Abstract
Growth factors are critical in neurodevelopment and neuroplasticity, and recent studies point to their involvement in addiction. We previously reported increased levels of basic fibroblast growth factor (FGF2) in high novelty/drug-seeking rats (bred High Responders, bHR) compared to low novelty/drug-seeking rats (bred Low Responders, bLRs). The present study asked whether an early life manipulation of the FGF system (a single FGF2 injection on postnatal day 2) can impact cocaine sensitization and associated neurobiological markers in adult bHR/bLR animals. Neonatal FGF2- and vehicle-treated bHR/bLR rats were sensitized to cocaine (7 daily injections, 15 mg/kg/day, i.p.) in adulthood. Neonatal FGF2 markedly increased bLRs’ typically low psychomotor sensitization to cocaine (day 7 locomotor response to cocaine), but had little effect on bHRs’ cocaine sensitization. Gene expression studies examined dopaminergic molecules as well as FGF2 and the FGFR1 receptor in cocaine naïve animals, to investigate possible neurobiological alterations induced by neonatal FGF2 exposure that may influence behavioral response to cocaine. bLRs showed decreased tyrosine hydroxylase in the ventral tegmental area (VTA), decreased D1 and increased D2 receptor expression in the nucleus accumbens core, as well as decreased FGF2 in the VTA, substantia nigra, accumbens core, and caudate putamen compared to bHRs. Neonatal FGF2 selectively increased D1 receptor and FGF2 mRNA in the accumbens core of bLRs, which may contribute to their heightened cocaine sensitization. Our results suggest increased FGF2 in the mesodopaminergic circuit (as in baseline bHRs and neonatal FGF2-exposed bLRs vs. baseline bLRs) enhances an individual’s susceptibility to cocaine sensitization and may increase vulnerability to drug seeking and addiction.
Keywords: bred High Responder, bred Low Responder, dopamine, FGF2, nucleus accumbens, cocaine
1. Introduction
Emerging evidence suggests that growth factors may contribute to the neurobiological mechanisms relevant to substance abuse and the development of addiction (Graham et al., 2007). For instance, manipulating brain-derived neurotrophic factor (BNDF) in mesolimbic areas influences cocaine-seeking behavior (Bahi et al., 2008, Berglind et al., 2007, Berglind et al., 2009, Liu et al., 2011, Lobo et al., 2010, Schoenbaum et al., 2007). Likewise, glial-derived neurotrophic factor (GDNF) shapes drug-taking propensity, with GDNF infusion into the ventral tegmental area (VTA) blocking cocaine’s rewarding effects, and GDNF blockade enhancing cocaine self-administration (Ron and Janak, 2005). However, growth factors are also critical in neural development, and relatively less is known about their developmental role in determining addiction vulnerability.
We previously developed a model of differential responsiveness to drugs of abuse by breeding rat lines that exhibit distinct behavioral sensitivity to cocaine-- the selectively-bred High Novelty Responder (bHR) versus Low Novelty Responder (bLR) rats (Cummings et al., 2011, Davis et al., 2008, Flagel et al., 2010, Garcia-Fuster et al., 2009, Garcia-Fuster et al., 2010). bHR rats show an enhanced proclivity to addictive behavior (e.g. cocaine self-administration and sensitization), while bLRs show low behavioral response to psychostimulants. The present study uses these rat lines to ask whether the animals also exhibit inborn differences in a growth factor family that has been implicated in addiction, and whether manipulating a member of this family can alter acute and/or sensitized behavioral response to cocaine, particularly in the bLR animals known to exhibit low levels of addiction-like behavior.
Fibroblast growth factor 2 (FGF2) has been implicated in the development of psychostimulant sensitization in adult animals (Flores et al., 2000, Flores and Stewart, 2000a, b). Blocking FGF2 in the VTA during the induction of amphetamine sensitization completely blocks the development of behavioral sensitization (Flores et al., 2000). But, is the role of FGF2 in sensitization primarily as mediator of downstream effects of chronic cocaine, or do naturally-occurring differences in FGF2 expression shape an individual’s behavioral response to cocaine?
We previously focused on the FGF family due to its profound perturbation in major depression (Evans et al., 2004). However, more recent efforts have uncovered a broader role for the FGF system in anxiety-related mechanisms (Perez et al., 2009) as well as hedonistic and reward-related pathways (Turner et al., 2009). We reported profound baseline differences in hippocampal FGF2 (Perez et al., 2009) and FGFR1 (Turner et al., 2008) in our bHR/bLR rats, with bHRs showing higher FGF levels compared to bLRs. But do these differences extend to brain circuitry classically implicated in drug reward? And, does altering the FGF2 system during development impact bHR and/or bLR’s behavioral response to cocaine?
Prior work showed that early life manipulation of the FGF system (a single systemic injection of FGF2 on the second day of life) induces life-long changes in hippocampal neurogenesis (Cheng et al., 2001, Wagner et al., 1999), structure, and gene expression (Turner et al., 2011). Interestingly, another study by our group found that this early life FGF2 manipulation produced “HR-like” rats that showed a slightly enhanced behavioral response to novelty and enhanced cocaine self-administration (Turner et al., 2009). A drawback of this earlier work was that it used commercially-available (non-selectively bred) rats, so we had no idea of whether pups were naturally inclined to grow up and exhibit a high novelty-seeking/cocaine sensitive (bHR-like) phenotype versus a low novelty-seeking/less cocaine-sensitive (bLR-like) phenotype. Thus, the present study enabled us to do two things. First, it allowed us to test whether this neonatal FGF2 treatment enhanced cocaine sensitization (in addition to increasing cocaine self-administration as previously shown (Turner et al., 2009)). Secondly, this new experiment also allowed us to determine whether the treatment differentially impacted individuals with distinct predispositions to addictive behavior. We hypothesized that neonatal FGF2 administration would enhance cocaine sensitization, particularly in bLRs known to exhibit both low basal FGF levels (at least in adulthood) as well as low cocaine self-administration and sensitization. Moreover, we predicted that neonatal FGF2 treatment may exert these effects via an altered mesolimbic dopamine system. We therefore conducted in situ hybridization studies in adult brains of neonatal FGF2- and vehicle-treated bHR/bLR rats to determine how this manipulation impacted dopamine and FGF system markers in the striatum, VTA, and substantia nigra.
2. Methods and Materials
All experiments were approved by the University Committee on the Use and Care of Animals at the University of Michigan and were conducted in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals, dictated by the National Research Council in 1996.
2.1 Animals
bHR/bLR male Sprague-Dawley rats were acquired from the 26th generation of our colony. We previously published a description of our breeding strategy and initial behavioral characterization of the bHR/bLR lines (Stead et al., 2006), and have continued to examine the many facets of the bHR-bLR behavioral phenotypes (Flagel et al., 2010). Rats were pair-housed housed in a 12:12 light–dark cycle (lights on/off at 6a.m./6p.m.) with food and water available ad libitum.
2.2 Neonatal FGF2 Injections
On the day after birth, postnatal day (P) 2, bHR/bLR litters were culled to 5 males and 5 females. Half of the litters were injected with FGF2 (Sigma-Aldrich, F0291; 20ng/g in 50μl in 0.1M PBS w/1% BSA, s.c.) and the other litters were injected with vehicle (0.1M PBS w/1% BSA, s.c.) into the axillary space. We chose to inject the axillary space because this is the largest subcutaneous space on a rat pup, and because this methodology was consistent with prior studies using neonatal FGF2 treatment (Cheng et al., 2001, Turner et al., 2009, Turner et al., 2011, Wagner et al., 1999). FGF2 delivered in this manner effectively crosses the blood brain barrier (Tao et al., 1996, Wagner et al., 1999), and has been shown to increase hippocampal neurogenesis (Cheng et al., 2001, Wagner et al., 1999), produce life-long changes in hippocampal gene expression patterns (Turner et al., 2011), and also improve anxiety-like behavior (Turner et al., 2011). Importantly, a prior study in commercially purchased (non-selectively bred) rats showed that this timing, dose and single-exposure of FGF2 lead animals to exhibit enhanced cocaine self-administration compared to vehicle-exposed rats (Turner et al., 2009). We therefore hypothesized in the present study that this same treatment regimen would enhance cocaine sensitization.
