Abstract
The stop-signal task probes agents’ ability to inhibit responding. A well-known race model affords estimation of the duration of the inhibition process. This powerful approach has yielded numerous insights into the neural circuitry underlying response control, the specificity of inhibition across effectors and response strategies, and executive processes such as performance monitoring. Translational research between human and non-human primates has been particularly useful in this venture. Continued progress with the stop-signal paradigm is contingent upon appreciating the dynamics of entire cortical and subcortical neural circuits and obtaining neurophysiological data from each node in the circuit. Progress can also be anticipated on extensions of the race model to account for selective stopping; we expect this will entail embedding behavioral inhibition in the broader context of executive control.
Introduction
Response control has been the subject of fruitful investigation using the stop-signal (or countermanding) task (reviewed by [1,2]). Subjects must respond quickly when targets appear but must cancel partially prepared movements when infrequent stop signals occur (Fig. 1). This task provides crucial leverage to investigate response control, because performance can be understood as a race between 2 processes that initiate (GO process) or cancel (STOP process) movement [3]. Using this race model, the duration of the covert STOP process can be derived from the proportion of successful stop trials and the distribution of reaction times (RT) on trials without stop signals. This stop-signal reaction time (SSRT) measures the time needed to cancel movements. This paradigm is very general, applying to simple and choice response tasks accomplished with any effector system.
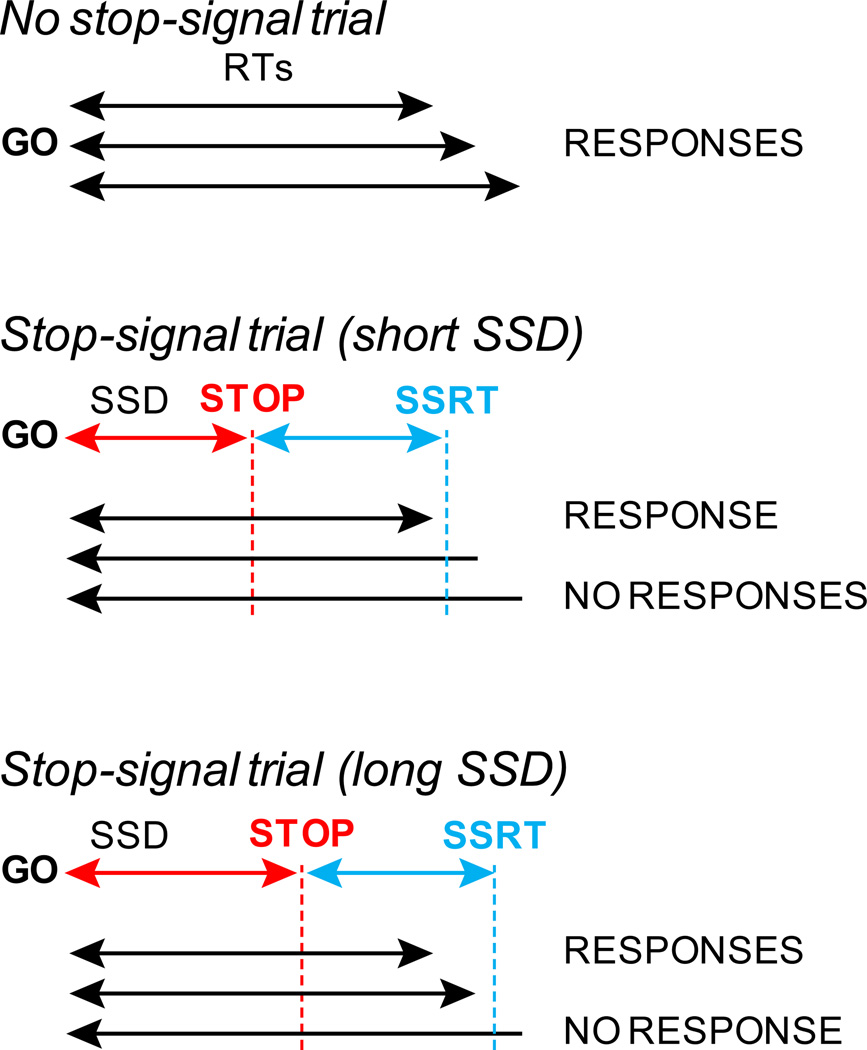
Figure 1.
The stop-signal task measures response inhibition. Two trial types are randomly interleaved. "No stop signal", "No signal, or simply "Go" trials do not contain stop signals (top). A GO signal instructs subjects to initiate a response. The times between GO signals and responses are the subject's reaction times (RTs). "Stop signal", "Signal", or "Stop" trials are randomly interleaved (middle and bottom). With some delay after the GO signal (stop-signal delay or SSD), a stop signal is presented. This cues subjects to cancel impending responses, which they are able to do with varying success. Short SSDs increase the probability of cancelation (middle) while long SSDs decrease the probability of cancelation (bottom). If responses are elicited in spite of the stop signal, trials are classified as "Signal respond", "Noncanceled", or "Stop failure" trials. If no response is elicited, trials are classified as "Signal inhibit", "Canceled", or "Stop success" trials. Stop-signal reaction time (SSRT) measures the time necessary for the covert inhibitory process to cancel responses. RTs faster than SSD plus SSRT will result in Signal respond trials, while RTs slower than the stop process will lead to Signal inhibit trials.
The stop-signal task enables powerful, translational research because it can be studied with humans, monkeys, and even rats, employing behavioral [4–6], neurochemical [7], neurophysiological [8,9], electrophysiological [10,11], magnetic and electrical stimulation [12–14], and functional imaging [15–18] techniques. Moreover, the task provides traction to understand the nature of clinical disorders of impulse control and response monitoring [19–22]. From such a large and growing literature, we can focus on only a few salient developments and emerging problems with emphasis on neurophysiological findings.
