Abstract
Angiogenesis is a hallmark of tumor development and metastatic progression, and antiangiogenic drugs targeting the VEGF pathway have shown to decrease the disease progression in cancer patients. In this study, we have analyzed the anti-proliferative and anti-angiogenic property of plumbagin in cisplatin sensitive, BRCA2 deficient, PEO-1 and cisplatin resistant, BRCA2 proficient PEO-4 ovarian cancer cells. Both PEO-1 and PEO-4 ovarian cancer cells are sensitive to plumbagin irrespective of BRCA2 status in both normoxia and hypoxia. Importantly, plumbagin treatment effectively inhibits VEGF-A and Glut-1 in PEO-1 and PEO-4 ovarian cancer cells. We have also analyzed the p53 mutant, cisplatin resistant, and BRCA2 proficient OVCAR-5 cells. Plumbagin challenge also restricts the VEGF induced pro-angiogenenic signaling in HUVECs and subsequently endothelial cell proliferation. In addition, we observe a significant effect on tumor regression among OVCAR-5 tumor-bearing mice treated with plumbagin, which is associated with significant inhibition of Ki67 and vWF expressions. Plumbagin also significantly reduces CD31 expression in an ear angiogenesis assay. Collectively, our studies indicate that plumbagin, as an anti-cancer agent disrupts growth of ovarian cancer cells through the inhibition of proliferation as well as angiogenesis.
Keywords: Plumbagin, Ovarian cancer, Cytotoxicity, Angiogenesis
INTRODUCTION
Angiogenesis, the growth of new blood vessels from pre-existing one plays a pivotal role in tumor growth and metastasis. Hence inhibiting angiogenesis is a promising strategy for the development of anticancer drugs. Natural and synthetic angiogenesis inhibitors are widely studied for their potential anticancer activities.1-3 Angiogenesis inhibitors have fewer side effects than many other cancer treatments, but they may only limit the growth of the cancer. A drug, which can inhibit tumor vasculature as well as kill the tumor cells, would definitely be a superior therapeutic choice for the cancer chemotherapy. Plumbagin (5-hydroxy, 3-methyl 1, 4-naphthaquinone) and its derivatives have been shown to have several biological activities including antitumour activity which has been recently reviewed.4 It has also been shown that plumbagin induces reactive oxygen species (ROS) resulting in apoptosis and cell cycle arrest.5, 6 Lately, plumbagin was shown to activate the Nrf2-ARE pathway and promote neuronal cell survival7 and at low concentrations plumbagin selectively induces the proliferation and differentiation of glial progenitor cells without affecting the proliferation or differentiation of neuron-restricted progenitors.8 Plumbagin was reported to inhibit tumor angiogenesis and tumor growth by VEGFR2 mediated Ras signaling pathway in endothelial cells.9 Plumbagin also selectively inhibited the growth of BRCA1 defective ER-α positive ovarian cancer cells.10 Both BRCA1 and BRCA2 regulate repair of the damaged DNA through Homologous Recombination (HR).11, 12 Although plumbagin was documented to have selective growth inhibition in BRCA1 defective condition, its effects on BRCA2 defective cells is not known.
In this study, we have analyzed the anti-proliferative and anti-angiogenic property of plumbagin in cisplatin sensitive, BRCA2 deficient, PEO-1 and cisplatin resistant, BRCA2 proficient PEO-4 ovarian cancer cells. Also this is the first study exploring the anticancer activity of plumbagin in hypoxic condition. Here we report that both PEO-1 and PEO-4 ovarian cancer cells are sensitive to plumbagin irrespective of BRCA2 status. We have shown that plumbagin significantly inhibited the growth of PEO-1 and PEO-4 cells both in normoxia and hypoxia and induced apoptosis. We have also included cisplatin resistant OVCAR-513, a human epithelial carcinoma cell line, established from the ascitic fluid of a patient with progressive ovarian adenocarcinoma in this study. OVCAR-5 cells are also sensitive to plumbagin treatment in normoxia as well as in hypoxia. Furthermore, to explain plumbagin’s anticancer activity, we assayed different signaling molecules associated with survival, proliferation, and chemo sensitivity of the tumor cells. Additionally, we have found that plumbagin treatment lowered the expression of potent angiogenenic molecules such as VEGF and Glut-1. Plumbagin challenge also inhibited the VEGF induced pro-angiogenenic signaling in HUVECs and subsequently endothelial cell proliferation. In addition, administration of plumbagin (1mg/kg/day) for three weeks beginning 28 days after OVCAR-5 tumor implantation showed significant regression of tumor volume and weight.
MATERIALS AND METHODS
Reagents
Reagents used are described in detail in the supplementary section.
Cell Culture
PEO-1 and PEO-414 cells were maintained under normoxic condition (5% CO2, 21% O2 and balance N2) or hypoxic condition (5% CO2, 1% O2 and balance N2). Immortalized normal Ovarian Surface Epithelial (OSEts/TERT),15, 16 OVCAR-5 ovarian cancer cells and HUVECs17 were also analyzed. For all cell culture experiments the incubation period with plumbagin (PB) was kept constant for 12 hr, unless otherwise specified.
In vitro cell viability assay
The cell viability studies were performed using a colorimetric MTS assay (Promega, Madison, WI) as described elsewhere.5 A detailed procedure is provided in the supplementary section.
In vitro Apoptosis Assay Using Annexin V-labeling
A detailed assay procedure is described in the supplementary section.
Colony formation assay
Cells were treated with 1 and 2.5 μM of plumbagin under normoxic condition and cultured for 14 days. The colonies/cells were then fixed in 4% paraformaldehyde (PFA) and stained with 0.2% crystal violet dissolved in 2% ethanol.
Western blot analysis
Western blot experiments were done with PEO-1, PEO-4, OSE, and HUVEC cell lysates and a detailed procedure has been included in the supplementary section.
Quantitative RT-PCR
Cells were treated with different concentrations of plumbagin and incubated under normoxic or hypoxic conditions. RNA isolation and Real-Time PCR (RT-PCR) were done as described previously.17 VEGF-A, Glut-1, and ACTB (β-Actin) primers were purchased from SABiosciences (Frederick, MD). Relative expression was calculated using the comparative Ct method.18
Thymidine incorporation assay
Thymidine incorporation assay was done with HUVEC cells and described in detail in the supplementary section.
