Abstract
Purpose
To use Fourier-domain optical coherence tomography (OCT) to measure the angle opening distance at Schwalbe's line (AOD-SL) and determine its value in anterior chamber angle assessment.
Methods
Horizontal scans of the nasal and temporal anterior chamber angles in glaucoma subjects were performed by 830 nm wavelength Fourier-domain OCT. Images were graded by two ophthalmologists who assessed the visibility of Schwalbe’s line (SL), anterior limbus (AL), scleral spur (SS), and angle recess (AR). AOD-SL was measured with computer calipers. SL was manually identified by the termination of the corneal endothelium. Gonioscopy was used to classify anterior chamber angles according to a modified Shaffer system. Spearman's rho analysis was performed to assess correlation between AOD-SL and modified Shaffer grade. A cut-off value of AOD-SL for diagnosing occludable angles (modified Shaffer grade ≤1) was determined by receiver operating characteristic (ROC) analyses.
Results
Thirty-five glaucoma subjects (65 eyes) were enrolled. SL, AL, AR, and SS were visible by OCT in 97.7%, 99.2%, 87.3%, and 80.8% of eyes, respectively. Nasal and temporal AOD-SLs were 322.6 ± 200.2 µm and 341.4 ± 197.4 µm, respectively. Correlation coefficients between AOD-SL and modified Shaffer grade were 0.80 (nasal) and 0.81 (temporal). The diagnostic cut-off value of AOD-SL for occludable angles was 290 µm. The areas under the ROC curve, sensitivity, specificity values were 0.90, 0.80, 0.87 (nasal) and 0.90, 0.85, 0.77 (temporal).
Conclusions
The measurement of AOD-SL by Fourier-domain OCT is highly correlated with gonioscopy and may be a useful noncontact method of assessing angle closure risk.
Keywords: glaucoma, anterior chamber angle imaging, optical coherence tomography
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide.1 Primary angle closure glaucoma (PACG) is the predominant glaucoma subtype in certain ethnic groups, including Asians and Eskimos.2–5 It has been estimated that in China alone, 3.5 million people are afflicted with PACG and 28 million have a predisposing condition of “occludable” angles.6 Progressive closure of the angle may be prevented if these subjects are treated with laser peripheral iridotomy.7 Screening these populations for at-risk eyes can have a significant impact on public health.
Currently, gonioscopy remains the reference standard for anterior chamber (AC) angle assessment. However, it has limitations such as requiring a highly skilled examiner and involving contact with the surface of the eye. This necessitates anesthetic agents and can cause artifacts. Anterior segment optical coherence tomography (OCT) is a relatively new method for imaging the angle. Many time-domain OCT studies used parameters of fixed distance from the scleral spur (SS) to quantitatively assess the AC angle. However, the SS cannot be identified in 20%–25% of the images due to limited resolution.8, 9
Fourier-domain OCT was initially used in observing the posterior segment of the eye.10, 11 More recently, it was developed to image the anterior segment as well. The shorter wavelength of 830 nm and broader bandwidth of the Fourier-domain OCT used in this study produced both better transverse and axial resolutions. Thus, more detailed anatomical structures such as the Schwalbe’s line (SL) and the trabecular meshwork (TM) can be identified. The distance from SL to the SS represents the TM and is the true aqueous humor filtration distance. Measuring the angle opening distance (AOD) from SL could more directly reflect the accessibility of the TM to aqueous humor compared with estimations based on the fixed distance from the SS. In this study, we use Fourier–domain OCT to explore the concept of AOD at SL (AOD-SL) as a parameter to assess angle characteristics with risk implications for angle closure glaucoma.
Materials and Methods
Subjects
This prospective, observational study recruited subjects diagnosed with either primary open-angle glaucoma (POAG) or PACG and who were treated at the Doheny Eye Institute, University of Southern California, Los Angeles, California, USA, during the period from October 2009 to March 2010. This study was approved by the Institutional Review Board of the University of Southern California. The study followed the tenets of the Declaration of Helsinki and was in accordance with the Health Insurance Portability Act of 1996. Written informed consent was obtained from each of the study subjects.
Enrolled subjects were diagnosed as either open angle or angle closure glaucoma. All subjects received a screening examination including visual acuity, slit-lamp biomicroscopy, Goldmann applanation tonometry, and dark-room gonioscopy. An experienced glaucoma specialist (BAF) performed all gonioscopy screening to ensure that the anterior chamber angle appearance of enrolled subjects were glaucoma related only. Subjects with previous intraocular surgeries, corneal opacity, or other corneal abnormalities were excluded. Subjects previously underwent laser iridotomy were not excluded.