Neural mechanism of response inhibition
The clearest mechanistic explanations of response inhibition are framed by neurophysiological studies in the frontal eye field (FEF) and superior colliculus (SC) of macaque monkeys performing an eye movement stopping task [23–25]. Two criteria must be met for neurons to participate in controlling movement initiation. First, neurons must discharge differently when movements are initiated or withheld; if neurons still discharge when movements are canceled, their activity was not affected by the stop process. Second, the differential modulation on canceled trials must occur before SSRT; otherwise, the neural modulation happens after the movement has already been canceled. Neurons that initiate or inhibit eye movements in FEF and SC modulate early enough to control movements directly (Figure 2A). These neurons project to brainstem structures that house ocular motor neurons, enabling them to influence response production directly [26,27]. After the target appears, movement-related activity begins to grow toward a threshold that triggers response initiation [28]. If the activity reaches threshold, a response is produced regardless of whether a stop-signal was presented. However, responses are canceled when the movement-related activity is inhibited so that it does not reach the threshold activation level. The source of this inhibition is a signal such as that conveyed by fixation neurons in FEF and SC. Crucially, the pronounced modulation of fixation- and movement-related activity precedes SSRT.
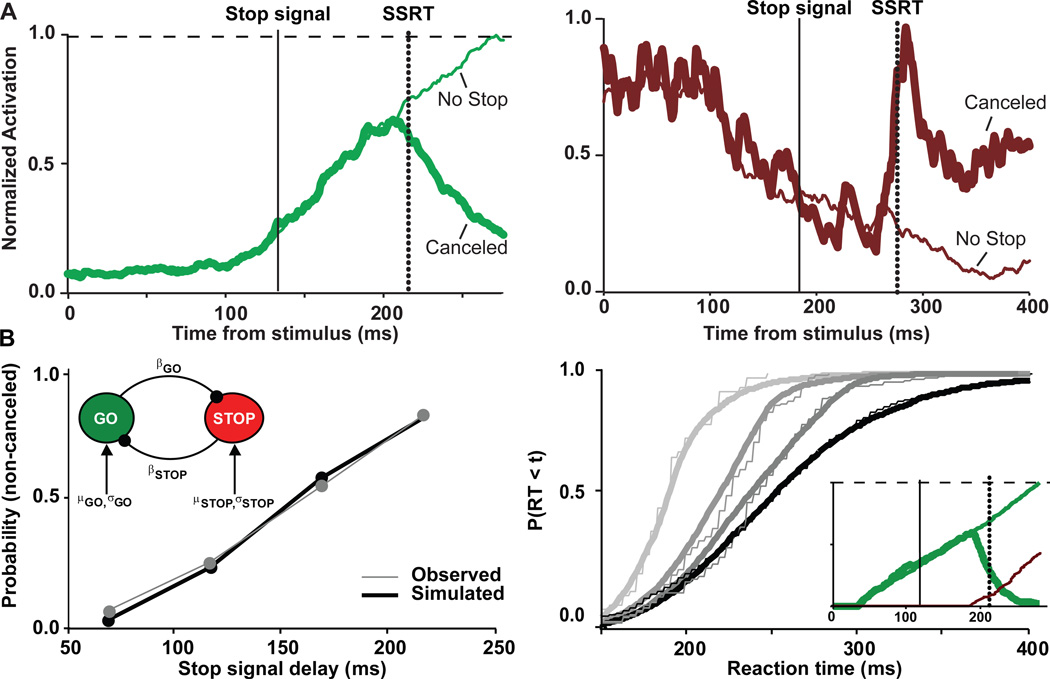
Figure 2.
Neural and computational mechanisms of movement inhibition. (A) Normalized activity of FEF gaze-shifting (left) and gaze-holding (right) neurons. Activity on trials in which movements were produced but would have been canceled if the stop signal had been presented (thin line) are compared with activity on trials when the planned saccade was canceled because the stop signal appeared (thick line). Presentation of the stop signal is indicated by the solid vertical line. The time needed to cancel the planned movement - stop signal reaction time (SSRT) - is indicated by the dashed vertical line. When the movement was canceled, gaze-holding activity increased and gaze-shifting activity decreased abruptly immediately before SSRT. The timing of this modulation demonstrates that FEF neurons convey signals sufficient to control the initiation of the movement. (B) The interactive race model elucidates how a network of mutually inhibitory GO and STOP units (left inset) can produce behavior consistent with the Logan race model. With proper parameters, the network produces error rates (left) and RT distributions (right) that are indistinguishable from observed values. Moreover, using the same parameters, GO and STOP unit modulation (right inset) correspond quantitatively to the form of actual neural activation. Movement inhibition can be accomplished only by late, potent interruption of the GO process by the STOP process. (Adapted from [37])
Single-neuron recordings resolve patterns of modulation within neural microcircuits that are invisible to noninvasive methods such as fMRI and event related potential (ERP). For example, neurons with purely visual responses in FEF do not satisfy the criteria necessary to participate in controlling movements [24], and visuomovement neurons exhibit a pattern of modulation distinct from that of movement neurons [29]. Because noninvasive techniques are unable to resolve such heterogeneous signals in the stop-signal task, associated claims framing mechanisms in terms of one gross anatomical structure influencing another must be interpreted cautiously (e.g., [30]).
The straightforward criteria laid forth by the race model identifies neurons that produce signals sufficient to control movement initiation. The results from FEF have been replicated and extended in a task requiring changing instead of just stopping saccades [31]. Meanwhile, neurons in other cortical areas, such as the supplementary eye field (SEF) and lateral intraparietal area (LIP), have also been described as movement-related. However, tests with the stop-signal paradigm have produced unambiguous results; vanishingly few neurons modulate before SSRT in SEF [9] or LIP [32]. Thus, SEF and LIP do not satisfy the logical criteria necessary to contribute directly to movement control. Similarly, neurons in supplementary motor area (SMA) do not modulate early enough to control limb movements [33]. Results from preSMA are mixed. One laboratory has recorded from preSMA [34] and subthalamic nucleus (STN) [35] during a "saccade overriding" and a go/no-go task. The latter task resembles a stop-signal task with SSD = 0 but lacks a measure like SSRT. These researchers identified some neurons in both structures with increased activity on successful no-go trials, similar to those modulating on canceled trials in SEF [9,36] and SMA [33]. However, whereas activity in SEF and SMA was too late to contribute to response inhibition in the stop signal task, the modulation of neurons in preSMA and STN did occur within the transition between error and correct response times. Another laboratory has described neural activity in preSMA during the stop-signal task. Similar to findings in SMA, the majority of these neurons modulate after SSRT [33]. Further research is needed to resolve this question in preSMA, and data must be recorded from STN while monkeys perform the stop-signal task to test for modulation before SSRT.