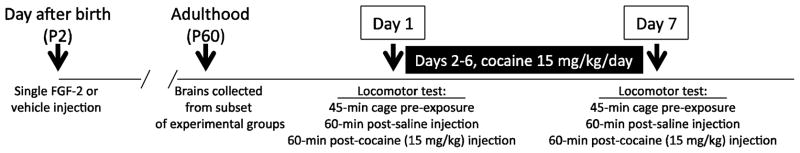
Although only male offspring were used for subsequent studies, litters were balanced by sex, and both male and female pups were injected with FGF2 or vehicle to protect against possible confounding effects on maternal care. All animals were weaned on P21, with males being housed 2–3 per cage for the duration of the experiment. Figure 1 depicts the timeline for neonatal FGF2 treatment and subsequent experimental manipulations.
Figure 1.
Experimental timeline for the neonatal FGF2 treatment and brain collection and behavioral studies in adult animals. On the day after birth (postnatal day (P) 2), bHR and bLR litters were randomly assigned to receive an injection of FGF2 (20ng/g in 50μl in 0.1M PBS w/1% BSA, s.c.) or vehicle (0.1M PBS w/1% BSA, s.c.). Once animals reached adulthood (P60), brains were collected from a subset of animals from each experimental group. The remaining animals were sensitized to cocaine using a paradigm that previously revealed bHR/bLR behavioral differences. On the first day of the paradigm, animals were placed in a novel test cage. Horizontal and rearing movements were monitored in 5-min intervals over three test phases: (1) a 45-min chamber pre-exposure period; (2) 60-min following saline injections; and (3) 60-min following cocaine injection (15 mg/kg, i.p.). Once a day for the next 5 days, rats received cocaine injections (15 mg/kg, i.p.) in a room adjacent to the housing quarters and then returned to home cages. On day 7, rats returned to the activity chambers to again monitor activity during the chamber pre-exposure, post-saline and post-cocaine injection periods.
2.3 Cocaine sensitization paradigm
Once animals reached adulthood (P60), a subset of animals (N=12/group) was sensitized to cocaine using a paradigm that previously resulted in bHR/bLR behavioral differences (Garcia-Fuster et al., 2010). On the first test day, rats were individually placed in clear acrylic cages (43 × 21.5 × 25.5 cm high) equipped with infrared photocell emitters to record movement. The testing room was separate from housing quarters, and rats were first exposed to the test chamber on this initial test day. Horizontal and rearing movements were monitored in 5-min intervals over three test phases: (1) a 45-min chamber pre-exposure period; (2) 60-min following saline injection; and (3) 60-min following cocaine injection (15 mg/kg, i.p.). Our subsequent data analysis examined horizontal and rearing movements as well as total activity scores, which were calculated by summing rearing and horizontal movements per 5-min as well as across an entire test phase. Once a day for the next 5 days, rats received cocaine injections (15 mg/kg, i.p.) in a room adjacent to the housing quarters and then returned to home cages. On day 7, rats returned to the activity chambers to again monitor locomotor activity during the chamber pre-exposure, post-saline and post-cocaine injection periods.
2.4 Data analysis for cocaine sensitization study
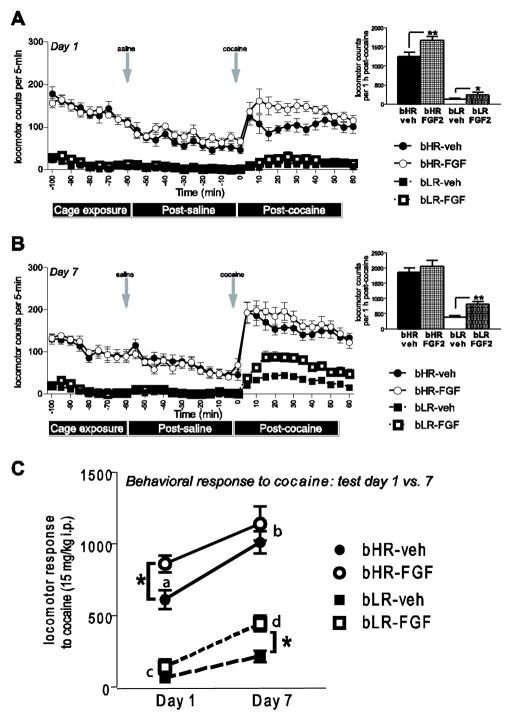
We first calculated total locomotor activity scores in 5-min intervals across the three test phases by summing an animal’s lateral and rearing movements per 5-min interval on test days 1 and 7 (Fig. 2A–B). We also calculated total locomotor activity over the 60-min period following cocaine injection on each test day 1 (acute behavioral response to cocaine; inset graph Fig. 2A) and test day 7 (sensitized response to cocaine; inset graph Fig. 2B). The graph in Fig. 2C illustrates the change in cocaine-induced total locomotor activity on test days 1 versus 7, indicating the degree of cocaine sensitization that develops in response to daily cocaine treatment. We also separately analyzed cocaine-induced rearing and lateral movements on test days 1 and 7 (Fig. 3). We assessed total cocaine-induced locomotor activity for consistency with several prior studies in our laboratory (e.g. (Flagel et al., 2008, Garcia-Fuster et al., 2010, Kabbaj et al., 2002, Wei et al., 2012)). However, given the fact that other work has demonstrated how cocaine can differentially impact rearing versus horizontal movement (Szumlinski et al., 2003, Walker et al., 2001), we also wanted to separately examine these measures.
Figure 2.
Locomotor activity during chamber pre-exposure, post-saline and post-cocaine test periods. On each test day, locomotor activity was monitored during a 45-min chamber pre-exposure period, 60-min following a saline injection, and 60-min following a cocaine injection. On test day 1 (when animals were first exposed to cocaine), bHRs were markedly more active than bLRs across test phases (A). Neonatal FGF2 exposure produced an enhanced behavioral response to acute cocaine in both phenotypes (inset A). On test day 7 (following a week of cocaine treatment), neonatal FGF2 treatment specifically increased bLRs’ locomotor response to cocaine, indicating enhanced cocaine sensitization compared to vehicle-treated bLRs (B and inset). Although all bHR and bLR rats became sensitized to cocaine by day 7 (showing enhanced cocaine-induced activity on day 1 versus 7; indicated by “a” versus “b” for bHR and “c” versus “d” for bLR), neonatal FGF2 treatment lead bLRs to exhibit a greatly enhanced cocaine sensitization compared to neonatal vehicle-exposed bLRs (C). n=12 for each experimental group; ** indicates p<0.01; * indicates p<0.05
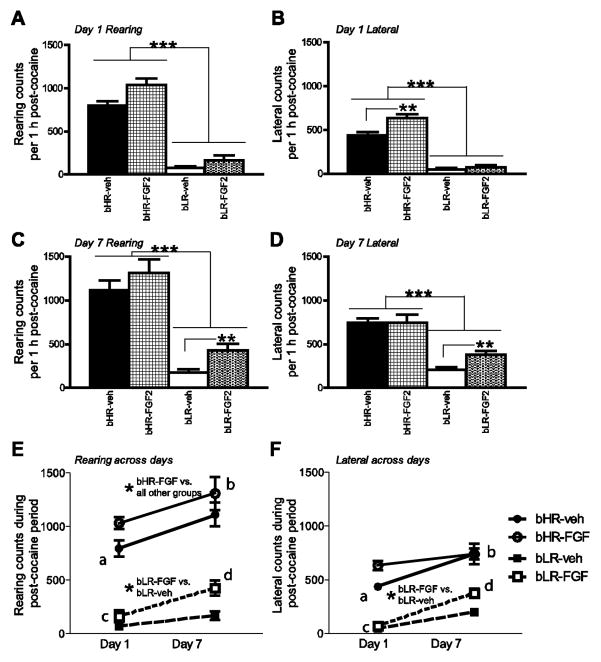
Figure 3.