Gonioscopy angle assessment
Gonioscopy was performed by a glaucoma specialist (BAF) using a Zeiss 4-mirror gonioscopy lens. The exam was carried out in a dark room (room light off), with the lowest intensity beam on a Haag Streit (Koeniz, Switzerland) BM 900 slit lamp with a width of 0.4 to 0.5 mm and length of 8 mm. Grading was recorded for 4 angle quadrants (superior, inferior, nasal, and temporal) in both eyes (if qualified) of each subject using the modified Shaffer grade which was based on the original Shaffer system.12 Each grade had the following properties: Grade 4, wide open angle (approximately 40 degrees) with a flat or concave iris surface and the SS visible without positioning the eye toward the gonioscopy mirror; Grade 3, wide open angle (30 degrees) with a slightly convex iris surface and the SS visible without positioning the eye toward the gonioscopy mirror; Grade 2, open angle (20 degrees) with a convex iris surface and the SS visible without positioning the eye toward the gonioscopy mirror; Grade 1, narrow angle (10 degrees) with a convex iris surface and the SS visible only with the positioning of the eye toward the gonioscopy mirror; Grade 0.5, slit angle (less than 10 degrees) and the SS not visible even with positioning the eye toward the gonioscopy mirror; and Grade 0, closed angle and the SS not visible even with positioning the eye toward the gonioscopy mirror. An occludable angle was defined as modified Shaffer Grade ≤1 in all quadrants.
Anterior chamber angle imaging
Imaging of the angle was performed with a Fourier-domain OCT system (RTVue, Optovue, Inc., Fremont, CA, USA). The instrument uses an 830 nm wavelength light source and has a scan speed of 26,000 A-scans per second. It has a transverse resolution of 15 µm and an axial resolution of 5 µm (in tissue). A corneal adapter module lens was mounted for anterior segment imaging. All scans were performed under uniform conditions of dim illumination of 6.6 foot candles that was standardized using a light meter (Model 810, AEMC instruments, Foxborough, MA, USA). The patient was guided to look to the side at an external fixation target. A 3 mm long and 2.3 mm in depth line scan pattern was used for imaging the angle. The scans were centered on the angle from either the nasal or temporal side. Each scan was averaged from 16 consecutive frames to reduce the speckle and background noise. Two sets of consecutive scans were performed. For each eye, the first set of images of the temporal and nasal AC angles was recorded in lateral gaze. If both eyes of the same patient were scanned, images from the right eye were acquired first. The second set of images was acquired using the same protocol. All images were acquired by the same examiner.
Anterior chamber angle assessment
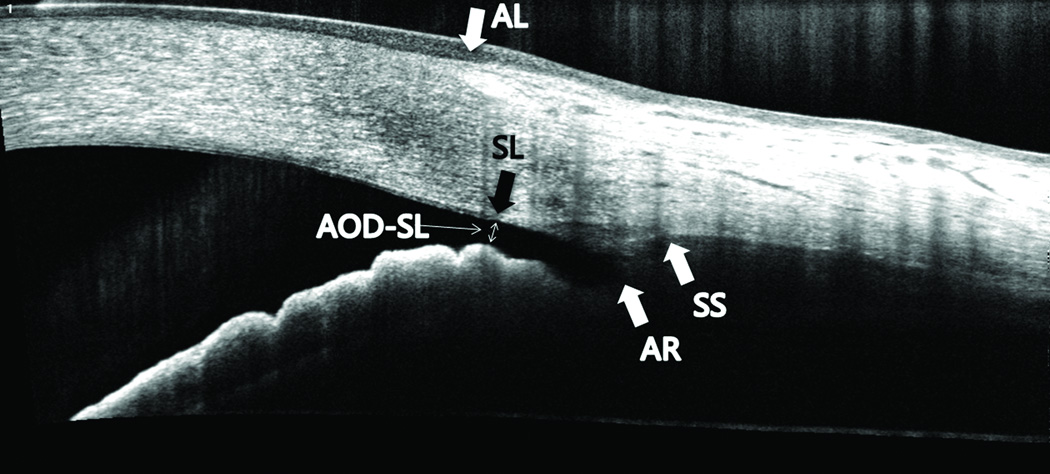
Low quality images in which landmarks were obscured by shadows from lash, lid, or limbal degeneration were excluded. All images were de-warped to correct refractive distortion and analyzed using OCT image analyses software ReVue version 4.0.7.9 (Optovue, Inc.). Two experienced ophthalmologists (BQ, CC) graded all of the images in random order with subject identity and clinical information masked. The two sets of images from consecutive scans were graded on different days and in random order. Visibility of SL, anterior limbus (AL), angle recess (AR), and SS were decided as either “visible” or “not visible”. The location of SL was manually identified by determining the posterior termination point of the corneal endothelium. The AOD-SL was defined as the distance from the SL to the iris surface that is perpendicular to a line drawn from SS to SL (Figure 1). The AOD-SL and the distance between SL and SS (SL-SS distance) were measured by computer calipers. The AOD-SL and SL-SS distances were only measured in images in which SL and the SS were decided as visible by both Observers.
