Abstract
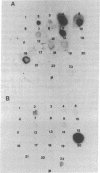
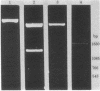
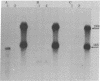
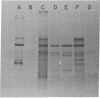
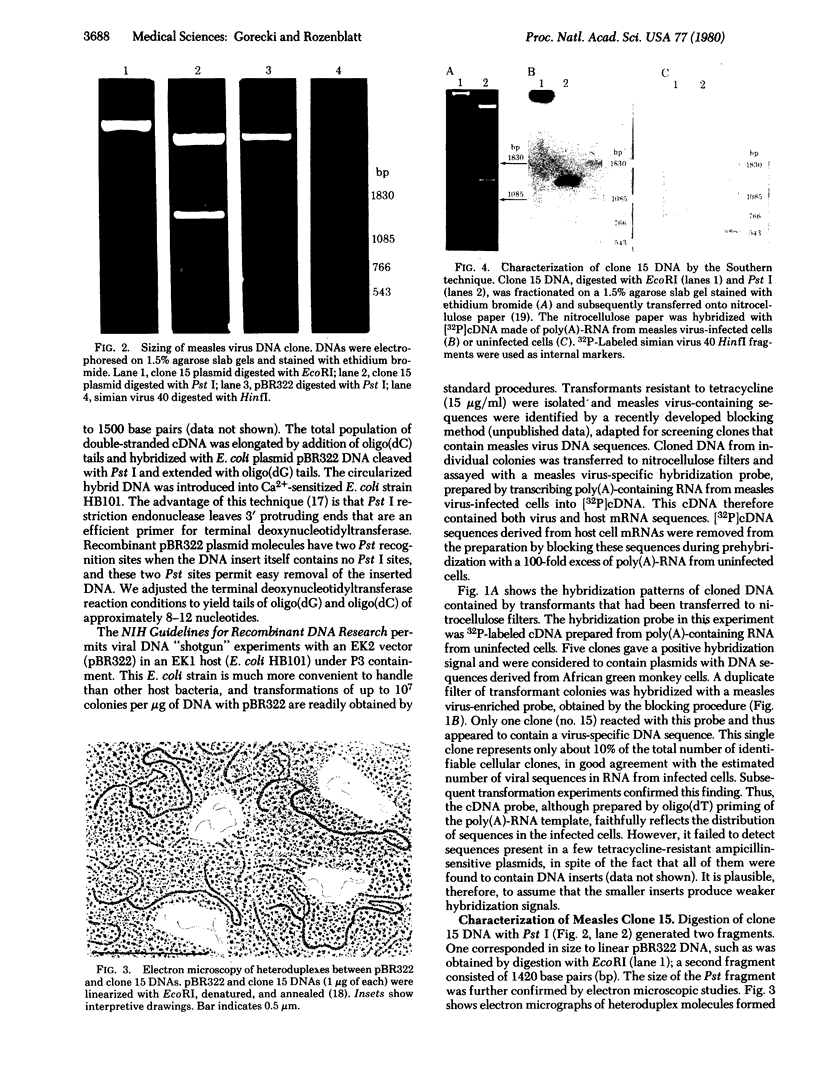
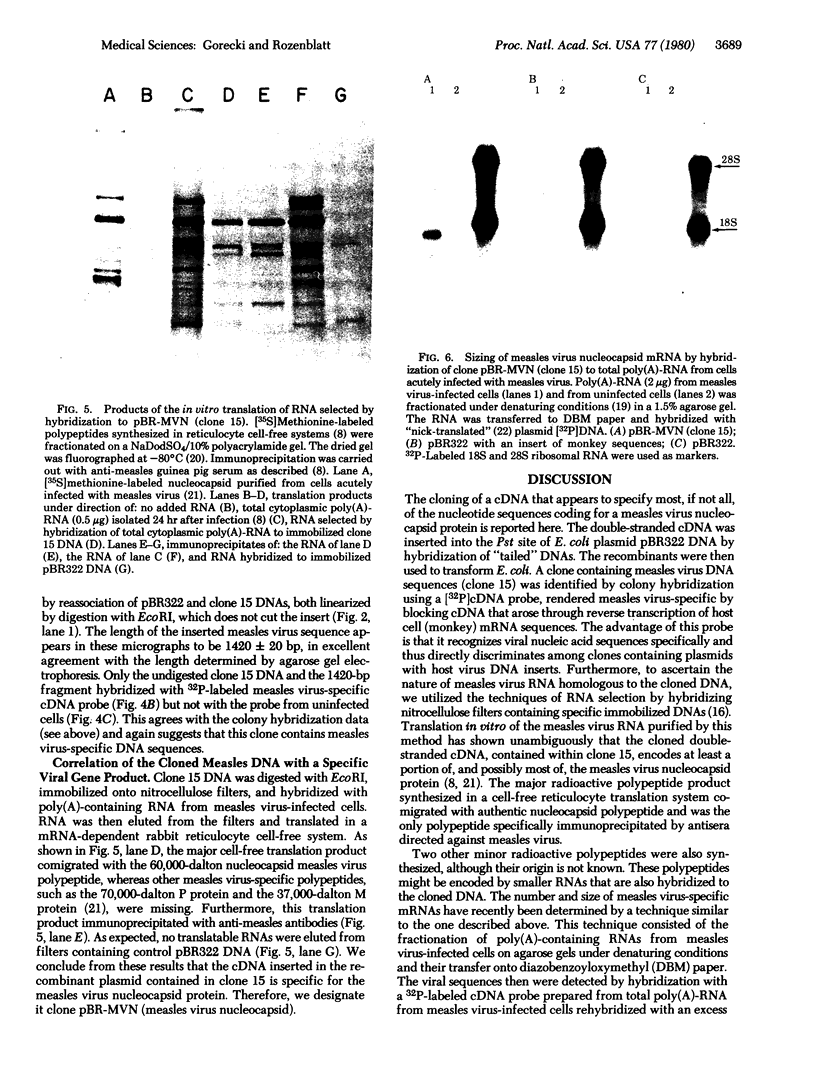
Double-stranded cDNA synthesized from total poly(A)-containing mRNA, extracted from monkey cells infected with measles virus, has been inserted into Pst cleavage site of Escherichia coli plasmid pBR322 and cloned. A clone containing measles virus DNA sequences was identified by hybridization to a measles virus-specific 32P-labeled cDNA probe prepared from the mRNA of measles virus-infected cells. Cellular sequences in the probe were neutralized by prehybridization with an excess of unlabeled mRNA from uninfected monkey cells. The insert of cloned cDNA isolated contans 1420 base pairs, as shown by agarose gel electrophoresis and electron microscopy. The size of the mRNA complementary to this cloned cDNA is 1750 nucleotides, as determined by the reverse Southern technique. The cloned DNA fragment was further identified as the reverse transcript of the mRNA coding for the nucleocapsid protein of measles virus on the basis that the major cell-free translation product of mRNA selected by hybridization to the cloned DNA comigrated with the nucleocapsid protein and was immunoprecipitated by measles virus-specific antibodies. Subsequently, the cloned DNA was used to detect specific measles virus sequences in the poly(A)-RNA extracted from brain autopsy material from a patient with subacute sclerosing panecephalitis. The cloned DNA can thus be used as a probe to study the structure and expression of the measles genome, and in particular, to study diseases of the central nervous system in which persistent infection with measles virus has been implicated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bratosin S., Horowitz M., Laub O., Aloni Y. Electron microscopic evidence for splicing of SV40 late mRNAs. Cell. 1978 Apr;13(4):783–790. doi: 10.1016/0092-8674(78)90228-3. [DOI] [PubMed] [Google Scholar]
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- Enea V., Vovis G. F., Zinder N. D. Genetic studies with heteroduplex DNA of bacteriophage fl. Asymmetric segregation, base correction and implications for the mechanism of genetic recombination. J Mol Biol. 1975 Aug 15;96(3):495–509. doi: 10.1016/0022-2836(75)90175-8. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Kiessling W., ter Meulen V. Membrane proteins of subacute sclerosing panencephalitis and measles viruses. Nature. 