Cocaine-induced rearing and lateral movement on test days 1 and 7. As an additional analysis, we examined cocaine-induced activity by separately evaluating rearing and lateral movement. On test day 1, bHRs showed dramatically more cocaine-induced rearing (A) and lateral (B) movement compared to bLRs. Early life FGF exposure increased acute cocaine-induced rearing movement in both groups (A), and selectively increased cocaine-induced lateral movement in bHRs (B). On test day 7 (following a week of cocaine treatment), bHRs again showed greater sensitized rearing (C) and lateral movement (D) compared to bLRs. However, neonatal FGF2 treatment specifically impacted bLRs’ cocaine sensitization, leading FGF2-exposed bLRs to exhibit greater sensitized rearing and lateral movement compared to vehicle-exposed bLRs (C–D). We evaluated cocaine sensitization by comparing animals’ rearing (E) and lateral movement (F) on test day 1 versus 7. All bHR and bLR rats became sensitized to cocaine by day 7, showing enhanced cocaine-induced rearing and lateral movement on day 1 versus 7 (indicated by “a” versus “b” for bHR and “c” versus “d” for bLR). For cocaine-induced rearing behavior, neonatal FGF2-treated bHRs showed more rearing compared to all other groups, and neonatal FGF2-treated bLRs exhibited more rearing compared to vehicle-exposed bLRs (E). Similarly, for cocaine-induced lateral movement, neonatal FGF2-treated bLRs exhibited more lateral movement compared to vehicle-exposed bLRs (F). n=12 for each experimental group; *** indicates p<0.0001; ** indicates p<0.01; * indicates p<0.05
2.5 In Situ Hybridization
A parallel group of adult bHR/bLR animals exposed to neonatal FGF2 (or vehicle) injection was used for a series of in situ hybridization studies to assess the long-term effects of this neonatal FGF2 treatment on gene expression differences in the mesolimbic reward system (without having cocaine on board). The animals used were naïve of any behavioral testing and were never exposed to cocaine to eliminate a possible confounding effect of these manipulations. Moreover, we felt this would allow us to, in a sense, determine what molecular antecedents were present in the brains of bHR/bLR rats exposed to neonatal FGF2 prior to cocaine sensitization. We focused on two broad classes of molecules: a) genes relevant to dopamine neurotransmission; and b) key genes in the FGF system. We examined tyrosine hydroxylase (TH), the dopamine transporter (DAT), and D2 dopamine receptor mRNA expression in the substantia nigra pars compacta (SNc) and VTA, as well as D1 and D2 dopamine receptor transcript expression in the caudate putamen and nucleus accumbens core and shell; and we also assessed the expression of FGF2 and its primary receptor, FGF receptor 1 (FGFR1) in these same brain regions.
At P60, rats (n=8/treatment group) were sacrificed by rapid decapitation between 9:00am–11:00am. Brains were removed, snap frozen, and stored at −80° C until processing for in situ hybridization. Brains were cryostat sectioned at 12 μm, and sections were immediately thaw-mounted onto Fisherbrand Superfrost/Plus microscope slides (Fisher Scientific, www.fishersi.com). Sections taken at 240 μm intervals from the frontal cortex through the midbrain were prepared for in situ hybridization as previously described (Clinton et al., 2008). These studies were conducted with 35S-labeled cRNA probes for each of the following genes: TH (length 274, accession # M10244, pos. 89–363); DAT (length 532 nt., accession # M80233, pos. 719–1251), D1 (length 480 nt., accession # M35077, pos. 420–900), D2 (length 495 nt., accession # M36831, pos. 865–1360), FGF2 (length 278 nt., accession # NM019305, pos. 716–994), or FGFR1 (length 657 nt., accession # NM024146, pos. 320–977). Following processing, slides were apposed to Kodak XAR film (Eastman Kodak, Rochester, NY) for a period of time that was optimized for each probe for each brain region to be analyzed. Accordingly, films for TH were exposed for 40 h, DAT for 40 h, D1 for 7 days, and D2 for 7 days. For FGF2, films were exposed for 56 days, and for FGFR1 films were exposed for 35 days.
Autoradiograms were digitized using a ScanMaker 1000XL Pro (Microtek, Carson, CA) with LaserSoft Imaging software (AG, Kiel, Germany). Digitized images were analyzed using ImageJ Analysis Software for PC (http://rsbweb.nih.gov/ij/). Specific signal, defined as 3.5 × the standard deviation of individual pixel signal values above mean background signal, was converted to optical density and multiplied by the area of signal to produce integrated optical density (IOD). Signal and IOD measurements for all 6 probes were taken across the entire rostro-caudal extent of each brain region.
2.6 Statistical Analyses
For the two behavioral test days (day 1 and 7), group differences in the total locomotor response to a) placement in the test cage; b) saline injection; and c) cocaine injection were separately examined by three-way ANOVAS – a repeated measures ANOVA with 5-min time interval as the within subject factor and bHR/bLR phenotype and FGF2 treatment as between subject factors (as in Fig. 2A–B). Rearing and lateral movement in response to cocaine injection were also examined in this way. To assess cocaine sensitization, we compared locomotor response (total locomotion, rearing, or lateral movement) to cocaine on day 1 versus day 7. Here we again used a three-way ANOVA - repeated measures ANOVA with test day as the within subject factor and bHR/bLR phenotype and FGF2 treatment as between subject factors (as in Fig. 2C and Fig. 3E–F). For the in situ hybridization studies, each gene of interest and each brain area of interest was analyzed separately. Here we used repeated measures ANOVA with rostro-caudal level of a given area of interest as within subject factor and bHR/bLR phenotype and FGF2 treatment included as between subject factors. ANOVAs were followed by Fisher’s post-hoc comparisons when necessary. All data were analyzed using Statview 5.0.1 for Windows, and for all tests, α=0.05.
3. Results
3.1 Neonatal FGF2 enhanced acute behavioral response to cocaine
On test day 1, there was a main effect of phenotype, with bHRs showing greater total locomotor activity compared to bLRs during chamber pre-exposure (F1, 52 = 415.01, p<0.0001), post-saline (F1, 52 = 188.73, p<0.0001), and post-cocaine (F1, 52 = 210.32, p<0.0001) test phases (n=12 per experimental group; Fig 2A). Interestingly, neonatal FGF2 treatment enhanced acute locomotor response to cocaine (main effect of treatment, F1, 52 = 9.94, p<0.01; inset Fig. 2A). Although there was no significant phenotype x treatment interaction, this effect was most obvious in bHR rats (inset Fig. 2A). There was no effect of neonatal FGF2 treatment and no phenotype x FGF2 interaction on total locomotor activity during the pre-cage exposure and post-saline test phases.
We had similar results when separately analyzing rearing and lateral movement data (Fig. 3A–B). On test day 1 (acute response to cocaine), there was a main effect of phenotype, with bHRs showing greater rearing in response to their initial cocaine exposure (F1, 52 = 132.30, p<0.0001). There was a main effect of neonatal FGF2 treatment (F1, 52 = 5.69, p<0.05) on rearing; although there was no significant phenotype x treatment interaction, this effect was most obvious in bHR rats (Fig. 3A). For day 1 acute cocaine-induced lateral movement, there was a main effect of bHR/bLR phenotype (F1, 52 = 250.31, p<0.0001), neonatal FGF2 treatment (F1, 52 = 13.74, p<0.01), and a significant phenotype x treatment interaction (F1, 52 = 8.14, p<0.05). bHRs clearly showed more acute cocaine-induced lateral movement compared to bLRs (p<0.0001), and neonatal FGF-treated bHRs exhibited greater lateral movement compared to vehicle-exposed bHRs (p<0.05; Fig. 3B).
3.2 Neonatal FGF2 enhanced cocaine sensitization particularly in bLRs
On test day 7 (sensitized response to cocaine), there was again a main effect of phenotype, with bHRs showing much greater total locomotor activity compared to bLRs across all test phases -- chamber pre-exposure (F1, 52 = 320.91, p<0.0001), post-saline injection (F1, 52 = 152.65, p<0.0001), and post-cocaine (F1, 52 = 111.08, p<0.0001) (Fig. 2B). On test day 7, there was no effect of FGF2 treatment and no FGF2 x phenotype interaction for the cage pre-exposure or post-saline test phases. While there was no main effect of FGF2 treatment on day 7 post-cocaine locomotor activity, there was an FGF2 treatment x phenotype interaction (F1, 52 = 5.92, p<0.005). Post-hoc analysis showed that the FGF2 treatment specifically affected bLR rats, with neonatal FGF2-treated bLRs showing greater cocaine-induced activity compared to vehicle-treated bLRs (p<0.05). Both bHR groups showed similar levels of cocaine-induced locomotion (Fig. 1B and inset).