Figure 1.
Anterior chamber angle anatomical landmarks and measurement of AOD-SL in an optical coherence tomography line scan image. (SL = Schwalbe’s line, AL = anterior limbus, SS = scleral spur, AR = angle recess, AOD-SL = angle opening distance at SL).
Statistical Analysis
Measurements of AOD-SL, SL-SS distances, and other continuous variables were described as mean values ± standard deviations. The test for two proportions was used to compare visibility among angle landmarks. Inter-observer and intra-observer repeatabilities were assessed using coefficient of variation (CV) analyses. Spearman's rho analysis was performed to evaluate correlation between AOD-SL and modified Shaffer grade. A linear trend test was performed to determine if the AOD-SL was linearly associated with modified Shaffer grade. A cut-off value of AOD-SL for angle occludability was determined by receiver operating characteristic (ROC) analyses. The area under the ROC curve (AROC), sensitivity, and specificity were used to assess the accuracy of AOD-SL in diagnosing angle closure risks according to gonioscopy standards. The generalized estimating equation was used to account for the inter-eye correlation.13 The level of significance was set at 0.05. All analyses were done in SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Of the 38 subjects who were recruited, 3 were excluded due to poor image quality. Among the remaining 35 subjects, 11 were clinically diagnosed with POAG and 24 were diagnosed with PACG. The OCT images from 65 eyes of the 35 subjects (22 female and 13 male, 25 Asians and 10 Caucasians, mean age 70.2 ± 10.7 years) were graded for the visibility of landmarks. Both eyes were scanned in 30 subjects, 3 had only the right eye scanned, and 2 only the left eye. The nasal and temporal SLs were visible in 100% and 96.9% of the eyes respectively for Observer 1 (Table 1). For Observer 2, the nasal and temporal SLs were visible in 96.9% and 96.9% respectively. All were more visible than either the AR or the SS (P < 0.05, Table 1). There were no significant differences in the visibility between SL and the AL (P-value range: 0.24 – 0.50). The averaged visibility in all eyes of SL, AL, AR, and SS were 97.7%, 99.2%, 87.3%, and 80.8%, respectively.
Table 1.
Visibility of anterior chamber angle anatomical landmarks.
| Schwalbe's line % |
Anterior limbus % (P) |
Angle recess % (P) |
Scleral spur % (P) |
||
|---|---|---|---|---|---|
| Observer 1 | Nasal | 100.0 | 98.5 (0.50) | 87.7 (<0.01) | 80.0 (<0.01) |
| Temporal | 96.9 | 100. (0.24) | 84.6 (0.02) | 84.6 (0.02) | |
| Observer 2 | Nasal | 96.9 | 100. (0.24) | 84.6 (0.02) | 78.5 (<0.01) |
| Temporal | 96.9 | 98.5 (0.50) | 86.2 (0.03) | 80.0 (<0.01) | |
%, percent of cases with visible anatomical landmark; P-values compare visibility of Schwalbe’s line with other anatomical landmarks.