1978 Mar 30;272(5652):460–462. doi: 10.1038/272460a0. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. Purification and characterization of measles virus. J Gen Virol. 1973 May;19(2):175–188. doi: 10.1099/0022-1317-19-2-175. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Katz M., Koprowski H. The significance of failure to isolate infectious viruses in cases of subacute sclerosing panencephalitis. Brief report. Arch Gesamte Virusforsch. 1973;41(4):390–393. doi: 10.1007/BF01250213. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Rapp F. Measles virus and its associated diseases. Bacteriol Rev. 1977 Sep;41(3):636–666. doi: 10.1128/br.41.3.636-666.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Gorecki M., Shure H., Prives C. L. Characterization of measles virus-specific proteins synthesized in vivo and in vitro from acutely and persistently infected cells. J Virol. 1979 Mar;29(3):1099–1016. doi: 10.1128/jvi.29.3.1099-1106.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Koch T., Pinhasi O., Bratosin S. Infective substructures of measles virus from acutely and persistently infected cells. J Virol. 1979 Oct;32(1):329–333. doi: 10.1128/jvi.32.1.329-333.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluederberg A. Measles virus RNA. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1012–1015. doi: 10.1016/0006-291x(71)90004-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tellez-Negal I., Harter D. H. Subacute sclerosing leukoencephalitis: ultrastructure of intranuclear and intracytoplasmic inclusions. Science. 1966 Nov 18;154(3751):899–901. doi: 10.1126/science.154.3751.899. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Polsky F. I., Seidman J. G., Leder A., Edgell M. H., Leder P. A comparison of two cloned mouse beta-globin genes and their surrounding and intervening sequences. Cell. 1978 Jun;14(2):237–245. doi: 10.1016/0092-8674(78)90110-1. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. L., Norrby E. Structural polypeptides of measles virus. J Gen Virol. 1978 May;39(2):219–229. doi: 10.1099/0022-1317-39-2-219. [DOI] [PubMed] [Google Scholar]
- Waters D. J., Bussell R. H. Polypeptide composition of measles and canine distemper viruses. Virology. 1973 Oct;55(2):554–557. doi: 10.1016/0042-6822(73)90202-x. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Intracellular synthesis of measles virus-specified polypeptides. J Virol. 1978 Jan;25(1):285–297. doi: 10.1128/jvi.25.1.285-297.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]