We assessed cocaine sensitization by comparing animals’ total locomotor response to cocaine on day 1 (their initial cocaine exposure) versus day 7 (after a week of cocaine treatments; Fig. 2C). By test day 7, all bHR and bLR animals were sensitized to cocaine, showing enhanced cocaine-induced activity compared to day 1 (Fig. 2C, main effect of test day, F1, 52 = 49.80, p<0.0001). Overall, bHRs showed greater cocaine-induced activity compared to bLRs (effect of phenotype, F1, 52 = 215.88, p<0.0001). There was also a main effect of neonatal FGF treatment (effect of FGF2 exposure, F1, 52 = 10.87, p<0.01) as well as a significant phenotype x neonatal FGF2 x test day interaction (F1, 52 = 4.72, p<0.05). Post hoc analysis separately examined bHR and bLR groups to determine how neonatal FGF2 differentially affected their behavior. Both bHR groups (FGF2- and vehicle-exposed) were sensitized to cocaine, showing enhanced cocaine-induced activity on day 7 versus 1 (main effect of test day F1, 26 = 17.48, p<0.001). However, there was no significant effect of FGF2 treatment on bHRs’ behavior, and no FGF2 x test day interaction. Both bLR groups (FGF2- and vehicle-treated) were sensitized to cocaine, showing enhanced cocaine-induced activity on day 7 versus 1 (main effect of test day F1, 26 = 73.06, p<0.0001). There was also a main effect of neonatal FGF2 exposure (F1, 26 = 10.59, p<0.01), and a significant test day x FGF2 treatment interaction, with neonatal FGF2 treatment leading to enhanced cocaine sensitization in FGF2-exposed bLRs compared to vehicle-exposed bLRs (p<0.01; evident in inset graph Fig. 2B and Fig. 2C).
We observed similar effects when separately analyzing sensitized rearing and lateral movements on test day 7 (Fig. 3C–F). For day 7 cocaine-induced rearing, we found a significant effect of bHR/bLR phenotype (F1, 52 = 77.48, p<0.0001), with bHRs showing greater sensitized rearing on test day 7 compared to bLRs. There was no effect of neonatal FGF treatment on day 7 cocaine-induced rearing, but a significant phenotype x treatment interaction (F1, 52 = 4.79, p<0.05); post-hoc analysis showed that neonatal FGF2 treatment specifically increased bLRs’ rearing behavior compared to vehicle-treated bLRs (p<0.05; Fig. 3C). For day 7 cocaine-induced lateral movement, we found a significant effect of bHR/bLR phenotype (F1, 52 = 56.47, p<0.0001), with bHRs showing greater sensitized lateral movement on test day 7 compared to bLRs. There was no effect of neonatal FGF treatment on sensitized lateral movement, but a significant phenotype x treatment interaction (F1, 52 = 2.79, p<0.05); post-hoc analysis showed that neonatal FGF2 treatment specifically increased bLRs’ lateral movement compared to vehicle-treated bLRs (p<0.05; Fig. 3D).
Finally, we compared rearing and lateral movement in bHR/bLR rats on test day 1 versus day 7 (Fig. 3E–F). By test day 7, all bHR and bLR animals showed increased cocaine-induced rearing and lateral movement compared to day 1 (main effect of test day on rearing, F1, 52 = 19.04, p<0.0001, Fig. 3E; on lateral movement F1, 52 = 64.14, p<0.0001, Fig. 3F). Overall, bHRs showed greater cocaine-induced rearing and lateral movement compared to bLRs (main effect of phenotype on rearing F1, 52 = 155.54, p<0.0001; on lateral movement F1, 52 = 143.18, p<0.0001). Although there was no main effect of neonatal FGF treatment, there was a treatment x phenotype interaction (rearing F1, 52 = 8.20, p<0.01; lateral movement F1, 52 = 6.61, p<0.05). There was no significant phenotype x neonatal FGF2 x test day interaction. For cocaine-induced rearing behavior, the post hoc analysis, which compared all bHR and bLR groups collapsed across test days, showed that neonatal FGF2-treated bHRs showed significantly higher rearing compared to all other groups (p<0.05 compared to vehicle-treated bHR; p<0.0001 compared to bLR groups). Vehicle-exposed bHRs showed more rearing compared to the bLR groups (p<0.0001), and the neonatal FGF2-exposed bLRs showed greater rearing compared to vehicle-treated bLR (p<0.05). For cocaine-induced lateral movement, the post hoc analysis showed that vehicle and neonatal FGF2-treated bHRs showed significantly higher rearing compared to the bLR groups (p<0.0001 for group comparisons). Neonatal FGF2-exposed bLRs showed greater lateral movement compared to vehicle-treated bLR (p<0.05).
3.3 Impact of bHR/bLR phenotype and neonatal FGF2 on mesolimbic dopamine system
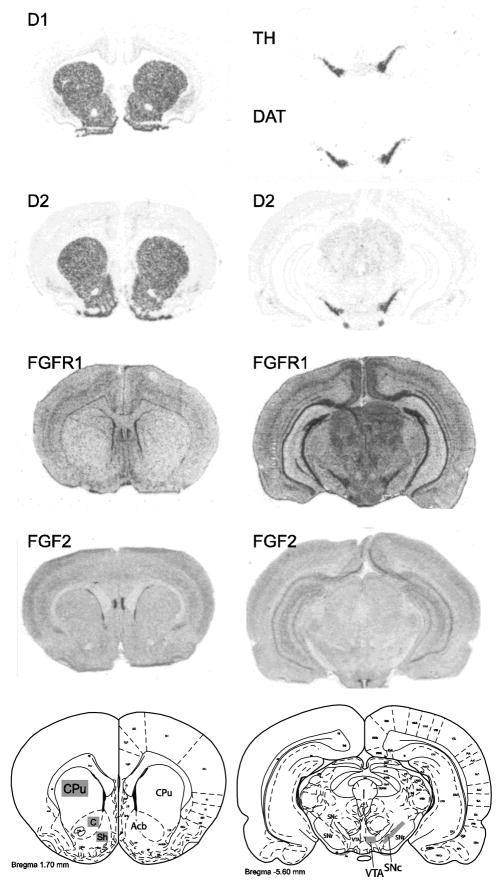
Since neonatal FGF2 treatment enhanced cocaine sensitization in bLRs, we examined how the treatment impacted components of the mesolimbic dopamine system to determine a possible neonatal FGF2-induced neurochemical change that might contribute to the observed behavioral findings. These studies were conducted in cocaine naïve animals (n=8 per experimental group) to avoid confounding effects of behavioral testing and cocaine treatment on gene expression. Figure 4 shows brain sections processed by in situ hybridization to detect the expression of D1 and D2 dopamine receptor mRNAs in the nucleus accumbens and caudate putamen, and TH, DAT, and D2 receptor mRNA in the VTA and SNc.
Figure 4.
Expression of dopamine and FGF system markers in mesolimbic brain structures. Left panels show autoradiograms from representative tissue sections through the caudate putamen (CPu) and nucleus accumbens core and shell (AcbC and AcbSh) processed by in situ hybridization with antisense riboprobes against rat dopamine receptor D1 and D2, FGF receptor 1 (FGFR1), or FGF2 mRNA. Right panels show autoradiograms from sections through the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA), processed for rat tyrosine hydroxylase (TH), dopamine transporter (DAT), D2, FGFR1, or FGF2. Since FGFR1 and FGF2 expression levels were relatively low in the VTA and SNc, the TH signal from adjacent sections was used to ensure precise identification of and measurement within these regions. Bottom panels indicate location of templates used to sample integrated optical density measurements within specific brain regions.