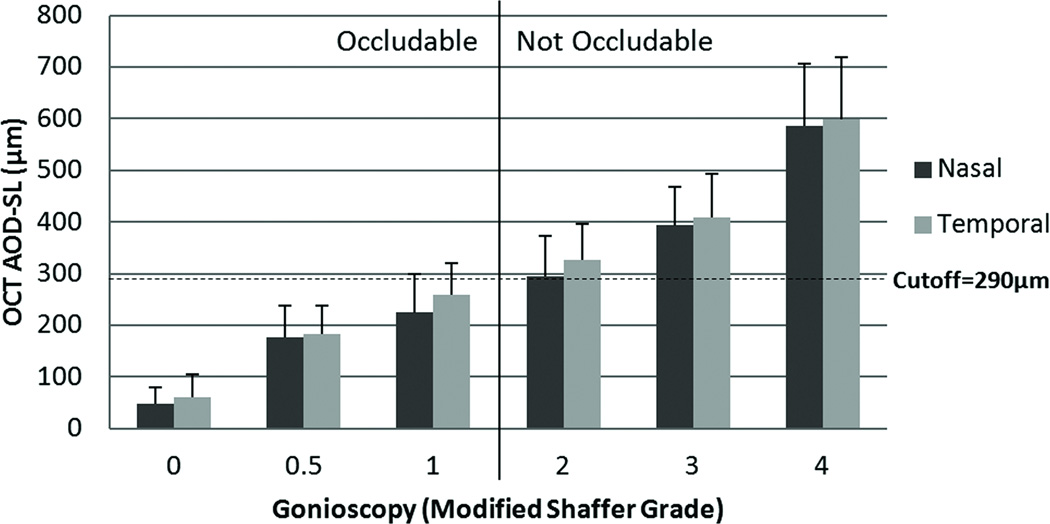
In OCT images, the AOD-SL was visible in 63 of the 65 eyes, and the SL-SS distance was visible in 50 eyes. Twenty-eight out of 63 eyes were considered to have modified Shaffer grade ≤1 angles (Figure 2). Table 2 showed the values of the AOD-SL and the SL-SS distances averaged from 2 observers, subjects with modified Shaffer grade ≤1 angles had significantly smaller AOD-SL compared with those with open angles ( P < 0.001) while the SL-SS distances showed no statistically significant difference (P = 0.068). The range of intra-observer CVs of AOD-SL, 9.8–12.0%, was slightly better than the range of inter-observer CVs, 10.1–13.3% (Table 3). The range of intra-observer CVs of SL-SS distance, 10.9–11.3%, was slightly better than the range of inter-observer CVs, 11.4–16.3%. The value of AOD-SL increased with higher modified Shaffer grades (Figure 3). The correlation coefficients between AOD-SL and gonioscopy grade were r = 0.80 (P < 0.001) for nasal angles and r = 0.81 (P < 0.001) for temporal angles. The linear trend test was significant for both nasal and temporal AOD-SL (P < 0.001). The AROC of the AOD-SL for detecting modified Shaffer grade ≤1 angles was 0.90 for both nasal and temporal angles. The optimized diagnostic cut-off point of AOD-SL for detecting modified Shaffer grade ≤1 angles was 290 µm. The sensitivity and specificity of the AOD-SL cut-off point for detecting modified Shaffer grade ≤1 angles were 0.80 and 0.87 for nasal angles and 0.85 and 0.77 for temporal angles, respectively.
Figure 2.
Distribution of occludable angles observed by gonioscopy. By modified Shaffer grade, 28 of 65 eyes had grades ≤1 and were considered to be occludable.
Table 2.
Measurements of angle opening distance at the Schwalbe’s line (AOD-SL) and distance between the Schwalbe’s line and the sclera spur (SL-SS).
| Angles with modified Shaffer grade ≤1 |
Angles with modified Shaffer grade ≥ 2 |
P-value | |
|---|---|---|---|
| µm | µm | ||
| (range) | (range) | ||
| AOD-SL | 194.4±99.7 | 445.9±196.0 | <0.001 |
| (0–350) | (192 – 922) | ||
| SL-SS | 700.6±134.1 | 738.9±184.7 | 0.068 |
| (333–902) | (344–1210) | ||
AOD-SL, angle opening distance at the Schwalbe’s line; SL-SS, distance between Schwalbe’s line (SL) and the sclera spur (SS).
Table 3.