Ca2+ release assay
Ca2+ release assay was performed with HUVEC cells described in the supplementary section.
Mouse ear angiogenesis assay
Nude mice ear angiogenesis assay was done as described previously.19 Details of the assay is provided in the supplementary section.
Tumor Model
Six-week-old female SCID mice were obtained from NIH and housed in the institutional animal facility. To establish tumor growth in mice, 5 × 106 OVCAR-5 cells, resuspended in 100 μL of PBS, were injected subcutaneously into the left flank.
In vivo Anti-tumor Activity
Tumors were allowed to grow for 28 days without treatment, and mice were randomized into two groups (ten animals per group). Group 1 was treated with 25% polyethylene glycol (PEG) alone, while group 2 was treated with plumbagin in 25% PEG at doses of 1 mg/kg/day intraperitoneally. Tumors were measured twice a week, and primary tumor volumes were calculated using the formula V = 1/2a × b2, where ‘a’ is the longest tumor axis, and ‘b’ is the shortest tumor axis. After 3 weeks of treatment, all OVCAR-5 tumor-bearing mice were sacrificed by asphyxiation with CO2; tumors were removed, measured, and prepared for immunochemistry.
Histological Study
Details of the histologilcal study are available in supplementary section.
Statistical analysis
The independent-sample t-test was used to test the probability of significant differences between two groups. Analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for multiple comparisons between multiple groups. Statistical significance was defined as (*) p ≤ 0.05; (**) p ≤ 0.001, and (***) p ≤ 0.0001. Error bars are given on the basis of calculated S.D values.
RESULTS
Plumbagin inhibits cell proliferation and induces apoptosis
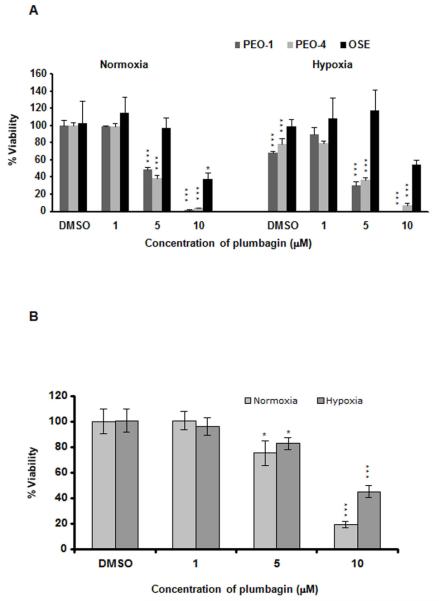
The specific growth inhibitory effects of plumbagin on BRCA1 defective cells10, 20, 21 prompted us to analyze the effect on PEO-1 and PEO-4 cells. 5 μM of plumbagin significantly inhibited the viability of both PEO-1 and PEO-4 cells irrespective of BRCA2 status under both normoxia and hypoxia compared to normal human ovarian surface epithelial (OSE) cells (Figure 1 A). We also assayed the viability of BRCA2 positive 22 OVCAR-5 cells (Figure 1 B) after plumbagin treatment. Inhibition of OVCAR-5 cell viability also has been detected in both normoxia and hypoxia with the 5 μM dose of plumbagin but less significantly compared to PEO cells (Figure 1 B). Hypoxia induced cytotoxicity has been observed in PEO-1 and PEO-4 cells but not in OSE or OVCAR-5 cells (Figure 1 A and B). The synergy between hypoxia-induced cell death and effect of plumbagin has only been witnessed in the case of PEO-1 cells.
Figure 1. Plumbagin inhibits ovarian cancer cell growth in vitro.
A. Ovarian cancer PEO-1, PEO-4 and OSE cells were treated with increasing concentrations of plumbagin under both normoxia and hypoxia. The cell viability was measured using MTS assay. B. MTS assay by plumbagin in OVCAR-5 cells. P values were calculated comparing with the respective DMSO treated cells in normoxia using two-way ANOVA followed by Tukey’s post hoc test. The figures are representative of three separate experiments (in quadruplicates) with similar results.
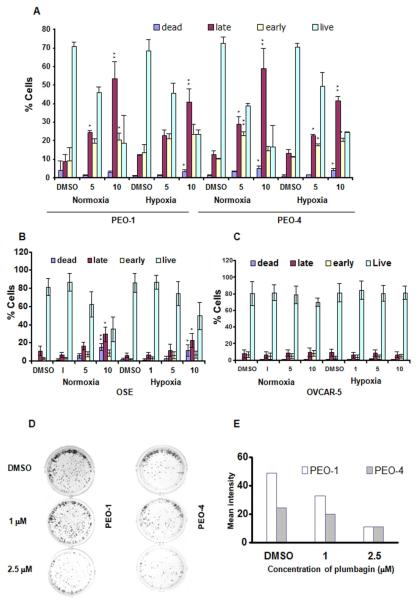
Induction of apoptosis is a critical part in the treatment of cancer. We observed a dose-dependent induction of apoptosis in both PEO-1 and PEO-4 cells after plumbagin treatment (Figure 2 A). A 5-μM dose of the compound induced significant apoptosis in these ovarian cancer cells compared to OSE cells in normoxia and in hypoxia (Figure 2 A and B). In OSE cells only 10 μM dose of plumbagin induced apoptosis (Figure 2 B). However, with OVCAR-5 cells no induction of apoptosis has been recorded (Figure 2 C). From the above data it seems that OVCAR-5 is more resistant to plumbagin treatment in vitro compared to PEO-1 or PEO-4 cells and effect on OVCAR-5 cell viability is possibly due to the plumbagin mediated cell cycle arrest.
Figure 2. Plumbagin induces apoptosis in vitro: A, B, and C.
PEO-1, PEO-4, OSE, and OVCAR-5 cells were treated with DMSO or 1/5/10 μM doses of plumbagin under normoxia and hypoxia. Apoptosis was measured by Annexin-FITC/PI method. Dead cells (PI-positive) were differentiated from late apoptotic cells (Annexin-positive and PI-positive), early apoptotic cells (Annexin-positive and PI-negative) and live cells (Annexin-negative and PI-negative). P values were calculated comparing with the respective DMSO treated cells in normoxia and hypoxia using one-way ANOVA followed by Tukey’s post hoc test. The figures are representative of three separate experiments with similar results. D. Colony formation assay after plumbagin treatment in PEO-1 and PEO-4 cells. The figures are representative of three separate experiments with similar results. E. Quantitation of clonogenic assay was done using adobe photoshop CS3.