It is tempting to hypothesize that movement and fixation neurons instantiate the race model. But the central assumption that finish times of the GO and STOP processes must be independent complicates this interpretation [3]. If circuits that instantiate the race model consist of interacting neurons, how can they produce behavior that appears to result from independent processes? This paradox has been resolved through a model network of interacting GO and STOP units with randomly accumulating activation ([37] see also [38]). The model fits performance data and replicates neural data if and only if the STOP unit inhibits the GO unit in a delayed and potent fashion (Figure 2B). Thus, a neurally plausible mechanism of interaction is the only way that the model naturally fits behavior. This interactive race model has since been extended to a network of biophysically realistic spiking neurons [39]. Others have modeled behavioral inhibition in Bayesian terms [40], but the utility of this approach for elucidating neural mechanisms is uncertain. Only the race model has afforded precise description at multiple levels, both neural and behavioral, during the stop-signal task. This rare coordination between psychophysics, formal mathematical modeling, and neurophysiology establishes a clear linking proposition between the GO and STOP processes of the race model and gaze-shifting and gaze-holding neurons in the ocular motor circuit. We are curious to know if this interactive race framework will extend gracefully to explain manual stopping, and we expect that this framework will be necessary to understand the diversity of selective stopping findings across stimulus conditions and effectors.
Meanwhile, a series of clinical and MRI studies have drawn attention to a specific region of ventral prefrontal cortex as a central focus of response inhibition in conjunction with preSMA and the STN. The evidence began with a correlational study relating frontal lesion location and size to SSRT [41]. Further work showed haemodynamic activation in right inferior frontal cortex (rIFC) and STN during response inhibition [42]. Based on findings that deep brain stimulation (DBS) of STN in Parkinson’s disease (PD) patients improved stopping performance [43], and evidence from diffusion tensor tract tracing for a direct connection between IFC and STN [44], a hypothesis was advanced that rIFC inhibits responses directly and immediately through the hyperdirect pathway by increasing β synchronization. β synchronization has been reported to decrease in primary motor cortex (M1) and STN locally during manual response initiation in patients [11,45,46]. Another study reported that DBS of STN in PD patients improved response inhibition and was associated with increased β power in surface EEG [10].
Confidence that rIFC is the seat of inhibition must be tempered, however, because key aspects of the data are uncertain and alternate hypotheses explain more observations.
The evidence for potent anatomical connectivity between rIFC and STN is uncertain. In monkeys, prefrontal inputs to STN arise from FEF and dorsal medial frontal areas but not from ventral PFC [47]. Moreover, the prefrontal inputs terminate in a ventral sector of STN which does not overlap with the M1 and SMA terminals [47,48]. In humans, a recent comprehensive survey of STN connectivity shows that fibers connecting IFC to STN are vanishingly weak in comparison to those connecting STN to caudate, putamen, globus pallidus, and thalamus [49]. Although some have hypothesized that a common inhibitory mechanism operates in humans and rats [50], comparison across species must be made with caution because STN connectivity differs between rodents and primates [51].
Numerous studies identify rIFC with functions other than response inhibition [52]. In particular during the stop-signal task, activation of rIFC may be caused by cognitive functions such as attentional capture [16,53] (although these effects are difficult to parse from increased RTs in these studies) or violations of event expectations [17]. Outside of the stop-signal task, IFC is consistently implicated in stimulus driven attentional capture [54,55] and this activation scales with the degree of stimulus unexpectedness [56,57]. In general, rIFC is often implicated as part of a network involved in orienting attention toward salient stimuli (reviewed by [58]). While the specific homologue of IFC has not been identified in nonhuman primates, it is probably located ventral to the principal sulcus and rostral to or in the arcuate sulcus. This region has not been explored extensively neurophysiologically, but an fMRI study in macaques identifies its activation with cognitive set shifting [59]. Thus, plausible alternative hypotheses about the role of IFC abound.
Confidence in the claim that β oscillations serve to synchronize rIFC and STN to interrupt movement preparation must also be tempered. One study reporting increased intracranial β power over rIFC when responses were canceled was clear in just one of four patients. In monkeys, increased β power has been observed in cortical areas in which no neurons modulate early enough to contribute directly to response inhibition, and such elevation is dissociated from increased γ power that is most commonly associated with spike rate modulation [60]. Another study reporting that DBS of STN in PD patients improved response inhibition with increased β power in surface EEG [10] has an inconsistent behavioral effect [61]. In fact, several studies have noted that DBS of STN reduces or reverses response inhibition in PD patients under conditions of decision conflict [62,63]. And others have recorded increased impulsivity measures in PD patients undergoing DBS ([64] but see [65] for a possible reconciliation). Causal links between β power, STN, and behavioral inhibition are therefore far from established.
Other cortical areas and basal ganglia pathways are known to contribute to response inhibition [18,66]. Of these, the SMA and preSMA have received considerable attention (e.g. [15]). But recent work suggests that medial frontal areas may play a more nuanced role in behavioral inhibition that will be discussed below. Meanwhile, as reviewed above, FEF and SC contain neurons with the connectivity and patterns of modulation sufficient to explain response inhibition and execution. Neurons in premotor cortex likewise modulate before SSRT [8], and parallel results are obtained in M1 (Stuphorn personal communication). Studies using paired pulse transcranial magnetic stimulation show that inhibition in M1 is recruited in humans carrying out the stop-signal task [12]. Ultimately, an effective model of response control cannot emphasize one node or pathway in a complex system at the exclusion of other, more powerful pathways; it must include the dynamics of the entire network.
General vs. specific inhibition mechanisms
Most stop-signal experiments with humans test manual responses. As detailed above, our laboratory has used this task to investigate gaze control [67]. To investigate the generality of stopping mechanisms across effectors, we tested whether human subjects could stop eye and hand movements independently [6]. SSRTs were longer for hand movements than for eye movements, and advanced knowledge of which effector to stop did not confer any stopping advantage. We concluded that there must be some independence between the processes which stop eye and hand movements. Additional evidence for differences between effectors comes from studies examining the fine dynamics of movements on stop trials. Several groups have reported partial muscle activation when overt manual responses are canceled [33] and one group has reported reduced response force on noncanceled trials [68]. Similarly, when combined eye and head gaze shifts are canceled, neck muscles are often active [69,70]. However, partial muscle activation is not observed when eye movements are canceled in isolation, and eye-movement dynamics are indistinguishable on no-stop and noncanceled trials [71]. This ballistic "all or nothing" property of saccadic eye movements highlights a difference between the control of eyes and hands in typical testing conditions.