Intra-observer and inter-observer repeatability of angle opening distance at the Schwalbe’s line (AOD-SL) and distance between the Schwalbe’s line and the sclera spur (SL-SS distance).
| Nasal | Temporal | |||||
|---|---|---|---|---|---|---|
| µm | CV% | µm | CV% | |||
| AOD-SL | Intra-observer | Observer 1 | 4.4±30.6 | 10.6 | 4.9±33.7 | 12.0 |
| Observer 2 | 4.6±23.5 | 9.8 | 4.4±30.7 | 10.6 | ||
| Inter-observer | 4.4±26.3 | 10.1 | 4.7±39.9 | 13.3 | ||
| SL-SS | Intra-observer | Observer 1 | 7.8±51.1 | 11.1 | 7.6±52.0 | 10.9 |
| Observer 2 | 8.0±52.4 | 11.3 | 7.8±52.5 | 11.0 | ||
| Inter-observer | 8.7±91.2 | 16.3 | 7.9±51.7 | 11.4 | ||
CV, coefficient of variation; AOD-SL, angle opening distance at Schwalbe’s line; SL-SS, distance between Schwalbe’s line (SL) and the sclera spur (SS).
Figure 3.
Comparison of optical coherence tomography (OCT) angle opening distance at Schwalbe’s line (AOD-SL) with modified Shaffer grade gonioscopy. The AOD-SL, based upon the average of two observers, was positively correlated with gonioscopy grades.
Discussion
Schwalbe’s line may be a more reliable anatomical landmark in angle assessment using Fourier-domain OCT than time-domain OCT because of its increased visibility. The Fourier-domain OCT used in our study had an axial resolution of 5 µm, which is more than 3 times higher than that of time-domain OCTs used in previous studies.14 The Fourier-domain OCT instruments can provide scan speeds 10–100 times faster than time-domain ones. This allows higher definition images (more axial scans in the same transverse scan length) to be acquired in less time. Moreover, the higher scan speed facilitates frame averaging which suppresses speckle noise. Small anatomical details such as SL, Schlemm’s canal, and the TM were not visible with previous generations of anterior segment OCT instruments.15, 16 The higher resolution and higher speed of Fourier-domain OCT improve image qualities and make visualization of these smaller anatomical details possible. In this study, SL was visible in more than 95% of the OCT images. The AL also had a high percentage of visibility. We chose not to use the AL to assess chamber angle because it occurs in the transition from the cornea to the sclera, and in some cases it can only be identified as a range instead of a single point. In other cases, it was difficult to identify the location of AL in patients having limbal degenerations and other corneal abnormalities.
The shorter wavelength of the 830 nm wavelength light source poses a negative impact in tissue penetration. This makes the visibility of the SS and AR not as good as SL and the AL due to the greater scattering of light at the scleral side of the limbus. A previous AC angle assessment study using 830 nm Fourier-domain OCT also reported better visibility of SL over the SS.17
The idea of using SS-based angle parameters in angle assessment was initially developed with ultrasound biomicroscopy.18 The advantage of OCT is that it requires neither contact nor immersion and is less user dependent.19 The area between the SS and SL represents the location of the TM where conventional aqueous humor outflow occurs.20 Previously, most OCT angle assessment studies used SS-based angle parameters solely, such as the AOD at 500 µm (AOD-500), AOD at 750 µm (AOD-750), and the trabecular iris space area at 500 µm and 750 µm.16, 21, 22 The 500 µm and 750 µm from the SS were estimations of the filtration area, and the true length of TM remained unknown due to limited resolution of 1310 nm OCT. Furthermore, several studies have reported that SS cannot be identified in 20%–25% of the time domain OCT images.8, 9 Because of its higher visibility, SL could be more reliable than the SS in assessing the angle using 830 nm high resolution OCT.
In our previous publication, we introduced AOD-SL as a new parameter that represents the valid filtration distance in angle assessment using Fourier-domain OCT.23 Cheung et al. also used OCT AOD-SL to assess the AC angle.24 They reported the visibility of SL and SS to be 95% and 85%, respectively. This is similar to our results of 97.7% and 80.8%. Their reported AOD-SL was 250 ± 150 µm which is smaller than ours at 330 ± 200 µm. In our study, the AOD-SL of closed angles ranged from 0 – 137 µm, and the open angles ranged from 192 – 922 µm. The distances in their study were 94 – 172 µm and 286 – 347 µm, respectively. The difference in AOD-SL measurements might be due to different patient populations. In Cheung et al.’s study, the patients were all Asian. Additionally, the mean age of their subjects was 60.6 ± 11.4 years, which is younger than ours. The correlation between AOD-SL and gonioscopy grade in Cheung et al.’s study, 0.709,24 was similar to our finding of 0.80. Their AROC for diagnosing angle closure was 0.88, which is also similar to our finding of 0.90. Similar to our own data, they also reported high intra-observer and inter-observer reliability in measuring AOD-SL and good correlation between AOD-SL and gonioscopy. All of these results showed that AOD-SL is a reliable parameter in the quantitative measurement of the angle.