Plumbagin inhibits clonal growth of ovarian cancer cells
Colony formation or clonogenic assay can be used to measure the sensitivity of human tumors to anticancer drugs. Treatment with plumbagin resulted in a dose-dependent decrease in both PEO-1 and PEO-4 ovarian cancer cells’ clonal growth in vitro under normoxia (Figure 2 D and E). Altogether these results suggest that the presence of BRCA2 might have no role in the anticancer activity of plumbagin.
Effect of plumbagin on Akt and MAPKinase pathway
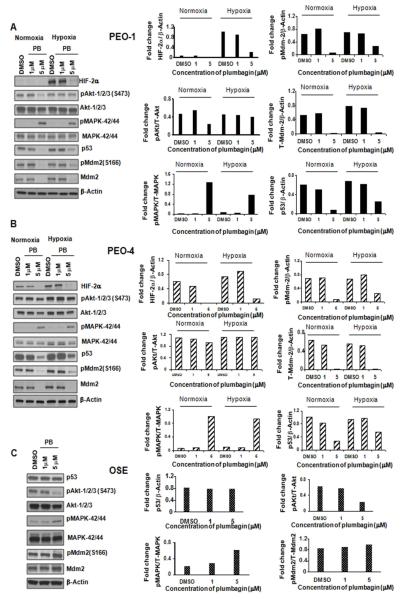
Plumbagin treatment induced phosphorylation of MAPK-42/44 (ERK1/2) in both PEO-1 and PEO-4 cells (Figure 3 A and B) as well as in normal OSE cells (Figure 3 C) at 5 μM concentration in both normoxia and hypoxia, possibly through ROS induction. In OVCAR-5 cells, 1 μM dose of plumbagin was sufficient to induce phosphorylation of MAPK-42/44 in normoxia but in hypoxia 5 μM of the drug was required (Figure S1 B). Akt is involved in cellular survival pathways, and inhibits apoptotic processes. We found that a 5 μM dose of plumbagin significantly inhibited Akt-1/2/3 phosphorylation at Ser-473 in PEO-1 (Figure 3 A) but not in PEO-4 (Figure 3 B) cells under normoxia. Moreover, the same dose of plumbagin had no effect on the Akt-1/2/3 phosphorylation in PEO-1 as well as in PEO-4 cells in hypoxia (Figure 3 A and B). A 5 μM dose of plumbagin had negligible effect on the survival/apoptosis of normal ovarian epithelial (OSE) cells (Figure 1 A and 2 B), but inhibited the phosphorylation of Akt-1/2/3 (Ser-473) (Figure 3 C) under normoxia in these cells.
Figure 3. Plumbagin modulates different signaling pathways. A, B, and C.
PEO-1, PEO-4 and OSE cells were treated with increased concentrations of plumbagin and were kept in normoxic or hypoxic conditions and western blotting was done with antibodies against HIF-2α, p-Akt1/2/3 (ser-473), Akt-1/2/3, p-MAPK-42/44, MAPK-42/44, p53, p-Mdm-2 (ser-166), and Mdm-2 (T-Mdm-2). β-Actin was used as a loading control. Expression levels of proteins have been shown with bar graphs. Adobe photoshop version 7.0 has been used for the western quantitation.
Effect of plumbagin on p53 and Mdm2 signaling pathway
A 5 μM dose of plumbagin on PEO-1 and PEO-4 ovarian cancer cells resulted in inhibition of p53 expression under both normoxia and hypoxia (Figure 3 A and B) compared to normal ovarian OSE cells where 5 μM of plumbagin had no effect on p53 expression (Figure 3 C). A 5 μM dose of plumbagin caused inhibition of both phosphorylation of Mdm2 at Ser-166 and total Mdm2 level in both PEO-1 and PEO-4 cells (Figure 3 A and B). On the other hand, the same dose of plumbagin could not inhibit the total Mdm2 level or phosphorylation of Mdm2 in OSE cells (Figure 3 C).
Plumbagin inhibits VEGF and Glut-1 expressions
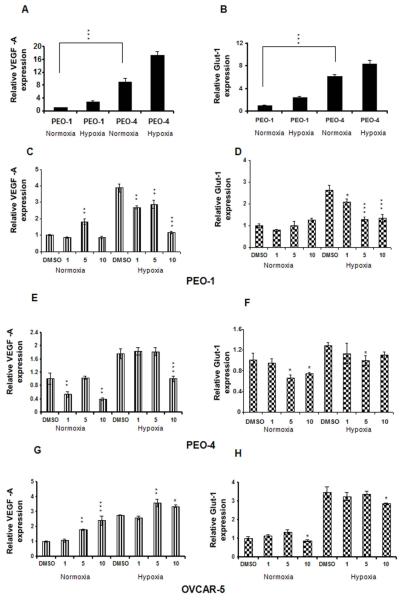
Hypoxia is well known to induce the expression of a large number of genes with VEGF-A and Glut-1, being critical targets of HIFα subunits affecting angiogenesis and glucose transport. PEO cells express HIF-2α but not HIF-1α (data not shown). By western blot analysis we found that plumbagin inhibited the HIF-2α expression in normoxia and even in hypoxia in PEO-1, PEO-4, and OVCAR-5 cells (Figure 3 A and B, and S1 B). Plumbagin had no effect on the HIF-1α expression in OVCAR-5 cells (data not shown). Therefore, we have analyzed the expressions of VEGF and Glut-1 after plumbagin treatment in PEO and OVCAR-5 cells under normoxia and hypoxia. Subjecting both PEO cell lines to hypoxia resulted in significant VEGF and Glut-1 mRNA accumulation and PEO-4 cells express considerably higher (more than 6 fold) amount of basal level of both VEGF and Glut-1 mRNA compared to PEO-1 cells (Figure 4 A and B). VEGF mRNA expression was significantly decreased as shown in Figure 4 C and E by 1 and 10 μM of plumbagin treatments in hypoxic PEO-1 and PEO-4 cells, respectively. In addition, 1μM concentration of plumbagin significantly inhibited the Glut-1 expression in PEO-1 cells in hypoxic conditions (Figure 4 D). In PEO-4 cells a 5 μM dose of plumbagin inhibited the Glut-1 mRNA expression in both normoxia and hypoxia (Figure 4 F). 5 μM concentration of plumbagin also decreased the Glut-1 protein expression significantly at normoxia compared to hypoxia (Figure S1 A) in PEO-4 cells. In normoxia, plumbagin reduced the expression of both VEGF and Glut-1 in PEO-4 cells but not in PEO-1 cells, compared to DMSO treated control samples (Figure 4 C, D, E and F). Although plumbagin treatment inhibited HIF-2α expression in OVCAR-5 cells, we observed increase in VEGF-A mRNA expression with the increasing dose of plumbagin in both normoxia and hypoxia (Figure 4 G). This finding indicates probability of ROS mediated increase in VEGF-A transcription possibly through Sp1-Sp3 dependent activation of promoter elements.23 However, we observed 10 μM concentration of plumbagin significantly inhibited the Glut-1 mRNA and protein expressions in normoxia as well in hypoxia (Figure 4 H and S1 A) in OVCAR-5 cells.