In addition to generality across effectors, how general is inhibition across strategies? And what is the relationship between slowing and stopping outright? These ethologically appealing issues have recently received attention under the headings of (1) global versus selective stopping, and (2) proactive versus reactive inhibition. Much work has focused on a role for the SMA and preSMA in proactive and selective inhibition in humans [10,17,72] and nonhuman primates [9,14,60,73]. These medial frontal areas exhibit single neuron and haemodynamic modulation that anticipates presentation of stop signals and relates to the quality of performance of the task. The contributions of other brain regions remains uncertain, though. Moving forward, we see issues needing clarification:
Many tasks confound proactive versus reactive stopping with selective versus global stopping (eg [74]). It is often implicitly assumed that proactive inhibition strategies are selective, whereas reactive inhibition is global (e.g. [1]). At least one study suggests that, under certain conditions, exerting proactive control leads to more selective inhibition [13], but care should be taken to manipulate one or the other of these variables until more data have been collected.
Logan’s original race model must be extended to understand selective stopping. For example, when different movements or effectors are used, multiple GO processes and possibly multiple STOP processes will be required [75]. In addition, many selective stopping studies have reported violations of race-model assumptions, with noncanceled RTs equal to or exceeding no-stop RTs (e.g. [76,77]). Such data invalidate the face-validity of SSRT calculated using Logan's original race model. Therefore, caution should guide interpretation of results until the underlying processes are understood through the next generation of race models. We believe that the interactive race model provides a foundation for models of selective stopping with different stimuli, rules and effectors. Like any RT, SSRT is comprised of successive stages, an initial encoding stage followed by an interactive stage during which the GO process is interrupted [37]. Encoding duration will vary based on stop-signal modality, task rules, history, or context. However, the interactive stage must be brief to produce noncanceled RTs faster than no-stop-signal RTs. Under selective stopping conditions, delayed noncanceled RTs can arise when the influence of the STOP process is weaker and prolonged. Thus, the interactive race model may extend naturally to selective stopping conditions. By whatever means the interaction between the GO and STOP processes is adjusted, an executive control circuit is necessary to register the need for these adjustments.
Executive control of stopping
An extensive body of work has associated areas of human medial frontal cortex with executive control. Consistent with this framework, SEF and anterior cingulate cortex (ACC) of monkeys both contain distinct populations of neurons active after errors or in association with reinforcement [36,73,78] (Figure 3). SEF also contains a population of neurons active after successful withholding of a partially prepared eye movement and proportional in their activation to the momentary co-activation of gaze-shifting and gaze-holding neurons. These neurons are hypothesized to signal response conflict. Several theories dealing with the precise functions carried out by medial frontal cortex have been advanced, ranging from error detection or prediction, to reinforcement learning to conflict monitoring. The existence of distinct populations of neurons signaling error, reinforcement, and putative response conflict indicates that each hypothesis has merit. But no neurons or LFPs have been recorded in monkey ACC that could signal conflict [78–80]. Some have proposed that macaque monkeys do not have the neural substrates necessary to generate performance monitoring ERPs similar to those observed in humans [81,82]. However, the presence of monitoring signals in both single units and LFPs as well as a monkey homologue of the error-related negativity (see below) call into question the merits of this proposal.
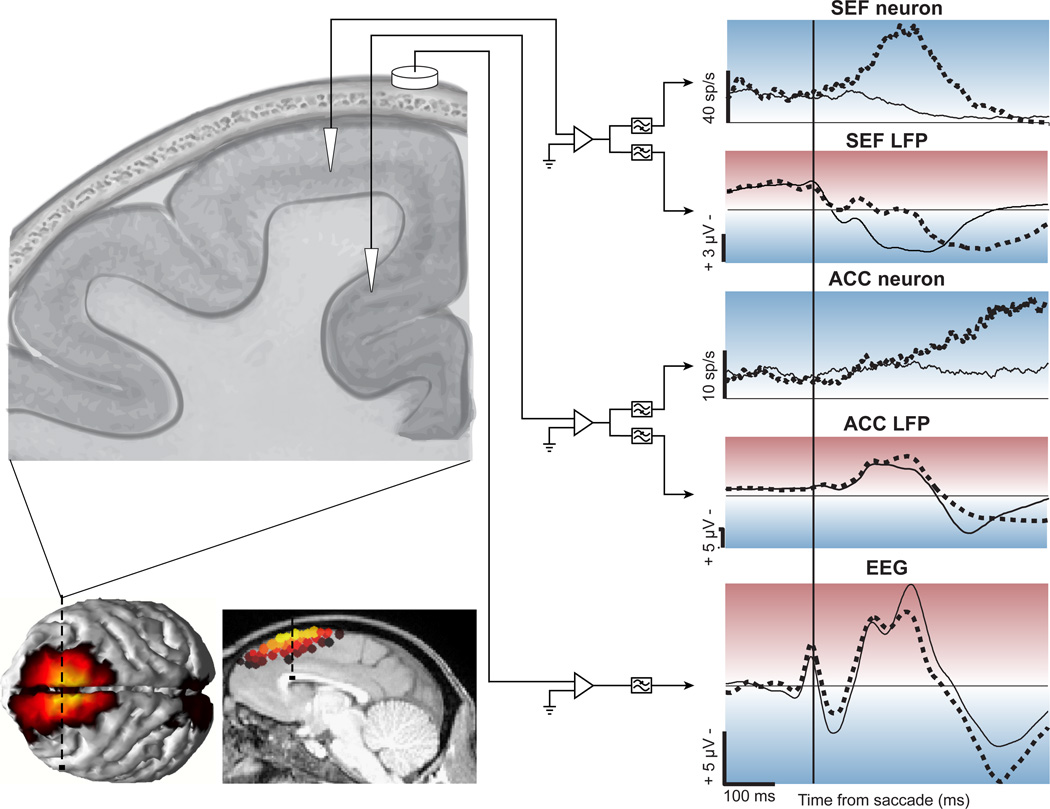
Figure 3.