The distance between SL and the SS represents the area of the TM, a distance we found to be 333–1210 µm. The only previous OCT AOD-SL study reported this distance to be 390–1230 µm.24 Another ultrasound biomicroscopy study reported this distance to be 400–800 µm among normal juvenile subjects.25 The length of the trabecular meshwork appears to vary over a wide range. For this reason, compared to using parameters such as AOD-500 or AOD-750 defined from the SS, measuring the angle opening distance at SL may more directly reflect whether or not a portion of the TM is accessible to the aqueous humor.
We found the AOD-SL to be a potentially useful method in assessing the angle. First, it could be identified in most eyes. Second, it was validated by the current clinical standard of gonioscopy. An occludable angle (modified Shaffer grade ≤1) suggests that the risk of angle-closure is higher. Identifying these at risk eyes can assist the clinician in making decisions that if alternative treatment or more frequent follow-ups with the patient are necessary. The cut-off value of AOD-SL for detecting modified Shaffer grade ≤1 angle was set at 290 µm with good diagnostic sensitivity and specificity. Thus, patients for whom the AOD-SL is under 290 µm may be at high risk of angle closure. Patients with AOD-SLs slightly over 290 µm could be monitored during follow-ups. Since the diagnostic cutoff was determined by correlation with gonioscopy, it has not yet being directly linked to the risk of angle closure. Further studies are needed to directly establish this link. Despite the preliminary nature of this diagnostic criterion, AOD-SL measurement by OCT is well suited for further studies in the screening of angle closure glaucoma due to its noncontact nature and ease of examination and digital documentation.
There were certain limitations of this study. It was difficult to acquire OCT images from the superior and inferior aspects of the angle. Eye lids and lashes can induce shadows on the images, making measurements impossible. During examination, the patients were asked to gaze at an external fixation target, but the axis of fixation for each individual could be slightly different. For that reason, fixation could not be completely standardized, and thus all images might not have been centered alike. All of the angle landmarks were manually identified on the images, and this is susceptible to subjective error. Software for automatic identification of the SL is under development. Unlike gonioscopy, which can observe the angle in a 360 degree fashion, OCT line-scan images can only observe certain axes of the angle. A new study using a three-dimensional OCT scan pattern will be conducted to better assess the angle.
In conclusion, SL could be identified in over 95% of OCT scans, and it may be a useful anatomical landmark for assessing the angle by 830 nm Fourier-domain OCT. The AOD-SL correlated well with gonioscopy and could be potentially useful in assessing angle-closure glaucoma risks, patients screening, and monitoring.
Acknowledgments
Financial support:
This study was supported by NIH grants R01EY013516 and R01EY018184.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial and proprietary interest:
Bing Qin, Brian A. Francis, Chunhui Jiang, and Catherine Cleary have no financial or proprietary interest in any material or method mentioned. Yan Li, Maolong Tang, Xinbo Zhang, and David Huang received grant support from Optovue Inc.; Yan Li and David Huang received travel support from Optovue, Inc. David Huang received patent royalty, speaker honorarium, and stock options from Optovue, Inc.