Figure 4. Plumbagin inhibits both VEGF and Glut-1 expressions in ovarian cancer cells.
RNA was collected from DMSO or plumbagin (1, 5 or 10 μM) treated PEO-1, PEO-4, and OVCAR-5 cells incubated under normoxic and hypoxic conditions. Real-time PCRs for VEGF-A and Glut-1 were done with the cDNAs from the respective samples, and β-actin was used for cDNA normalization. A and B. Subjecting both PEO cell lines to hypoxic growth conditions resulted in significant VEGF and Glut-1 mRNA accumulation and PEO-4 cells express considerably higher amount of basal level of both VEGF and Glut-1 mRNA compared to PEO-1 cells [***p<0.0001; paired t test, 2 tailed]. C, E and G represent VEGF-A mRNA expression and D, F and H represent Glut-1 mRNA expression in PEO-1, PEO-4 and OVCAR-5 cells respectively. P values were calculated comparing with the respective DMSO treated cells in normoxia and hypoxia using one-way ANOVA followed by Tukey’s post hoc test. The figures are representative of three separate experiments with similar results.
Plumbagin inhibits VEGF-mediated angiogenesis
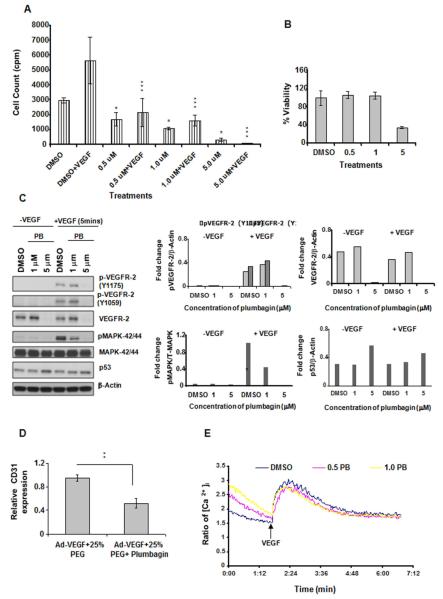
Tumor angiogenesis involves a complex interplay between tumor and supportive cells like endothelial cells. Tumor secreted VEGF activates endothelial cells which are subsequently recruited to form new blood vessels. Next, the effect of plumbagin on VEGF induced proliferation of endothelial cells was analyzed. Starved HUVEC cells were simultaneously treated with increasing concentration of plumbagin and 10ng/ml of VEGF. 0.5 μM of plumbagin was sufficient to inhibit VEGF induced cells in S phase as observed by thymidine incorporation assay (Figure 5 A). Additionally, Figure 5 B shows the effect of plumbagin on HUVEC cell viability. 0.5 and 1.0 μM concentrations of plumbagin had no effect on the cell viability but interfered with the thymidine incorporation at S phase of the cell cycle. Higher dose of plumbagin (5.0 μM) decreased HUVEC cell viability and also inhibited the total VEGFR-2 expression and VEGF induced VEGFR-2 phosphorylation at tyrosine residues 1175 and 1059 (Figure 5 C). 1.0 μM concentration of plumbagin blocked VEGF stimulated phosphorylation of pro-proliferative molecule MAPK-42/44 (Figure 5 C). Finally, we observed that plumbagin treatment increased the expression of p53 thereby possibly exerting additional inhibitory effects on proliferation by arresting cell cycle (Figure 5 C). Furthermore, to support our hypothesis that plumbagin inhibits VEGF-mediated angiogenesis in vivo, we studied the nude mouse ear model. We injected Ad-VEGF-A one day before the injection of plumbagin into nude mice. Animals were sacrificed at 1 week after injection of VEGF-A, and both treated and untreated ears were processed for RNA extraction and RT-PCR was performed for CD31 expression. We observed a marked decrease in CD31 mRNA expression in the plumbagin treated group compared to the untreated group [p<0.001] (Figure 5 D).
Figure 5. Effect of plumbagin on HUVECs. A. Plumbagin inhibits VEGF- induced proliferation of HUVECs.
Serum starved HUVECs were treated with DMSO or 0.5/1/5 μM of plumbagin in the presence or absence of VEGF (10 ng/ml) and thymidine incorporation assay was done. P values were calculated comparing with the respective DMSO treated cells using one-way ANOVA followed by Tukey’s post hoc test. The figures are representative of three separate experiments with similar results. B. Effect of plumbagin on HUVEC cell viability was measured using MTS assay. C. Effect of plumbagin on signaling pathway in HUVEC’s. Serum starved HUVEC cells were pre-treated with DMSO or 1/5 μM of plumbagin and then stimulated with VEGF (10 ng/ml) for 5 min. Protein expression was analysed by western blot. β-Actin was used as a loading control. Expression levels have been shown with bar graphs. Adobe photoshop version 7.0 has been used for western quantitation. D. Relative CD31 mRNA expression in nude mouse ears: 1mg/kg intraperitoneal injection of plumbagin in 25% PEG significantly reduced CD31 mRNA expression [**p<0.001, paired t test, 2 tailed] compared to 25% PEG treatment only in mouse ear pre-treated with Ad-VEGF. E. Plumbagin induces Ca2+ mobilization. Serum starved HUVEC cells were pre-treated with DMSO or 500 nM/1 μM of plumbagin and then used for intracellular Ca2+ mobilization assay as described in ‘Materials and Methods’ section.
