vivo. 2003;17(3):213–217. [PubMed] [Google Scholar]
- 49.Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol. 2001;280(6):H2891–H2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31(5):1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enriori PJ, Sinnayah P, Simonds SE, Garcia RC, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31(34):12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leinninger GM. Lateral thinking about leptin: a review of leptin action via the lateral hypothalamus. Physiol Behav. 2011;104(4):572–581. doi: 10.1016/j.physbeh.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG., Jr Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14(3):313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shih CD, Au LC, Chan JY. Differential role of leptin receptors at the hypothalamic paraventricular nucleus in tonic regulation of food intake and cardiovascular functions. J Biomed Sci. 2003;10(4):367–378. doi: 10.1159/000071156. [DOI] [PubMed] [Google Scholar]
- 55.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53(2):375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143(1):239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 57.Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology. 2006;147(7):3190–3195. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- 58.Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55(3):567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- 59.Smith PM, Ferguson AV. Cardiovascular actions of leptin in the subfornical organ are abolished by diet induced obesity. J Neuroendocrinol. 2011;24(3):504–510. doi: 10.1111/j.1365-2826.2011.02257.x. [DOI] [PubMed] [Google Scholar]
- 60.Dulloo AG. Biomedicine. A sympathetic defense against obesity. Science. 2002;297(5582):780–781. doi: 10.1126/science.1074923. [DOI] [PubMed] [Google Scholar]