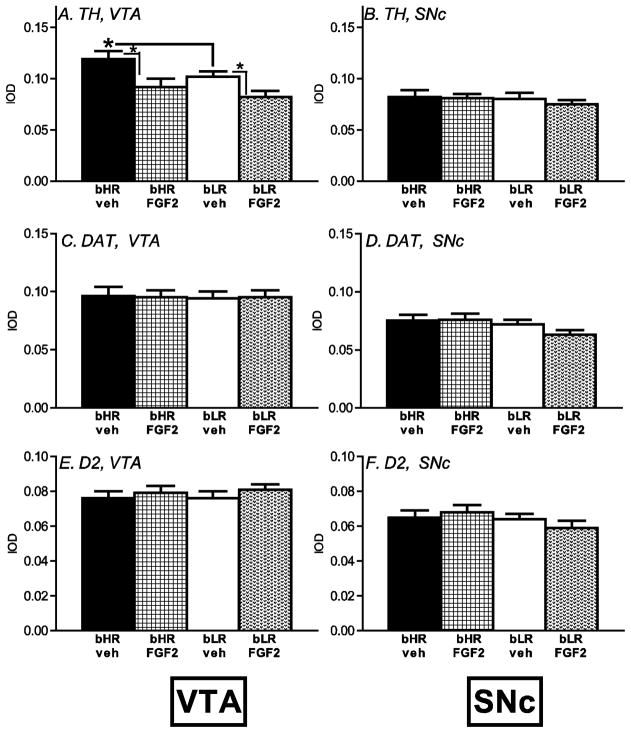
VTA and SNc
D2, DAT and TH transcripts were measured in the VTA and SNc of neonatal vehicle- and FGF2-treated bHR/bLR rats. bLRs exhibited lower TH levels in the VTA compared to bHRs (effect of phenotype [F1, 59 = 6.78, p<0.01]), and FGF2 treatment reduced TH levels in the VTA of both strains (effect of treatment [F1, 59 = 3.77, p<0.05]; Fig. 5A). There was no phenotype x FGF2 treatment interaction on this measure. All groups showed similar TH expression in the SNc (Fig. 5A–B). For DAT (Fig. 5C–D) and D2 (Fig. 5E–F) in the VTA and SNc, there were no effects of bHR/bLR phenotype or neonatal FGF2 treatment, and no significant phenotype x FGF2 treatment interactions.
Figure 5.
Midbrain dopamine marker expression in bHR and bLR rats exposed to neonatal FGF2. Tyrosine hydroxylase (TH), dopamine receptor D2, and the dopamine transporter (DAT) mRNA expression was assessed in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) of bHR and bLR animals exposed to neonatal FGF2 or vehicle injection (n=8 per experimental group). At baseline, bLRs showed reduced TH in the VTA compared to bHRs, and neonatal FGF2 treatment reduced TH expression in the VTA of both strains (A). There were no TH differences in the SNc (B), and no differences in D2 or DAT in the VTA or SNc (C–D). * indicates p<0.05
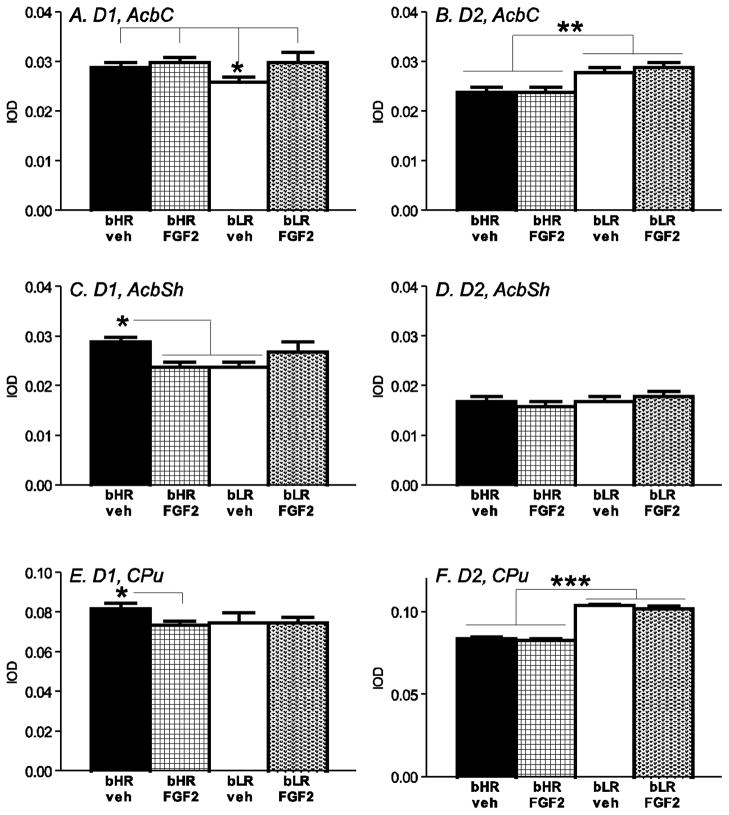
Nucleus accumbens
D1 and D2 dopamine receptor mRNAs were assessed in the nucleus accumbens core (Fig. 6A–B) and shell (Fig. 6C–D). For D1 mRNA in the accumbens core, there was no effect of phenotype, and no effect of neonatal FGF2 treatment. However, there was a phenotype x FGF2 interaction [F1, 217 = 3.70, p<0.05]. Post-hoc analysis revealed lower D1 expression in the core of neonatal vehicle-treated bLRs compared to other groups (p<0.05; Fig. 6A). For D1 mRNA in the accumbens shell, there was no effect of phenotype, and no effect of FGF2 treatment. As in the core, though, there was also a phenotype x FGF2 interaction [F1, 194 = 7.96, p<0.01]. Post-hoc tests revealed higher D1 in the shell of neonatal FGF-treated bHRs versus vehicle-treated bHRs and bLRs (p<0.05 for each comparison; Fig. 6C).
Figure 6.
Striatal dopamine receptor expression in bHR and bLR rats exposed to neonatal FGF2. At baseline, bLRs showed reduced D1 receptor mRNA in the nucleus accumbens core (AcbC; A) and shell (AcbSh; C), as well as increased D2 receptor mRNA in the AcbC (B) and caudate putamen (CPu; F) compared to bHRs. Neonatal FGF2 treatment differentially affected gene expression in bLR versus bHRs, leading to selectively increased D1 levels in the AcbC of bLRs (A), but decreased D1 in the AcbSh (C) and CPu (E) of bHRs. n=8 per experimental group; *** indicates p<0.0001; * indicates p<0.05
For D2 mRNA, bLRs had greater D2 levels in the accumbens core compared to bHRs (main effect of phenotype [F1, 188 = 26.90, p<0.0001]; Fig. 6B), but levels were not affected by neonatal FGF2. There was no phenotype x FGF2 treatment interaction on D2 expression in the accumbens core. For D2 expression in the accumbens shell, there was no effect of phenotype, no effect of FGF2 treatment, and no phenotype x FGF2 treatment interaction (Fig. 6D).
Caudate putamen
In the caudate putamen, there was no effect of bHR/bLR phenotype on D1 levels, however there was a main effect of FGF2 treatment [F1, 284 = 6.12, p<0.05] and a phenotype x treatment interaction [F1, 284 = 4.94, p<0.05] (Fig. 6E). Post-hoc analysis showed that bHRs exposed to neonatal FGF2 had lower levels of D1 in the caudate compared to vehicle-treated bHRs (p<0.05). bLRs generally expressed higher D2 in the caudate compared to bHRs (effect of phenotype [F1, 284 = 191.09, p<0.0001]), but there was no effect of FGF2 treatment, and no treatment x phenotype interaction (Fig. 6F).
3.4 Impact of bHR/bLR phenotype and neonatal FGF2 on FGF system markers
Besides examining the impact of neonatal FGF2 on bHR/bLR’s dopamine system, we also assessed its effect on the FGF system markers within the same brain structures. We therefore measured expression of FGF2 and its primary receptor FGFR1 in the accumbens, caudate putamen, VTA, and SNc. As reported in Table 1, there were no effects of bHR/bLR phenotype or FGF2 treatment, and no phenotype x treatment interactions on FGFR1 expression in any brain region examined.
Table 1.
FGFR1 mRNA expression
| Brain region | bHR-veh | bLR-veh | bHR-FGF2 | bLR-FGF2 |
|---|---|---|---|---|
| AcbC | 0.018 ± 0.001 | 0.019 ± 0.002 | 0.019 ± 0.001 | 0.020± 0.001 |
| AcbSh | 0.030 ± 0.001 | 0.024 ± 0.002 | 0.023 ± 0.001 | 0.022 ± 0.001 |
| Cpu | 0.039 ± 0.002 | 0.037 ± 0.002 | 0.037 ± 0.002 | 0.038 ± 0.002 |
| VTA | 0.113 ± 0.006 | 0.116 ± 0.005 | 0.111 ± 0.006 | 0.115 ± 0.006 |
| SN | 0.098 ± 0.003 | 0.100 ± 0.004 | 0.091 ± 0.004 | 0.092 ± 0.005 |
Values represent average integrated optical density values ± standard errors.