Plumbagin promotes intracellular Ca2+ mobilization
VEGF stimulates VEGFR-2 phosphorylation, intracellular Ca2+ mobilization, MAPK-42/44 phosphorylation, and increases proliferation of endothelial cells. A 1.0 μM concentration of plumbagin significantly inhibited VEGF induced MAPK-42/44 phosphorylation and number of HUVEC cells in S phase (Figure 5 C and A). We performed the Ca2+ mobilization experiment in presence of 0.5 and 1.0 μM doses of plumbagin in HUVECs. Both doses of plumbagin treatment increased VEGF-uninduced basal level of Ca2+ (Figure 5 E) most likely as a result of ROS generation. However, the same doses of plumbagin treatment hindered the VEGF-stimulated release of intracellular Ca2+ ion compared to DMSO treated cells (Figure 5 E), corroborating with the MAPK-42/44 phosphorylation data (Figure 5 C). Next we pretreated the HUVECs with NAC (10 mM) which is an ROS inhibitor, for 1.5 hr and then treated with plumbagin. Pretreatment with NAC increased the basal level of calcium in DMSO treated HUVEC cells but blocked the further plumbagin mediated increase in Ca2+ level, proved the role of ROS in plumbagin mediated Ca2+ mobilization (Figure S1 C). Furthermore, we repeated the Ca2+ experiment in presence of BAPTA-AM (10 μM) (Figure S1 D). We observed moderate effect of BAPTA-AM on plumbagin mediated basal increase in Ca2+ level. Therefore, plumbagin induced basal increase of Ca2+ level may be the combinatorial effect of both intracellular Ca2+ mobilization and extracellular Ca2+ influx.
Effect of plumbagin on ovarian cancer cell line OVCAR-5 growth in vivo
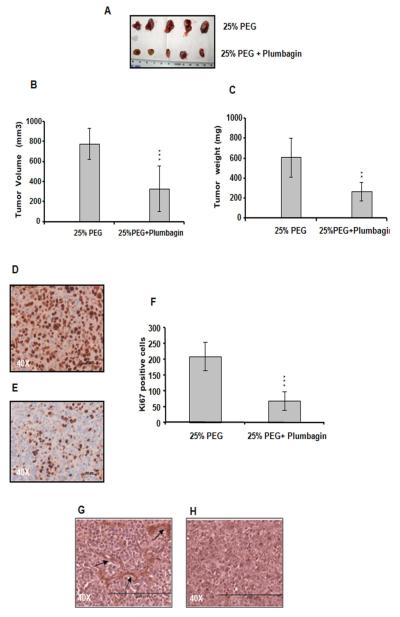
To study the effect of plumbagin on ovarian tumor growth, we injected OVCAR-5 human ovarian cancer cells subcutaneously into female SCID mice. After 28 days, plumbagin in 25% PEG was administered intraperitoneally. Treatments were continued for three consecutive weeks for mice bearing OVCAR-5 tumors. All control mice received an equal volume of the 25% PEG only. We observed a significant effect on tumor suppression among the OVCAR-5 tumor-bearing mice treated with plumbagin compared to mice treated with PEG alone (p <0.0001) (Figure 6 A and B). The average tumor volume was observed to be 776.8 ± 155.7 mm3 in the control group, 330.0 ± 227.0 mm3 in the plumbagin-treated group. We also found a significant difference in tumor weight between the control and plumbagin treated groups [p<0.001] (Figure 6 C).
Figure 6. Plumbagin inhibits OVCAR-5 tumor growth in SCID mice.
A. Images depicting differences in OVCAR-5 tumor growth with and without plumbagin treatment. B. Average OVCAR-5 tumor volume after 3 weeks of treatment in control and treatment groups. C. Significant regression in tumor weight has been observed. Plumbagin inhibits tumor cell proliferation in vivo. Ki67-stained tumor sections: D. Control group received only 25% PEG, E. received plumbagin in 25% PEG. Plumbagin alone demonstrated a significant effect on the inhibition of the Ki67 level. F. Quantitation of the Ki67 positive cells. Plumbagin inhibits angiogenesis in vivo. vWF stained sections: G. Control group received only 25% PEG, H. received plumbagin in 25% PEG, showing no staining of vWF. P value (Paired t test, 2 tailed) was calculated by comparing treated group vs. control group.
Effect of plumbagin on Ki67 Expression in vivo
We investigated the effect of plumbagin on tumor cell proliferation as measured by Ki67 staining (Figure 6 D, E and F). The Ki67 protein is a cellular marker for proliferation. 24 The average number of Ki67-positive nuclei in ten randomly selected microscopic fields is shown in Figure 6 F. There was significant reduction in Ki67 positive cells in the plumbagin-treated group (p < 0.0001, treated group vs. control group).
Effect of Plumbagin on Tumor Angiogenesis in vivo
Finally, the effect of plumbagin on tumor angiogenesis was analyzed in in vivo models. For this, tumor sections from the OVCAR-5 ovarian cancer model were stained with anti-vWF antibody (Figure 6 G and H). Tissue sections from plumbagin treated group did not show any staining with anti-vWF antibody (Figure 6 H) where as untreated group shows vessel formation (Figure 6 G) indicating that plumbagin can inhibit blood vessel formation in tumors.