Performance monitoring signals in and over medial frontal cortex of macaque monkeys during the stop-signal task. Schematized coronal section on top left illustrates location of intracranial recordings from SEF, ACC, and cranial surface recordings. Intracranial signals from microelectrodes were amplified and filtered to provide spikes or LFP, and cranial signal was processed as typical EEG (right panels). Solid lines plot activity on no-stop (correct) trials, and broken lines plot activity on noncanceled (error) trials. In both SEF and ACC, many neurons show increased activity following noncanceled error responses. Simultaneously, the LFP at some sites in SEF and most sites in ACC exhibit greater polarization after errors. These LFPs contribute to the greater polarization recorded on the surface, corresponding to the ERN, as indicated by the current sources calculated from the surface voltage distribution projected onto dorsal and medial surfaces of structural MRI data (lower left). Adapted from [36,78,79,84,85].
Error-related activity in SEF and ACC may contribute to an ERP recorded over medial frontal cortex known as the error-related negativity (ERN) [83]. This ERP was the first physiological signature of a supervisory control system. A bridge between the monkey single-neuron results and the human ERN began with a series of studies showing that LFPs in ACC and SEF exhibit polarization corresponding precisely to the ERN [79,84]. This was followed by the keystone finding that macaque monkeys exhibit an ERN recorded from the cranial surface consistent with a current source in medial frontal cortex [85] (Figure 3), and nearly identical in form and distribution to that recorded from humans performing the same task [86]. The neural origins and cognitive correlates of the ERN have been subjects of intense research, and an animal model of the ERN will certainly provide effective leverage on elucidating mechanisms.
As soon as performance monitoring signals were discovered, their relationship to performance adjustment was explored [83]. After mastering the stop-signal task, fine adjustments of performance continue [4,87,88]. For example, RT is delayed as more stop-signal trials are encountered or expected [4,89]. The role of medial frontal cortex in performance monitoring has been tested through intracortical microstimulation of SEF while monkeys performed the stop-signal task [14]. Stimulation was delivered simultaneous with stop-signal presentation, at current levels well below threshold for eliciting eye movements. Microstimulation of sites in SEF improve performance by reducing the fraction of non-canceled responses.
A reanalysis of data from FEF and SC shows how this behavioral slowing is accomplished [90]. RTs are presumably adapted to minimize errors and maximize rewards. Stochastic accumulator models commonly account for this adaptation through changes in response threshold. However, Pouget et al. [90] demonstrated that RT adaptation is accomplished not through a change of threshold, baseline, or accumulation rate, but instead through a change in the time when presaccadic movement activity first begins to accumulate. This result highlights the subtlety of mapping computational models onto neural processes.
Of course, performance adjustments need not be implemented via RT adaptation alone. In the stop-signal task, subjects may also adjust SSRT. The question of how motivational factors may influence SSRT is particularly interesting. To date, only one study has manipulated motivational state and tested for changes in SSRT [91]. These researchers manipulated reward contingencies to favor either speed or accurate inhibition, and found that SSRTs were lower when subjects were encouraged to value stopping. Unfortunately, SSRT estimates in this study were unreliable since only a single SSD was used. When estimating SSRT from a single SSD, it is important to use the SSD which yields 50% errors. Since the probability of making a noncanceled error was higher in the motivated speed condition, sampling error rates in both conditions at a single SSD means that SSRT was estimated from different tails of the RT distribution. Thus, the observed SSRT differences in this study probably represent well-known confounds in SSRT estimates rather than motivational factors. This study should be repeated using standard staircasing procedures. Until then, the effect of motivation on SSRT remains an open topic of great interest and importance.
Conclusions
Investigation of the stop-signal task in humans and animal models will likely accelerate because of the paradigm's powerful utility for elucidating mechanisms of response control from muscles to cognition. Its unique translational leverage is afforded by the race model, which provides a common language linking behavior, models, and neurons. The race model also provides powerful constraints on mechanisms and predictions, encouraging the use of strong inference. We look forward to new insights pinpointing neural circuitry responsible for interrupting responses in multiple effector systems, resolving mechanisms of proactive and reactive inhibition, and placing behavioral inhibition in the broader context of performance monitoring and executive control.
Highlights.
The stop-signal task probes response inhibition in multiple effector systems and species.
Neurophysiological studies in monkeys demonstrate mechanisms for stopping in motor structures.
Clinical and imaging studies in humans indicate complex circuits accomplishing stopping.
Differences in stopping mechanisms across effectors and task conditions are under active study.
Stop-signal studies afford effective investigation of executive control.
Acknowledgements
The authors gratefully acknowledge the efforts of our colleagues at Vanderbilt and elsewhere that frame this review and enjoy knowing that disagreements are entertained without disrespect. We are grateful to A Aron, P Bissett, M Franks, A Heritage, G Logan, P Middlebrooks, B Neggers, G Samanez-Larkin, and B Zandbelt for helpful comments. Research has been supported by the National Eye Institute, the National Institute of Mental Health, the National Science Foundation, the McKnight Endowment Fund for Neuroscience, the Air Force Office of Scientific Research and by Robin and Richard Patton through the E. Bronson Ingram Chair in Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological Psychiatry. 2011;69:E55–E68. doi: 10.1016/j.biopsych.2010.07.024.. This review covers recent developments in the cognitive neuroscience of stopping responses, arguing that studies of proactive rather than reactive control are of more translational value.
- 2.Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan GD, Cowan WB. On the ability to inhibit thought and action - a theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 4. Bissett PG, Logan GD. Balancing cognitive demands: control adjustments in the stop-signal paradigm. Journal of Experimental Psychology. 2011;37:392–404. doi: 10.1037/a0021800.. This paper presents a thorough exploration of post-stop-signal performance adjustments. The authors tested 5 competing hypotheses for post-stop-signal adjustments: goal priority, error detection, conflict monitoring, surprise, and memory. They propose that goal priority drives adjustments in reaction time.
- 5.Bissett PG, Logan GD. Post-stop-signal adjustments: Inhibition improves subsequent inhibition. Journal of Experimental Psychology: Learning, Memory & Cognition. 2012 doi: 10.1037/a0026778. [DOI] [PubMed] [Google Scholar]
- 6.Boucher L, Stuphorn V, Logan GD, Schall JD, Palmeri TJ. Stopping eye and hand movements: Are the processes independent? Perception & Psychophysics. 2007;69:785–801. doi: 10.3758/bf03193779. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mirabella G, Pani P, Ferraina S. Neural correlates of cognitive control of reaching movements in the dorsal premotor cortex of rhesus monkeys. Journal of Neurophysiology. 2011;106:1454–1466. doi: 10.1152/jn.00995.2010.. This paper provides the first data from dorsal premotor cortex of monkeys performing a manual stop-signal task. It shows that some neurons modulate before SSRT and can therefore contribute to response inhibition.