Abbreviations ventral tegmental area (VTA); substantia nigra (SN); nucleus accumbens core (AcbC); nucleus accumbens shell (AcbSh); caudate putamen (Cpu)
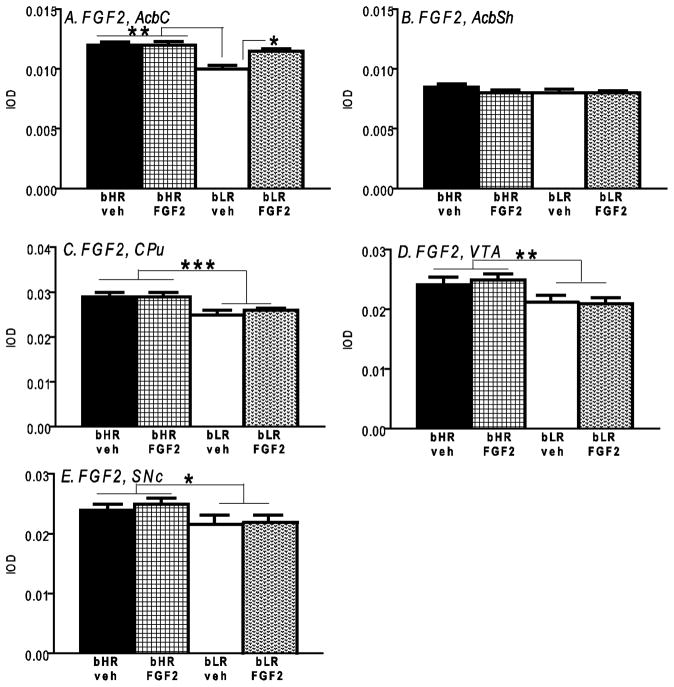
FGF2 mRNA in nucleus accumbens
There was a main effect of phenotype on FGF2 mRNA levels in the nucleus accumbens core [F1, 220 = 33.06, p<0.0001], with bLRs having lower levels compared to bHR. While there was no main effect of neonatal FGF2 treatment on this measure, there was a significant phenotype x neonatal FGF2 treatment interaction [F1, 220 = 7.88, p<0.01]. Post-hoc analysis showed that neonatal FGF2 treatment increased FGF2 mRNA in the accumbens core specifically in LRs (p<0.05 for bLR neonatal FGF2-treated versus bLR vehicle-treated; Fig. 7A). There was no effect of phenotype, no effect of neonatal FGF2 treatment, and no phenotype x treatment interaction for FGF2 mRNA expression in the accumbens shell (Fig. 7B).
Figure 7.
FGF2 mRNA expression in bHR and bLR rats exposed to neonatal FGF2 treatment. At baseline, bLRs showed reduced FGF2 mRNA in the nucleus accumbens core (AcbC; A), caudate putamen (CPu; C), ventral tegmental area (VTA; D), and substantia nigra pars compacta (SNc; E) compared to bHRs. Neonatal FGF2 treatment selectively increased FGF2 levels in the AcbC of bLRs (A), but had no effect in the other brain areas within bLRs, and did not affect any measures in bHRs. n=8 per experimental group; *** indicates p<0.0001; ** indicates p<0.01; * indicates p<0.05
FGF2 mRNA in caudate putamen
There was a main effect of phenotype on FGF2 levels in the caudate putamen [F1, 188 = 35.14, p<0.0001], with bLRs exhibiting reduced FGF2 mRNA compared to bHRs (Fig. 7C). There was no effect of neonatal FGF2 treatment, and no treatment x phenotype interaction on this measure.
FGF2 mRNA in VTA and SNc
There was a main effect of phenotype on FGF2 expression in the VTA [F1, 124 = 7.46, p<0.01] and SNc [F1, 156 = 3.98, p<0.005], with LRs showing lowever FGF2 mRNA in both regions compared to bHRs (Fig. 7D–E). In both the VTA and SNc, there was no effect of neonatal FGF2 treatment, and no treatment x phenotype interaction for this measure.
4. Discussion
The present study used selectively-bred rats that differ in propensity to addictive behavior and exhibit basal differences in “FGF tone” (at least in the adult brain), and asked whether manipulating their FGF system in early life produced lasting changes in cocaine sensitization. We found that “addiction-resistant” bLRs exhibit: (a) reduced cocaine sensitization compared to bHRs (consistent with prior studies (Cummings et al., 2011, Davis et al., 2008, Flagel et al., 2010, Garcia-Fuster et al., 2009, Garcia-Fuster et al., 2010)); (b) reduced FGF2 levels across several key brain areas relevant to addiction - the VTA, SNc, nucleus accumbens core and caudate putamen; and (c) altered dopaminergic markers compared to bHRs (mRNA findings summarized in Table 2), which is consistent with prior reports of baseline bHR/bLR dopamine system differences (Flagel et al., 2011, Flagel et al., 2010, Hooks et al., 1992, Hooks et al., 1994, Marinelli and White, 2000, Piazza et al., 1991). Interestingly, the single neonatal FGF2 injection partially reverses some of these effects, leading FGF2-exposed bLRs to exhibit greater cocaine sensitization and enhanced FGF2 and D1 dopamine receptor expression in the adult nucleus accumbens core compared to vehicle-exposed bLRs. Our data support the notion of FGF2 playing an important role in the neurobiology of addiction. Prior work demonstrated how FGF2 mediates the effects of chronic cocaine (Flores et al., 2000), and our new data suggest that baseline FGF2 differences may shape an individual’s acute and sensitized response to cocaine. Thus, bHR rats’ naturally high levels of FGF2 (at least during adulthood) may contribute to their increased vulnerability to addictive behavior, but pharmacologically enhancing the FGF system in early life may shift brain development and render individuals more susceptible to developing enhanced cocaine sensitivity. Future experiments will be required to test this hypothesis, aiming to block FGF2 in the bHR brain (or in neonatal FGF2-exposed brain) and see whether this dampens their proclivity to cocaine sensitization and/or self-administration.
Table 2.
Summary of Gene Expression Findings
| Gene | Brain Region | Basal bHR/bLR differences | Impact of neonatal FGF2 |
|---|---|---|---|
|
| |||
| TH | VTA | decreased in bLR vs bHR | decreased |
| DAT | VTA | - | - |
| D2 | VTA | - | - |
| FGF2 | VTA | decreased in bLR vs bHR | - |
| FGFR1 | VTA | - | - |
|
| |||
| TH | SN | - | - |
| DAT | SN | - | - |
| D2 | SN | - | - |
| FGF2 | SN | decreased in bLR vs bHR | - |
| FGFR1 | SN | - | - |
|
| |||
| D1 | AcbC | decreased in bLR vs bHR | increased in bLR ONLY |
| D2 | AcbC | increased in bLR vs bHR | - |
| FGF2 | AcbC | decreased in bLR vs bHR | increased in bLR ONLY |
| FGFR1 | AcbC | - | - |
|
| |||
| D1 | AcbSh | decreased in bLR vs bHR | decreased in bHR ONLY |
| D2 | AcbSh | - | - |
| FGF2 | AcbSh | - | - |
| FGFR1 | AcbSh | - | - |
|
| |||
| D1 | Cpu | - | decreased in bHR ONLY |
| D2 | Cpu | increased in bLR vs bHR | - |
| FGF2 | Cpu | decreased in bLR vs bHR | - |
| FGFR1 | Cpu | - | - |
Abbreviations: tyrosine hydroxylase (TH); dopamine transporter (DAT); dopamine receptor 2 (D2); Fibroblast Growth Factor 2 (FGF2); Fibroblast Growth Factor Receptor 1 (FGFR1); ventral tegmental area (VTA); substantia nigra (SN); nucleus accumbens core (AcbC); nucleus accumbens shell (AcbSh); caudate putament (Cpu)
4.1 FGF2 and psychostimulant sensitization
Numerous studies demonstrate that chronic drug administration triggers a variety of neuroplasticity mechanisms likely involved in the development of addiction (Nestler, 2005). These mechanisms include changes in growth factors such as FGF2 (Flores et al., 2000, Flores and Stewart, 2000a, b, Forget et al., 2006, Mueller et al., 2006), since blocking FGF2 in the VTA impaired psychostimulant sensitization and reversed amphetamine-induced changes in VTA dendritic morphology (Mueller et al 2006a). Our current findings show that adult bHRs, which are genetically predisposed to manifest high levels of cocaine self-administration and sensitization, express higher FGF2 in the VTA, SN, nucleus accumbens core and caudate putamen compared to bLRs. Flores et al also found that repeated amphetamine treatment persistently increased FGF2 in the VTA and SN (Flores and Stewart, 2000b). It is therefore reasonable to hypothesize that bHRs’ high baseline FGF2 levels contribute to their increased propensity to cocaine sensitization relative to bLRs. Moreover, since neonatal FGF2 treatment increased bLRs’ FGF2 mRNA levels in the adult nucleus accumbens core, it seems possible that this change may contribute to their increased cocaine sensitization (enhance day 7 cocaine-induced locomotion) relative to vehicle-exposed bLRs. Future experiments will explore these possibilities by blocking FGF2 in the nucleus accumbens of either bHRs or neonatal FGF2-exopsed bLRs and determine its effect on cocaine sensitization. Our data suggest that neonatal FGF2 treatment enhances cocaine sensitization in bLRs, while bHRs appear unaffected, however, this may be due to a ceiling effect on cocaine-induced locomotion in bHRs. Moreover, it will be important in future studies to also evaluate cocaine-induced stereotypy that typically occurs in highly sensitized animals (Flagel and Robinson, 2007). Recent data from our laboratory suggest that bHRs may be particularly prone to stereotypy following chronic cocaine treatment (Waselus et al, 2012 In Preparation); therefore, future studies will need to determine whether early life FGF treatment in bHRs influences this aspect of their sensitized response to cocaine. It is also interesting to note that while neonatal FGF2-treatment had little effect on bHRs’ sensitized locomotor response to cocaine, it enhanced their acute locomotor response to cocaine (day 1 cocaine-induced locomotion). The in situ hybridization study revealed a possible molecular change that may contribute to this effect - decreased D1 receptor mRNA in the nucleus accumbens shell and caudate putamen of bHRs exposed to neonatal FGF2 versus vehicle-treated bHRs (Table 2).