DISCUSSION
Here we describe the anti-tumorigenic and anti-angiogenic property of plumbagin in BRCA2 deficient and BRCA2 proficient ovarian cancer cells, which could potentially be of significant therapeutic benefit in treating a wide range of malignancies. For the past 30 years, cisplatin has been considered as one of the gold standard chemotherapeutic agent against cancers of various organs like lung, ovary and prostate.25 Although cisplatin is very effective when administered in naïve patients, the repeated challenge leads to resistance and consequently the drug loses its efficacy. It has been shown that acquired resistance to cisplatin can be mediated by secondary intragenic mutations in BRCA2 that restore the wild-type BRCA2 reading frame.26 PEO-1, derived from BRCA2 mutated ovarian cancer, is an example where a second mutation reinstated the BRCA2 function making it resistant to cisplatin (PEO-4 cells). It has also been described earlier that cisplatin/ AG14361 selection of PEO-1 led to restoration of BRCA2 due to another secondary mutation.26 In the current study, it has clearly been shown that plumbagin treatment caused cell growth inhibition and induced apoptosis in both PEO-1 and PEO-4 cells, independent of their BRCA2 status. However, BRCA2 positive OVCAR-5 cells were found to be comparatively resistant to plumbagin compared to PEO cells. Hypoxia is one of the key regulators in tumor growth and survival. Tumor hypoxia is also associated with poor prognosis and resistance to therapy.27 Importantly, in our study we have demonstrated that plumbagin could inhibit the ovarian cancer cell growth even in hypoxia.
Mitogen-activated protein kinases (MAPKs), including extracelluar signal-regulated kinase 1/2 (ERK1/2), c-Jun NH2-terminal kinase (JNK) and p38, are a family of central signaling molecules that are known to participate in cell survival.28 Previous reports have shown that plumbagin treatment causes cytotoxicity by the induction of MAPK phosphorylation through ROS generation in a variety of cancer cell lines.6, 29-31 It has also been described that plumbagin activates ERK1/2 via ROS.6, 31 In this study we found that plumbagin increased ERK1/2 or MAPK-42/44 phosphorylation in PEO-1, PEO-4, OVCAR-5, and normal ovarian OSE cells in normoxic as well as hypoxic condition, possibly through ROS generation.
As p53 is an important apoptosis inducer, we analysed the expression of p53 in normoxic and hypoxic condition. We found that in PEO cells, plumbagin could inhibit p53 expression irrespective of their oxygen status. It is not reported if the p53 in PEO cells are mutant or wild type. It has been described that plumbagin treatment caused p53 accumulation and subsequent cell cycle arrest in lung cancer cells.32 Being an oxidative stress inducer, plumbagin could also act in a p53 independent pathway. It has been described earlier that oxidative stress induces WAF1/CIP1 expression and arrests cell cycle progression through a mechanism that is independent of p53.33 It also has been reported that plumbagin specifically inhibits the p300 mediated acetylation of p53.34 Acetylation of p53 prevents it from ubiquitination. Another explanation for the decreased expression of p53 by plumbagin is that it might as well act as an antiestrogen.10 By using breast adenocarcinoma cell lines, antiestrogen treatment leads to a dramatic decrease in the p53 protein levels.35,36 Moreover, plumbagin did not seem to inhibit the p53 (wild-type) expression in normal OSE/HUVEC cells indicating that changes in p53 by plumbagin may be cancer cell specific. It seems that p53 deficiency is of some value at times and inhibiting this protein might minify adverse side effects of chemotherapy and radiotherapy.37 Mouse studies support this strategy, suggesting that many side effects of acute genotoxic insult might be avoided by a short-term inhibition of p53, without causing a substantial loss in tumor suppressor activity.38 Therefore, inhibition of p53 by plumbagin might be one of the important aspects, which might be useful for its anticancer activity. We have also shown that plumbagin also inhibits Mdm-2 expression and therefore p53 regulation by plumbagin is independent of Mdm-2 pathway, as Mdm2 is a negative regulator of p53.39-41 Moreover, our results also suggest that expression of the p53 mutant protein (loss of function) in OVCAR-5 cells42 might enhance their resistance to plumbagin treatment compared to PEO cells.
Since plumbagin is reported to inhibit VEGF expression,43, 44 we analyzed whether plumbagin could inhibit the expression of hypoxia-inducible factor (HIFα) subunit and subsequently HIF- regulated hypoxia response genes VEGF and Glut-145, 46, 47 expressions in ovarian cancer cells under both normoxia and hypoxia. PEO-1 and PEO-4 cells do not express HIF-1α but HIF-2α and OVCAR-5 cells express both. We observed that plumbagin could inhibit the HIF-2α expression, even upon hypoxic stress in PEO and OVCAR-5 cells. Plumbagin also effectively inhibited both VEGF and Glut-1 expressions in these ovarian cancer cells even under hypoxia. These are of immense importance as most of the cells in the tumor remains in hypoxic condition. It is known that VEGF stimulates VEGFR-2 phosphorylation, intracellular Ca2+ mobilization, MAPK-42/44 phosphorylation, and increases proliferation of endothelial cells.48, 49 We also observed that plumbagin interfered with the VEGF induced calcium mobilization, MAPK-42/44 phosphorylation and proliferation in endothelial cells. Plumbagin has a differential action in endothelial cells where there is an inhibition of MAPK-42/44 phosphorylation and induction of p53, whereas in ovarian cancer cells, there is induction of MAPK-42/44 phosphorylation and inhibition of p53 expression.
Ovarian cancer is the seventh-most common cancer among women in the United States. It occurs most frequently in women who are between 40 and 65 years of age. Among the chemotherapy agents most commonly used in the treatment of ovarian cancer are paclitaxel, and one of the platinum-type agents (carboplatin or cisplatin).50 In this study, we failed to develop xenograft model with PEO cells in mice. So we used OVCAR-5 ovarian cancer cells to establish subcutaneous model in female SCID mice. It is important to note that in our study, we have been able to get tumor inhibition while using half (1mg/kg/day) of the normal dose of the plumbagin.30, 43 We found that a single-agent treatment with plumbagin produced significant OVCAR-5 tumor growth inhibition in mice compared to the untreated group and we also found significant reduction of vascular structure.
Taken together, we have shown the novel property of plumbagin, which can sensitize the ovarian cancer cell growth in vivo through the inhibition of both cellular growth as well as angiogenesis.
Supplementary Material
Acknowledgements
This study was supported by an Overseas Associateship grant to PS by the Department of Biotechnology, Government of India (San No. 102/IFD/SAN/415/2009-10). This work is also supported by NIH Grants CA78383 and HL70567 and a generous gift from Bruce and Martha Atwater to DM. DM is a visiting professor of King Saudi University. We also acknowledge Dr. S. Langdon, Western General Hospital, University of Edinburgh, Edinburgh, United Kingdom and Cancer Research Technology Ltd, UK for the PEO1 and PEO4 cell lines.