- 9. Stuphorn V, Brown JW, Schall JD. Role of supplementary eye field in saccade initiation: executive, not direct, control. Journal of Neurophysiology. 2010;103:801–816. doi: 10.1152/jn.00221.2009.. This paper shows that, although neurons in SEF are strongly modulated during the stop signal task, they do not modulate in a manner sufficient to contribute directly to response inhibition. Instead, some neurons generate signals that can contribute to proactive stopping and executive control.
- 10.Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai WD, Strunk J, George J, Aron AR. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson's disease. Journal of Neuroscience. 2011;31:5721–5729. doi: 10.1523/JNEUROSCI.6135-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. Journal of Neuroscience. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. Journal of Neurophysiology. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- 13.Greenhouse I, Oldenkamp CL, Aron AR. Stopping a response has global or nonglobal effects on the motor system depending on preparation. Journal of Neurophysiology. 2012;107:384–392. doi: 10.1152/jn.00704.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nature Neuroscience. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- 15. Duann JR, Ide JS, Luo X, Li CR. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. Journal of Neuroscience. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009.. Using Granger causality with fMRI data, the authors of this study concluded that rIFC serves a role in detecting stop signals, while preSMA plays a direct role in inhibiting action.
- 16. Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107.. This paper contrasts trials containing task relevant stop signals with those containing task irrelevant signals in an effort to obtain pure measures of response inhibition, attentional capture, and error processing in an fMRI paradigm. The authors conclude that pre-SMA, not rIFC is responsible for response inhibition.
- 17. Zandbelt BB, Bloemendaal M, Neggers SFW, Kahn RS, Vink M. Expectations and violations: delineating the neural network of proactive inhibitory control. Human Brain Mapping. 2012 doi: 10.1002/hbm.22047.. This paper dissociates proactive behavioral adjustments from violations of expectations during the stop-signal task. By presenting a stop-signal probability cue and a GO signal separated in time the authors show that rIFC is active when trial expectations are violated rather than during response inhibition per se.
- 18. Zandbelt BB, Vink M. On the role of the striatum in response inhibition. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013848.. This fMRI study of human manual stopping demonstrated the contribution toward stopping of the striatum to suppression of motor cortex in conjunction with supplementary motor complex and rIFC.
- 19. Lipszyc J, Schachar R. Inhibitory control and psychopathology A meta-analysis of studies using the stop signal task. Journal of the International Neuropsychological Society. 2010;16:1064–1076. doi: 10.1017/S1355617710000895.. This paper reports the outcome of a meta-analysis of stop signal studies in patients with various psychiatric disorders, assessing the methodological quality of studies. Results confirm an inhibition deficit in ADHD with differences comorbid with other disorders but indicate need for further studies to establish conclusively an inhibition deficit in obsessive-compulsive disorder and schizophrenia.
- 20. Thakkar KN, Schall JD, Boucher L, Logan GD, Park S. Response inhibition and response monitoring in a saccadic countermanding task in schizophrenia. Biological Psychiatry. 2011;69:55–62. doi: 10.1016/j.biopsych.2010.08.016.. This paper shows that, when tested with the same task used in monkey neurophysiology experiments, schizophrenics exhibited longer SSRT and inflated response adjustments. SSRT was correlated with the severity of negative symptoms and poorer occupational functioning.
- 21.Zandbelt BB, van Buuren M, Kahn RS, Vink M. Reduced proactive inhibition in schizophrenia is related to corticostriatal dysfunction and poor working memory. Biological Psychiatry. 2011;70:1151–1158. doi: 10.1016/j.biopsych.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW. CNTRICS Final task selection: executive control. Schizophrenia Bulletin. 2009;35:115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown JW, Hanes DP, Schall JD, Stuphorn V. Relation of frontal eye field activity to saccade initiation during a countermanding task. Experimental Brain Research. 2008;190:135–151. doi: 10.1007/s00221-008-1455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanes DP, Patterson WF, Schall JD. Role of frontal eye fields in countermanding saccades: Visual, movement, and fixation activity. Journal of Neurophysiology. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- 25.Paré M, Hanes DP. Controlled movement processing: Superior colliculus activity associated with countermanded saccades. Journal of Neuroscience. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segraves MA. Activity of monkey frontal eye field neurons projecting to oculomotor regions of the pons. Journal of Neurophysiology. 1992;68:1967–1985. doi: 10.1152/jn.1992.68.6.1967. [DOI] [PubMed] [Google Scholar]
- 27.Shinoda Y, Sugiuchi Y, Takahashi M, Izawa Y. Basic and Clinical Ocular Motor and Vestibular Research. vol 1233. Annals of the New York Academy of Sciences; 2011. Neural substrate for suppression of omnipause neurons at the onset of saccades; pp. 100–106. Edited by; [DOI] [PubMed] [Google Scholar]
- 28.Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- 29.Ray S, Pouget P, Schall JD. Functional distinction between visuomovement and movement neurons in macaque frontal eye field during saccade countermanding. Journal of Neurophysiology. 2009;102:3091–3100. doi: 10.1152/jn.00270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O'Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murthy A, Ray S, Shorter SM, Schall JD, Thompson KG. Neural control of visual search by frontal eye field: effects of unexpected target displacement on visual selection and saccade preparation. Journal of Neurophysiology. 2009;101:2485–2506. doi: 10.1152/jn.90824.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunamonti E, Thomas NWD, Paré M. The activity patterns of lateral intraparietal area neurons is not sufficient to control visually guided saccadic eye movements. Soc Neurosci Abstract. 2008 855.18.2008. [Google Scholar]
- 33. Scangos KW, Stuphorn V. Medial frontal cortex motivates but does not control movement initiation in the countermanding task. Journal of Neuroscience. 2010;30:1968–1982. doi: 10.1523/JNEUROSCI.4509-09.2010.. This paper shows that although neurons in SMA and pre-SMA are strongly modulated during a manual stop signal task, most do not modulate in a manner sufficient to contribute directly to response inhibition. Instead, the majority of neurons signaled expectation of reward, modulated by the amount of expected reward.