The present study and majority of our work to-date has evaluated bHR/bLR differences in FGF2 and its primary receptor, FGFR1. As demonstrated here and elsewhere, we find prominent differences in FGF2 in bHR/bLR animals across several brain regions, with minimal differences in FGFR1. Considering the complexity of the FGF gene family, which includes more than 20 separate proteins (Itoh and Ornitz, 2011), it will be interesting to examine other aspects of this system (e.g. other FGF ligands and receptors), to determine how these may differ in bHR/bLR animals, and how they may be involved in emotional and addictive behavior.
4.2 FGF and dopamine interactions
Our in situ hybridization data highlight multiple baseline differences in dopamine and FGF system markers in bHR versus bLR rats (summarized in Table 2). The baseline differences in dopamine system markers are consistent with previous reports of altered dopamine transmission in high versus low novelty-reactive animals (Flagel et al., 2011, Flagel et al., 2010, Hooks et al., 1992, Hooks et al., 1994, Marinelli and White, 2000, Piazza et al., 1991). Our data also highlight profound FGF2 differences in adult bHR/bLR animals within the mesolimbic system. While these findings and previous work (e.g. (Perez et al., 2009) demonstrated FGF system differences in adult bHR/bLR rats, it would be extremely interesting to know whether these differences are also present in neonatal and developing bHR/bLR animals. Future work in developing bHR/bLR animals will pursue this question, and also examine how neonatal FGF2 treatment may alter the trajectory of FGF system development.
Our in situ hybridization results show that neonatal FGF2 treatment leads to increased FGF2 and D1 dopamine receptor expression in the nucleus accumbens core specifically in bLRs – molecular changes that may contribute the enhanced cocaine sensitization observed in neonatal FGF2-exposed bLR rats. These data suggest that exogenous FGF2 administered on the second day of life may “rescue” bLRs’ low FGF2 state, leading to downstream changes that render them more bHR-like. It is important to note, however, that although the neonatal FGF2 treatment brings bLRs’ levels of D1 and FGF2 mRNA in the accumbens up to bHR-like levels, it does not completely bring their level of cocaine sensitization up to the extraordinarily high bHR levels. Therefore, while these changes may contribute to FGF2-treated bLRs showing enhanced cocaine sensitization relative to vehicle-treated bLRs, there are still a multitude of other biological and genetic factors that likely differ between bHR/bLR rats and underlie their vastly disparate behavioral phenotypes.
There is a growing literature examining functional interactions between the FGF and dopaminergic systems (Grothe and Timmer, 2007, Terwisscha van Scheltinga et al., 2010). In vitro cultures of embryonic day 12 rat dopaminergic neurons exhibited increased cell proliferation and delayed cell differentiation following FGF2 treatment (Bouvier and Mytilineou, 1995). Another study showed that FGF receptors can directly interact with adenosine A(2A) receptors to antagonize dopamine D2 receptor signaling, activate the MAPK/ERK pathway, and ultimately lead to several changes in neuroplasticity (Flajolet et al., 2008). Pharmacologically-boosting dopamine levels with psychostimulants influences FGF2 levels, with cocaine treatment leading to persistently increased FGF2 levels in the striatum and prefrontal cortex (Fumagalli et al., 2008, Fumagalli et al., 2006, Molteni et al., 2001). Data from our laboratory adds further support to this body of work, demonstrating (a) that animals with naturally-occurring differences in dopaminergic tone (Flagel et al., 2011, Flagel et al., 2010) also exhibit differences in the FGF system ((Perez et al., 2009) and current data); and (b) that manipulating the FGF system has the capacity to shift an individual’s behavioral response to cocaine (self-administration and/or sensitization) (Turner et al., 2009, Turner et al., 2008). Our current findings suggest that this is particularly true in individuals that typically exhibit very low levels of cocaine sensitization (bLRs). Since bHRs already exhibit such high levels of cocaine sensitization, a ceiling effect may prevent us from seeing any further enhancement.
As noted above, we find that neonatal FGF2 treatment selectively increases FGF2 and D1 dopamine receptor mRNA in the nucleus accumbens core of bLRs, suggesting a possible neurobiological substrate that may contribute to their increased acute response to cocaine as well as cocaine sensitization following neonatal FGF2 exposure. Future experiments could explore this possible mechanism by blocking FGF2 in the accumbens core (akin to the previous immunoneutralization studies by Stewart et al in the VTA (Flores et al., 2000)). Additionally, it is important to consider how chronic cocaine treatment itself differentially shapes gene expression in bHR versus bLR animals we well as in neonatal FGF2-exposed animals, and how the interplay between these factors may shape both the acute behavioral response to cocaine as well as sensitization. Finally, we must also keep in mind other brain areas that are changed by the neonatal FGF2 treatment and could potentially shape addictive behaviors, or more generally an organism’s manner of interacting with the environment. For example, we recently showed how neonatal FGF2 treatment shifts the developmental trajectory of the hippocampus, leading to a life-long change in neurogenesis, hippocampal morphology and gene expression (Turner et al., 2011). Considering the myriad roles of the hippocampus in guiding emotional behavior and exploration of the environment, which are certainly relevant to addiction-related behavior (Bannerman et al., 2002, Gray, 1982, Jacobson and Sapolsky, 1991, Jeewajee et al., 2008, Lever et al., 2006, McNaughton and Gray, 2000, Roullet and Lassalle, 1990, Sapolsky et al., 1984), we cannot exclude the possibility that increased cocaine sensitization in neonatal FGF2-treated bLRs is linked to changes in their hippocampus. Future experiments are warranted to explore these possibilities.
4.3 Conclusions
In conclusion, a single dose of FGF2 on the second day of life resulted in enhanced cocaine sensitization in animals that typically exhibit blunted behavioral response to drugs of abuse (bLRs) (e.g. blunted cocaine sensitization and self-administration). Our gene expression data demonstrate the marked downregulation of FGF2 across several brain regions in bLR versus bHRs under baseline conditions. The neonatal FGF2 injection may offer an early “boost” to bLRs’ flagging FGF2 system, shifting the developmental trajectory of select brain circuits (such as the accumbens and hippocampus), and ultimately pushing their behavior towards a more “bHR-like” phenotype.
Prior work demonstrated a role for FGF2 in mediating cocaine sensitization (e.g. (Flores et al., 2000)), and showed that early life manipulation of the FGF system leads to enhanced cocaine self-administration (Turner et al., 2009). Our current results show that naturally enhanced FGF2 levels in the adult mesolimbic system (as in bHR rats or neonatal FGF2-exposed bLRs compared to vehicle-exposed bLRs) is associated with enhanced cocaine sensitization. These data suggest that FGF2 may act as an early molecular organizer that sets the stage for enhanced propensity to respond to repeated cocaine exposure, and perhaps in doing so, increase drug addiction vulnerability.