Glossary
Abbreviations
- VEGF
Vascular Edothelial Growth Factor
- HUVECs
human umbilical vein endothelial cells
- BRCA1
Breast Cancer Susceptibility Gene-1
- Glut-1
Glucose transporter-1
Footnotes
Novelty and Impact Statements: In this study, the anti-proliferative and anti-angiogenic properties of plumbagin in BRCA2 deficient and BRCA2 proficient ovarian cancer cells have been analyzed. Our studies indicate that ovarian cancer cells are sensitive to plumbagin irrespective of BRCA2 status in normoxia as well as in hypoxia. This is the first ever study performed that investigates the effect of plumbagin on cellular growth, apoptosis, and different signaling pathways in hypoxic condition.
REFERENCES
- 1.Satchi-Fainaro R, Puder M, Davies JW, Tran HT, Sampson DA, Greene AK, Corfas G, Folkman J. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat Med. 2004;10:255–61. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- 2.Maeda N, Matsubara K, Yoshida H, Mizushina Y. Anti-cancer effect of spinach glycoglycerolipids as angiogenesis inhibitors based on the selective inhibition of DNA polymerase activity. Mini Rev Med Chem. 2011;11:32–8. doi: 10.2174/138955711793564042. [DOI] [PubMed] [Google Scholar]
- 3.Mizushina Y, Saito A, Horikawa K, Nakajima N, Tanaka A, Yoshida H, Matsubara K. Acylated catechin derivatives: inhibitors of DNA polymerase and angiogenesis. Front Biosci (Elite Ed) 2011;3:1337–48. doi: 10.2741/e337. [DOI] [PubMed] [Google Scholar]
- 4.Padhye S, Dandawate P, Yusufi M, Ahmad A, Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med. Res. Rev. 2010 doi: 10.1002/med.20235. doi: 10.1002/med.20235. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas G, Annab LA, Gopinath G, Banerji A, Srinivas P. Antisense blocking of BRCA1 enhances sensitivity to plumbagin but not tamoxifen in BG-1 ovarian cancer cells. Mol Carcinog. 2004;39:15–25. doi: 10.1002/mc.10164. [DOI] [PubMed] [Google Scholar]
- 6.Wang CC, Chiang YM, Sung SC, Hsu YL, Chang JK, Kuo PL. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008;259:82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA, Johnson JA, Greig NH, et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–26. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo Y, Mughal MR, Ouyang TG, Jiang H, Luo W, Yu QS, Greig NH, Mattson MP. Plumbagin promotes the generation of astrocytes from rat spinal cord neural progenitors via activation of the transcription factor Stat3. J Neurochem. 2010;115:1337–49. doi: 10.1111/j.1471-4159.2010.06780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai L, Liu J, Zhai D, Lin Q, He L, Dong Y, Zhang J, Lu B, Chen Y, Yi Z, Liu M. Plumbagin Inhibits Tumor Angiogenesis and Tumor Growth through VEGFR2-mediated Ras Signaling Pathway. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01532.x. DOI: 10.1111/j.1476-5381.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thasni KA, Rakesh S, Rojini G, Ratheeshkumar T, Srinivas G, Priya S. Estrogen-dependent cell signaling and apoptosis in BRCA1-blocked BG1 ovarian cancer cells in response to plumbagin and other chemotherapeutic agents. Ann Oncol. 2008;19:696–705. doi: 10.1093/annonc/mdm557. [DOI] [PubMed] [Google Scholar]
- 11.Foray N, Marot D, Gabriel A, Randrianarison V, Carr AM, Perricaudet M, Ashworth A, Jeggo P. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. Embo J. 2003;22:2860–71. doi: 10.1093/emboj/cdg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell SN, Kachnic LA. Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene. 2003;22:5784–91. doi: 10.1038/sj.onc.1206678. [DOI] [PubMed] [Google Scholar]
- 13.Godwin AK, Meister A, O’Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A. 1992;89:3070–4. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP, Schol DJ, Hilgers J, Leonard RCF, Smyth JF. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988;48:6166–72. [PubMed] [Google Scholar]
- 15.Liu P, Khurana A, Rattan R, He X, Kalloger S, Dowdy S, Gilks B, Shridhar V. Regulation of HSulf-1 expression by variant hepatic nuclear factor 1 in ovarian cancer. Cancer Res. 2009;69:4843–50. doi: 10.1158/0008-5472.CAN-08-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalli KR, Falowo OI, Bale LK, Zschunke MA, Roche PC, Conover CA. Functional insulin receptors on human epithelial ovarian carcinoma cells: implications for IGF-II mitogenic signaling. Endocrinology. 2002;143:3259–67. doi: 10.1210/en.2001-211408. [DOI] [PubMed] [Google Scholar]
- 17.Sinha S, Dutta S, Datta K, Ghosh AK, Mukhopadhyay D. Von Hippel-Lindau gene product modulates TIS11B expression in renal cell carcinoma: impact on vascular endothelial growth factor expression in hypoxia. J Biol Chem. 2009;284:32610–8. doi: 10.1074/jbc.M109.058065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichiorri F, Suh SS, Ladetto M, Kuehl M, Palumbo T, Drandi D, Taccioli C, Zanesi N, Alder H, Hagan JP, Munker R, Volinia S, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci USA. 2008;105:12885–90. doi: 10.1073/pnas.0806202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, et al. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest. 2000;80:99–115. doi: 10.1038/labinvest.3780013. [DOI] [PubMed] [Google Scholar]
- 20.Srinivas P, Gopinath G, Banerji A, Dinakar A, Srinivas G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol Carcinog. 2004;40(4):201–11. doi: 10.1002/mc.20031. [DOI] [PubMed] [Google Scholar]
- 21.Nair S, Nair RR, Srinivas P, Srinivas G, Pillai MR. Radiosensitizing effects of plumbagin in cervical cancer cells is through modulation of apoptotic pathway. Mol Carcinog. 2008;47:22–33. doi: 10.1002/mc.20359. [DOI] [PubMed] [Google Scholar]