- 34.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nature Neuroscience. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- 35.Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. Journal of Neuroscience. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- 37. Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: An interactive race model of countermanding Saccades. Psychological Review. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376.. This paper presents the interactive race model that demonstrates mechanistically how the STOP process can interrupt the GO process. While accounting for the behavior, this model replicated essential characteristics of the modulation of the neurons accomplishing response inhibition. Providing a bridge between the abstract computational race model and neural mechanisms, it has sparked renewed interest in modeling this task.
- 38.Wong-Lin K, Eckhoff P, Holmes P, Cohen JD. Optimal performance in a countermanding saccade task. Brain Research. 2010;1318:178–187. doi: 10.1016/j.brainres.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lo CC, Boucher L, Paré M, Schall JD, Wang XJ. Proactive inhibitory control and attractor dynamics in countermanding action: A spiking neural circuit model. Journal of Neuroscience. 2009;29:9059–9071. doi: 10.1523/JNEUROSCI.6164-08.2009.. This paper demonstrates that a network of biophysically plausible spiking neurons can instantiate the interactive race model of response inhibition and predicted a correlation between the magnitude of fixation activity and behavioral outcome that was verified but remained unnoticed in the neurophysiological data recorded from monkeys.
- 40.Shenoy P, Yu AJ. Rational decision-making in inhibitory control. Frontiers in Human Neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 42.Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Wildenberg WPM, van Boxtel GJM, van der Molen MW, Bosch DA, Speelman JD, Brunia CHM. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson's disease. Journal of Cognitive Neuroscience. 2006;18:626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- 44.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- 46.Ray NJ, Brittain JS, Holland P, Joundi RA, Stein J, Aziz TZ, Jenkinson N. The role of the subthalamic nucleus in response inhibition: Evidence from local field potential recordings in the human subthalamic nucleus. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann-von Monakow K, Akert K, Kunzle H. Projections of the precentral motor cortex and other cortical areas of the frontal-lobe to the subthalamic nucleus in the monkey. Acta Anatomica. 1978;105:112–113. doi: 10.1007/BF00235561. [DOI] [PubMed] [Google Scholar]
- 48.Nambu A. Somatotopic organization of the primate basal ganglia. Frontiers in Neuroanatomy. 2011;5 doi: 10.3389/fnana.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambert C, Zrinzo L, Nagy Z, Lutti A, Hariz M, Foltynie T, Draganski B, Ashburner J, Frackowiak R. Confirmation of functional zones within the human subthalamic nucleus: Patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage. 2012;60:83–94. doi: 10.1016/j.neuroimage.2011.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neuroscience and Biobehavioral Reviews. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003.. This paper reviews rat studies of stopping. While experiments with rats afford manipulations that are difficult with monkeys and nearly impossible with humans, qualitative differences between the rodent and primate brain (in particular in connectivity of the subthalamic nucleus) and the distinction between stopping initiation of a movement (as in primates) versus stopping execution of a movement should not be overlooked.
- 51.Parent A. Extrinsic connections of the basal ganglia. Trends in Neurosciences. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- 52.Duncan J. EPS Mid-Career Award 2004 - Brain mechanisms of attention. Quarterly Journal of Experimental Psychology. 2006;59:2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- 53.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: Object-based selection of a region in space. Journal of Cognitive Neuroscience. 2000;12:106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- 55.Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nature Neuroscience. 2010;13 doi: 10.1038/nn.2509. 507-U136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shulman GL, Astafiev SV, Franke D, Pope DLW, Snyder AZ, McAvoy MP, Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. Journal of Neuroscience. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vossel S, Thiel CM, Fink GR. Cue validity modulates the neural correlates of covert endogenous orienting of attention in parietal and frontal cortex. Neuroimage. 2006;32:1257–1264. doi: 10.1016/j.neuroimage.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakahara K, Hayashi T, Konishi S, Miyashita Y. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- 60. Chen XM, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. Journal of Neuroscience. 2010;30:14657–14675. doi: 10.1523/JNEUROSCI.2669-10.2010.. This paper demonstrates how SMA contributes to proactive response inhibition in monkeys during a manual stop-signal task. Single-unit, multi-unit activity and LFP power in gamma bands were all correlated with RT, even before the target, and were modulated according to adjustment derived from trial history. Curiously, before SSRT when movements were canceled, elevated LFP power in the beta but not gamma band was observed.
- 61.Ray NJ, Jenkinson N, Brittain JS, Holland P, Joint C, Nandi D, Bain PG, Yousif N, Green A, Stein JS, et al. The role of the subthalamic nucleus in response inhibition: Evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia. 2009;47:2828–2834. doi: 10.1016/j.neuropsychologia.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nature Neuroscience. 2011;14 doi: 10.1038/nn.2925. 1462-U1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: Impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 64.Hälbig TD, Tse W, Frisina PG, Baker BR, Hollander E, Shapiro H, Tagliati M, Koller WC, Olanow CW. Subthalamic deep brain stimulation and impulse control in Parkinson's disease. European Journal of Neurology. 2009;16:493–497. doi: 10.1111/j.1468-1331.2008.02509.x. [DOI] [PubMed] [Google Scholar]
- 65.Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, van Wouwe NC, van den Wildenberg WPM. Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson's disease. Brain. 2010;133:3611–3624. doi: 10.1093/brain/awq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li CSR, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanes DP, Schall JD. Countermanding saccades in macaque. Visual Neuroscience. 1995;12:929–937. doi: 10.1017/s0952523800009482. [DOI] [PubMed] [Google Scholar]
- 68.Ko YT, Miller J. Nonselective motor-level changes associated with selective response inhibition: evidence from response force measurements. Psychonomic Bulletin & Review. 2011;18:813–819. doi: 10.3758/s13423-011-0090-0. [DOI] [PubMed] [Google Scholar]
- 69.Corneil BD, Elsley JK. Countermanding eye-head gaze shifts in humans: Marching orders are delivered to the head first. Journal of Neurophysiology. 2005;94:883–895. doi: 10.1152/jn.01171.2004. [DOI] [PubMed] [Google Scholar]
- 70. Goonetilleke SC, Doherty TJ, Corneil BD. A within trial measure of the stop signal reaction time in a head-unrestrained oculomotor countermanding task. Journal of Neurophysiology. 2010;104:3677–3690. doi: 10.1152/jn.00495.2010.. This paper demonstrates a novel measure of the time needed to cancel a gaze shift by probing neck muscle activity. When subjects generated small head movements without a gaze shift a burst of antagonist neck muscle activity occurred synchronized on SSRT. This finding highlights the importance of recognizing the effector-specific embodiment of stopping.