Highlights.
Fibroblast growth factor 2 (FGF2) plays an important role in addictive behavior.
Low (bLR) versus high (bHR) novelty-reactive rats exhibit marked FGF2 differences.
bLR/bHR rats also exhibit marked differences in behavioral response to cocaine.
Early life manipulation of bLRs’ FGF system increases their cocaine reactivity.
Neonatal FGF2 elevates bLR dopamine receptor and FGF2 levels in nucleus accumbens.
Acknowledgments
The authors would like to thank Hailey Orr, Kate Mills, Sharon Burke, and Jennifer Fitzpatrick for excellent technical assistance. This study was supported by NIMH R00 MH085859-03 (SMC), Office of Naval Research N00014-02-1-0879 (HA and SJW), and NIDA PPG 5P01DA021633-02 (HA and SJW).
Footnotes
Disclosure of Financial Interests and Potential Conflicts of Interest
None of the authors have biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahi A, Boyer F, Chandrasekar V, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 2008;199:169–82. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–66. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:3715–9. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier MM, Mytilineou C. Basic fibroblast growth factor increases division and delays differentiation of dopamine precursors in vitro. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:7141–9. doi: 10.1523/JNEUROSCI.15-11-07141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Tao Y, Black IB, DiCicco-Bloom E. A single peripheral injection of basic fibroblast growth factor (bFGF) stimulates granule cell production and increases cerebellar growth in newborn rats. J Neurobiol. 2001;46:220–9. doi: 10.1002/1097-4695(20010215)46:3<220::aid-neu1004>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Clinton S, Miller S, Watson SJ, Akil H. Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psychoneuroendocrinology. 2008;33:162–77. doi: 10.1016/j.psyneuen.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2:3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–8. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004;101:15506–11. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE. Quantifying the psychomotor activating effects of cocaine in the rat. Behav Pharmacol. 2007;18:297–302. doi: 10.1097/FBP.0b013e3281f522a4. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, et al. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nature neuroscience. 2008;11:1402–9. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Samaha AN, Stewart J. Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:RC55. doi: 10.1523/JNEUROSCI.20-02-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Stewart J. Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat. Psychopharmacology (Berl) 2000a;151:152–65. doi: 10.1007/s002130000417. [DOI] [PubMed] [Google Scholar]
- Flores C, Stewart J. Changes in astrocytic basic fibroblast growth factor expression during and after prolonged exposure to escalating doses of amphetamine. Neuroscience. 2000b;98:287–93. doi: 10.1016/s0306-4522(00)00115-9. [DOI] [PubMed] [Google Scholar]
- Forget C, Stewart J, Trudeau LE. Impact of basic FGF expression in astrocytes on dopamine neuron synaptic function and development. Eur J Neurosci. 2006;23:608–16. doi: 10.1111/j.1460-9568.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Stress and cocaine interact to modulate basic fibroblast growth factor (FGF-2) expression in rat brain. Psychopharmacology (Berl) 2008;196:357–64. doi: 10.1007/s00213-007-0966-x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Pasquale L, Racagni G, Riva MA. Dynamic regulation of fibroblast growth factor 2 (FGF-2) gene expression in the rat brain following single and repeated cocaine administration. J Neurochem. 2006;96:996–1004. doi: 10.1111/j.1471-4159.2005.03627.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Clinton SM, Watson SJ, Akil H. Effect of cocaine on Fas-associated protein with death domain in the rat brain: individual differences in a model of differential vulnerability to drug abuse. Neuropsychopharmacology. 2009;34:1123–34. doi: 10.1038/npp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Perez JA, Clinton SM, Watson SJ, Akil H. Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. Eur J Neurosci. 2010;31:79–89. doi: 10.1111/j.1460-9568.2009.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nature neuroscience. 2007;10:1029–37. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford, UK: Oxford University Press; 1982. [Google Scholar]
- Grothe C, Timmer M. The physiological and pharmacological role of basic fibroblast growth factor in the dopaminergic nigrostriatal system. Brain Res Rev. 2007;54:80–91. doi: 10.1016/j.brainresrev.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Colvin AC, Juncos JL, Justice JB., Jr Individual differences in basal and cocaine-stimulated extracellular dopamine in the nucleus accumbens using quantitative microdialysis. Brain Res. 1992;587:306–12. doi: 10.1016/0006-8993(92)91012-4. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Juncos JL, Justice JB, Jr, Meiergerd SM, Povlock SL, Schenk JO, et al. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. Journal of Neuroscience. 1994;14:6144–52. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–30. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–34. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jeewajee A, Lever C, Burton S, O’Keefe J, Burgess N. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus. 2008;18:340–8. doi: 10.1002/hipo.20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Isgor C, Watson SJ, Akil H. Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience. 2002;113:395–400. doi: 10.1016/s0306-4522(02)00188-4. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, O’Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17:111–33. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- Liu S, Zheng D, Peng XX, Cabeza de Vaca S, Carr KD. Enhanced cocaine-conditioned place preference and associated brain regional levels of BDNF, p-ERK1/2 and p-Ser845-GluA1 in food-restricted rats. Brain Res. 2011;1400:31–41. doi: 10.1016/j.brainres.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:8876–85. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord. 2000;61:161–76. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, et al. Modulation of fibroblast growth factor-2 by stress and corticosteroids: from developmental events to adult brain plasticity. Brain Res Brain Res Rev. 2001;37:249–58. doi: 10.1016/s0165-0173(01)00128-x. [DOI] [PubMed] [Google Scholar]
- Mueller D, Chapman CA, Stewart J. Amphetamine induces dendritic growth in ventral tegmental area dopaminergic neurons in vivo via basic fibroblast growth factor. Neuroscience. 2006;137:727–35. doi: 10.1016/j.neuroscience.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–9. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–87. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–74. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Ron D, Janak PH. GDNF and addiction. Rev Neurosci. 2005;16:277–85. doi: 10.1515/revneuro.2005.16.4.277. [DOI] [PubMed] [Google Scholar]
- Roullet P, Lassalle JM. Genetic variation, hippocampal mossy fibres distribution, novelty reactions and spatial representation in mice. Behav Brain Res. 1990;41:61–70. doi: 10.1016/0166-4328(90)90054-i. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proceedings of the National Academy of Sciences of the U S A. 1984;81:6174–7. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Stalnaker TA, Shaham Y. A role for BDNF in cocaine reward and relapse. Nature neuroscience. 2007;10:935–6. doi: 10.1038/nn0807-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, et al. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Frys KA, Kalivas PW. Pretreatment with serotonin 5-HT(3) receptor antagonists produces no observable blockade of long-term motor sensitization to cocaine in rats. Psychopharmacology (Berl) 2003;165:329–36. doi: 10.1007/s00213-002-1274-0. [DOI] [PubMed] [Google Scholar]
- Tao Y, Black IB, DiCicco-Bloom E. Neurogenesis in neonatal rat brain is regulated by peripheral injection of basic fibroblast growth factor (bFGF) J Comp Neurol. 1996;376:653–63. doi: 10.1002/(SICI)1096-9861(19961223)376:4<653::AID-CNE11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Terwisscha van Scheltinga AF, Bakker SC, Kahn RS. Fibroblast growth factors in schizophrenia. Schizophr Bull. 2010;36:1157–66. doi: 10.1093/schbul/sbp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Capriles N, Flagel SB, Perez JA, Clinton SM, Watson SJ, et al. Neonatal FGF2 alters cocaine self-administration in the adult rat. Pharmacol Biochem Behav. 2009;92:100–4. doi: 10.1016/j.pbb.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8021–5. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Flagel SB, Clinton SM, Akil H, Watson SJ. Cocaine interacts with the novelty-seeking trait to modulate FGFR1 gene expression in the rat. Neurosci Lett. 2008;446:105–7. doi: 10.1016/j.neulet.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:6006–16. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, et al. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25:118–30. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Wei Q, Fentress HM, Hoversten MT, Zhang L, Hebda-Bauer EK, Watson SJ, et al. Early-life forebrain glucocorticoid receptor overexpression increases anxiety behavior and cocaine sensitization. Biological psychiatry. 2012;71:224–31. doi: 10.1016/j.biopsych.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]