References
- 1.Thylefors B, Negrel AD. The global impact of glaucoma. Bull World Health Organ. 1994;72:323–326. [PMC free article] [PubMed] [Google Scholar]
- 2.Dandona L, Dandona R, Mandal P, et al. Angle-closure glaucoma in an urban population in southern India. The Andhra Pradesh eye disease study. Ophthalmology. 2000;107:1710–1716. doi: 10.1016/s0161-6420(00)00274-8. [DOI] [PubMed] [Google Scholar]
- 3.Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001;85:1277–1282. doi: 10.1136/bjo.85.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew PT, Aung T. Primary angle-closure glaucoma in Asia. J Glaucoma. 2001;10:S7–S8. doi: 10.1097/00061198-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Alsbirk PH. Anterior chamber depth, genes and environment. A population study among long-term Greenland Eskimo immigrants in Copenhagen. Acta Ophthalmol (Copenh) 1982;60:223–224. doi: 10.1111/j.1755-3768.1982.tb08376.x. [DOI] [PubMed] [Google Scholar]
- 6.Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? The British journal of ophthalmology. 2001;85:1277–1282. doi: 10.1136/bjo.85.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim LS, Aung T, Husain R, et al. Acute primary angle closure: configuration of the drainage angle in the first year after laser peripheral iridotomy. Ophthalmology. 2004;111:1470–1474. doi: 10.1016/j.ophtha.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Sakata LM, Lavanya R, Friedman DS, et al. Assessment of the scleral spur in anterior segment optical coherence tomography images. Arch Ophthalmol. 2008;126:181–185. doi: 10.1001/archophthalmol.2007.46. [DOI] [PubMed] [Google Scholar]
- 9.Narayanaswamy A, Sakata LM, He MG, et al. Diagnostic performance of anterior chamber angle measurements for detecting eyes with narrow angles: an anterior segment OCT study. Arch Ophthalmol. 2010;128:1321–1327. doi: 10.1001/archophthalmol.2010.231. [DOI] [PubMed] [Google Scholar]
- 10.Tan O, Chopra V, Lu AT, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:2305–2314. e1–e2. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim JI, Tan O, Fawzi AA, et al. A pilot study of Fourier-domain optical coherence tomography of retinal dystrophy patients. Am J Ophthalmol. 2008;146:417–426. doi: 10.1016/j.ajo.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer RN, editor. Stereoscopic manual of gonioscopy. Saint Louis: Mosby; 1962. [Google Scholar]
- 13.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 14.Li Y, Tang M, Zhang X, et al. Pachymetric mapping with Fourier-domain optical coherence tomography. J Cataract Refract Surg. 2010;36:826–831. doi: 10.1016/j.jcrs.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang D, Li Y, Radhakrishnan S. Optical coherence tomography of the anterior segment of the eye. Ophthalmol Clin North Am. 2004;17:1–6. doi: 10.1016/S0896-1549(03)00103-2. [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123:1053–1059. doi: 10.1001/archopht.123.8.1053. [DOI] [PubMed] [Google Scholar]
- 17.Wong HT, Lim MC, Sakata LM, et al. High-definition optical coherence tomography imaging of the iridocorneal angle of the eye. Arch Ophthalmol. 2009;127:256–260. doi: 10.1001/archophthalmol.2009.22. [DOI] [PubMed] [Google Scholar]
- 18.Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992;113:381–389. doi: 10.1016/s0002-9394(14)76159-8. [DOI] [PubMed] [Google Scholar]
- 19.Memarzadeh F, Li Y, Francis BA, et al. Optical coherence tomography of the anterior segment in secondary glaucoma with corneal opacity after penetrating keratoplasty. Br J Ophthalmol. 2007;91:189–192. doi: 10.1136/bjo.2006.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields MB, editor. Textbook of glaucoma. 4th ed. Philadelphia: Williams & Wilkins; 2000. [Google Scholar]
- 21.Li H, Leung CK, Cheung CY, et al. Repeatability and reproducibility of anterior chamber angle measurement with anterior segment optical coherence tomography. Br J Ophthalmol. 2007;91:1490–1492. doi: 10.1136/bjo.2007.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Memarzadeh F, Tang M, Li Y, et al. Optical coherence tomography assessment of angle anatomy changes after cataract surgery. Am J Ophthalmol. 2007;144:464–465. doi: 10.1016/j.ajo.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memarzadeh F, Mahdaviani S, Li Y. Interpretation of angle images. In: Huang D, Duker JS, Fujimoto JG, editors. Imaging the eye from front to back with RTVue Fourier-domain optical coherence tomography. Thorofare, NJ: SLACK; 2010. pp. 47–51. [Google Scholar]
- 24.Cheung CY, Zheng C, Ho CL, et al. Novel anterior-chamber angle measurements by high-definition optical coherence tomography using the Schwalbe line as the landmark. The British journal of ophthalmology. 2011;95:955–959. doi: 10.1136/bjo.2010.189217. [DOI] [PubMed] [Google Scholar]
- 25.Stegman Z, Sokol J, Liebmann JM, et al. Reduced trabecular meshwork height in juvenile primary open-angle glaucoma. Arch Ophthalmol. 1996;114:660–663. doi: 10.1001/archopht.1996.01100130652003. [DOI] [PubMed] [Google Scholar]