- 71.Godlove DC, Garr AK, Woodman GF, Schall JD. Measurement of the extraocular spike potential during saccade countermanding. J Neurophysiol. 2011;106:104–114. doi: 10.1152/jn.00896.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to Inhibit a Response Complements Response Inhibition during Performance of a Stop-Signal Task. Journal of Neuroscience. 2009;29:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.So NY, Stuphorn V. Supplementary eye field encodes option and action value for saccades with variable reward. Journal of Neurophysiology. 2010;104:2634–2653. doi: 10.1152/jn.00430.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aron AR, Verbruggen F. Stop the presses: dissociating a selective from a global mechanism for stopping. Psychological Science. 2008;19:1146–1153. doi: 10.1111/j.1467-9280.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- 75.Camalier CR, Gotler A, Murthy A, Thompson KG, Logan GD, Palmeri TJ, Schall JD. Dynamics of saccade target selection: Race model analysis of double step and search step saccade production in human and macaque. Vision Research. 2007;47:2187–2211. doi: 10.1016/j.visres.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Jong R, Coles MGH, Logan GD. Strategies and mechanisms in nonselective and selective inhibitory motor control. Journal of Experimental Psychology-Human Perception and Performance. 1995;21:498–511. doi: 10.1037//0096-1523.21.3.498. [DOI] [PubMed] [Google Scholar]
- 77.van de Laar MC, van den Wildenberg WPM, van Boxtel GJM, van der Molen MW. Processing of global and selective stop signals application of donders' subtraction method to stop-signal task performance. Experimental Psychology. 2010;57:149–159. doi: 10.1027/1618-3169/a000019. [DOI] [PubMed] [Google Scholar]
- 78.Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 79. Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: Anterior cingulate cortex. Journal of Neurophysiology. 2008;99:759–772. doi: 10.1152/jn.00896.2006.. Following a study in anterior cingulate cortex, this paper described LFPs in SEF of monkeys performing a saccade stopping task. Prominent visual responses and saccade-related polarization were observed. When saccades were canceled, LFP polarization was correlated with the magnitude of response conflict derived from the coactivation of gaze-shifting and gaze-holding neurons. At a minority of sites, a pronounced negative-going polarization occurred after errors that could contribute to the error-related negativity.
- 80.Nakamura K, Roesch MR, Olson C R. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol. 2005;93:884–908. doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- 81.Cole M, Yeung N, Freiwald WA, Botvinick M. Cingulate cortex: diverging data from humans and monkeys. Trends Neurosci. 2009;32:566–574. doi: 10.1016/j.tins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schall JD, Emeric EE. Conflict in cingulate cortex function between humans and macaque monkeys: more apparent than real. Comment on "Cingulate cortex: diverging data from humans and monkeys. Brain, Behavior and Evolution. 2010;75:237–238. doi: 10.1159/000313862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gehring WJ, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne) In: Luck SJ, Kappenman E, editors. Oxford handbook of event-related potential components. Oxford University Press; 2012. . A current and comprehensive review of the ERN and related components.
- 84.Emeric EE, Leslie M, Pouget P, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: Supplementary eye field. J Neurophysiol. 2010;104:1523–1537. doi: 10.1152/jn.01001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Godlove DC, Emeric EE, Segovis CM, Young MS, Schall JD, Woodman GF. Event-related potentials elicited by errors during the stop-signal task. I. macaque monkeys. Journal of Neuroscience. 2011;31:15640–15649. doi: 10.1523/JNEUROSCI.3349-11.2011.. This paper is the first demonstration of the ERN in nonhuman primates, forming a keystone in the bridge linking human and nonhuman primate studies on the neural basis of performance monitoring.
- 86. Reinhart RMG, Carlisle NB, Kang MS, Woodman GF. Event-related potentials elicited by errors during the stop-signal task. II: Human effector specific error responses. J Neurophysiol. 2012 doi: 10.1152/jn.00803.2011.. This companion to [85] describes the ERN from humans performing both saccade and manual stop-signal tasks. Waveforms and distributions varied slightly between tasks, but the ERN waveform and current source distribution during saccade stopping looked virtually identical to that of monkeys.
- 87.Emeric EE, Brown JW, Boucher L, Carpenter RHS, Hanes DP, Harris R, Logan GD, Mashru RN, Paré M, Pouget P, et al. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Research. 2007;47:35–49. doi: 10.1016/j.visres.2006.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nelson M, Boucher L, Logan GD, Palmeri TJ, Schall JD. Nonindependent and nonstationary response times in stopping and stepping saccade tasks. Attention, Perception & Psychophysics. 2010;72:1913–1929. doi: 10.3758/APP.72.7.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bissett PG, Logan GD. Post-stop-signal slowing: Strategies dominate reflexes and implicit learning. Journal of Experimental Psychology: Human Perception and Performance. doi: 10.1037/a0025429. in press.. This study manipulated the probability of stop-trial repetition to study post-stop-signal slowing. The authors demonstrate that post-stop-signal slowing can be eliminated or reversed when the probability of stop-trial repetition is reduced, and that this effect is more pronounced after the contingency is made explicit to subjects.
- 90. Pouget P, Logan GD, Palmeri TJ, Boucher L, Paré M, Schall JD. Neural basis of adaptive response time adjustment during saccade countermanding. Journal of Neuroscience. 2011;31:12604–12612. doi: 10.1523/JNEUROSCI.1868-11.2011.. This paper examined for the first time how proactive adjustments of stop-signal task performance are accomplished. The systematic delay in response time after stop-signal trials was accomplished not through a change of threshold, baseline, or accumulation rate, but instead through a change in the time when movement activity first began to accumulate. This result was not anticipated by standard accumulator models of RT.
- 91.Leotti LA, Wager TD. Motivational influences on response inhibition measures. Journal of Experimental Psychology-Human Perception and Performance. 2010;36:430–447. doi: 10.1037/a0016802. [DOI] [PMC free article] [PubMed] [Google Scholar]