- 22.Sakai W, Swisher EM, Jacquemont C, Chandramohan KV, Couch FJ, Langdon SP, Wurz K, Higgins J, Villegas E, Taniguchi T. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69:6381–6. doi: 10.1158/0008-5472.CAN-09-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgia Schäfer, Thorsten Cramer, Guntram Suske, Wolfgang Kemmner, Bertram Wiedenmann, Michael Höcker. Oxidative Stress Regulates Vascular Endothelial Growth Factor-A Gene Transcription through Sp1- and Sp3-dependent Activation of Two Proximal GC-rich Promoter Elements. J. Biol. Chem. 2003;278:8190–8198. doi: 10.1074/jbc.M211999200. [DOI] [PubMed] [Google Scholar]
- 24.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Wang L, Xing Z, Liu D, Sun J, Li X, Zhang Y. Status of bi- and multi-nuclear platinum anticancer drug development. Anticancer Agents Med Chem. 2010;10:272–82. doi: 10.2174/187152010791162270. [DOI] [PubMed] [Google Scholar]
- 26.Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, Villegas E, Jacquemon C, Farrugia DJ, Couch FJ, Urban N, Taniguchi T. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–20. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 28.Nickischer D, Laethem C, Trask OJ, Jr., Williams RG, Kandasamy R, Johnston PA. Development and implementation of three mitogen-activated protein kinase (MAPK) signaling pathway imaging assays to provide MAPK module selectivity profiling for kinase inhibitors: MK2-EGFP translocation, c-Jun, and ERK activation. Methods Enzymol. 2006;414:389–418. doi: 10.1016/S0076-6879(06)14022-7. [DOI] [PubMed] [Google Scholar]
- 29.Powolny AA, Singh SV. Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm Res. 2008;25:2171–80. doi: 10.1007/s11095-008-9533-3. [DOI] [PubMed] [Google Scholar]
- 30.Xu KH, Lu DP. Plumbagin induces ROS-mediated apoptosis in human promyelocytic leukemia cells in vivo. Leuk Res. 2010;34:658–65. doi: 10.1016/j.leukres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Yang SJ, Chang SC, Wen HC, Chen CY, Liao JF, Chang CH. Plumbagin activates ERK1/2 and Akt via superoxide, Src and PI3-kinase in 3T3-L1 cells. Eur J Pharmacol. 2010;638:21–8. doi: 10.1016/j.ejphar.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Gomathinayagam R, Sowmyalakshmi S, Mardhatillah F, Kumar R, Akbarsha MA, Damodaran C. Anticancer mechanism of plumbagin, a natural compound, on non-small cell lung cancer cells. Anticancer Res. 2008;28:785–92. [PubMed] [Google Scholar]
- 33.Russo T, Zambrano N, Esposito F, Ammendola R, Cimino F, Fiscella M, et al. A p53-independent pathway for activation of WAF1/CIP1 expression following oxidative stress. J Biol Chem. 1995;270:29386–91. doi: 10.1074/jbc.270.49.29386. [DOI] [PubMed] [Google Scholar]
- 34.Ravindra KC, Selvi BR, Arif M, Reddy BA, Thanuja GR, Agrawal S, Pradhan SK, Nagashayana N, Dasgupta D, Kundu TK. Inhibition of lysine acetyltransferase KAT3B/p300 activity by a naturally occurring hydroxynaphthoquinone, plumbagin. J Biol Chem. 2009;284:24453–64. doi: 10.1074/jbc.M109.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillot C, Falette N, Courtois S, Voeltzel T, Garcia E, Ozturk M, et al. Alteration of p53 damage response by tamoxifen treatment. Clin Cancer Res. 1996;2:1439–44. [PubMed] [Google Scholar]
- 36.Mi Q, Kim S, Hwang BY, Su BN, Chai H, Arbieva ZH, et al. Silvestrol regulates G2/M checkpoint genes independent of p53 activity. Anticancer Res. 2006;26:3349–56. [PubMed] [Google Scholar]
- 37.Berns A. Cancer biology: can less be more for p53? Nature. 2006;443:153–4. doi: 10.1038/443153a. [DOI] [PubMed] [Google Scholar]
- 38.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 39.Feng J, Tamaskovic R, Yang Z, Brazil DP, Merlo A, Hess D, et al. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J Biol Chem. 2004;279:35510–7. doi: 10.1074/jbc.M404936200. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299–1303. doi: 10.1038/sj.onc.1205181. [DOI] [PubMed] [Google Scholar]
- 41.Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–50. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 42.Astanehe A, Arenillas D, Wasserman WW, Leung PC, Dunn SE, Davies BR, Mills GB, Auersperg N. Mechanisms underlying p53 regulation of PIK3CA transcription in ovarian surface epithelium and in ovarian cancer. J Cell Sci. 2008;121:664–674. doi: 10.1242/jcs.013029. [DOI] [PubMed] [Google Scholar]
- 43.Aziz MH, Dreckschmidt NE, Verma AK. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008;68:9024–32. doi: 10.1158/0008-5472.CAN-08-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–33. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 45.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 46.Behrooz A, Ismail-Beigi F. Dual control of glut1 glucose transporter gene expression by hypoxia and by inhibition of oxidative phosphorylation. J Biol Chem. 1997;272:5555–62. doi: 10.1074/jbc.272.9.5555. [DOI] [PubMed] [Google Scholar]
- 47.Wang GL, Chen CH, Pore N, Behrooz A, Ismail BF, Maity A. Regulation of Glut-1 mRNA by hypoxia inducible factor-1: interaction between H-ras and hypoxia. JBC. 2001:9519–9525. doi: 10.1074/jbc.M010144200. 1995. [DOI] [PubMed] [Google Scholar]
- 48.Petrova TV, Makinen T, Alitalo K. Signaling via vascular endothelial growth factor receptors. Exp Cell Res. 1999;253:117–30. doi: 10.1006/excr.1999.4707. [DOI] [PubMed] [Google Scholar]
- 49.Zeng H, Sanyal S, Mukhopadhyay D. Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J Biol Chem. 2001;276:32714–9. doi: 10.1074/jbc.M103130200. [DOI] [PubMed] [Google Scholar]
- 50.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.