Abstract
Although liquid chromatography/electrospray ionization tandem mass spectrometry-based assays have been reported for the measurement of the antiviral oseltamivir (OS) in human samples, these assays either involve complicated sample pretreatment or lack sensitivity. Here we introduce a straightforward approach to improve the assay performance for OS and its metabolite oseltamivir carboxylate (OSC) in human plasma. A very low concentration of mobile phase modifier can improve the ionization efficiency of both analytes, thus enabling a high sensitivity without any matrix effect. The fast LC gradient further increases the sensitivity by narrowing the peak width (6–9 seconds) and eluting the analytes at higher organic content. The increased ionization efficiency and minimized matrix effects enabled us to introduce a one-step protein precipitation for sample clean-up without compromising the sensitivity. The lower limit of quantification was 0.34 ng/mL for both analytes, which was at least 3 times more sensitive than published assays that involve complicated sample pretreatment. The assay involves measurement of analytes and their stable-isotope internal standards in small-volume (30-µL) plasma. Sodium fluoride was utilized to prevent the hydrolysis of OS during and after sampling. The calibration curve was linear over the range of 0.34–1000 ng/mL. Accuracy was 95–110% and the precision was 2.2–11.0%. This method was applied successfully to the human pharmacokinetic study of OS, and can estimate the relevant pharmacokinetic parameters of OS with more accuracy. The approach utilized in the optimization of assay performance can be extended to the measurement of other drugs in biomatrices.
Keywords: liquid chromatography, electrospray ionization, mass spectrometry, oseltamivir, pharmacokinetics
1. Introduction
Oseltamivir (OS), an ethyl ester prodrug that is rapidly converted in vivo to the active neuraminidase inhibitor oseltamivir carboxylate (OSC), is the first-line therapy for patients with influenza virus infection [1]. Consequently, post marketing clinical study continues to fulfill a need to improve our understanding of the therapeutics and toxicity especially in specific subpopulations. For example to assess pharmacokinetics and safety of oseltamivir in high-risk populations such as young infants, immunocompromised patients and the severely ill [2]. Determinations of drug concentration in these patient subgroups are often hampered by inadequately sensitive analytical methods and blood sample volume limitations. Accordingly, a very sensitive assay for the measurement of OS and OSC in small-volume human plasma specimens is required. A technically simple assay would reduce costs and increase availability to labs with limited resources. Therefore, a simple and routine assay for the measurement of oseltamivir in human plasma without compromising high sensitivity is particularly attractive.
Currently, liquid chromatography/electrospray ionization tandem mass spectrometry (LC-MS/MS)-based assays have been reported for the measurement of OS and OSC in plasma or blood [3–10]. Six of these assays measured OS and OSC in human plasma [3–6, 9, 10], while one assay measured the analytes in human dried blood spots (DBS) [7]. The assays used offline or online extraction for sample pretreatment, which can be time consuming and costly. In these studies, the lower limit of quantification (LLOQ) for OS and OSC was 1–5 ng/mL and 1–20 ng/mL, respectively. The assay developed by Heinig et al. [5] was the most sensitive one with a LLOQ of 1 ng/mL for both analytes. Only one recently reported assay incorporates a simple protein precipitation as sample pretreatment [9]. Unfortunately, the sensitivity of this assay was not satisfactory (LLOQ was 3 ng/mL for OS and 10 ng/mL for OSC), and significant matrix effects were observed for OS and OSC.
This paper describes the optimization of electrolyte and chromatographic method to enhance the assay performance for oseltamivir and its active metabolite oseltamivir carboxylate in human plasma using high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry.
2. Experimental
A detailed description of experimental procedures is provided in the Supplementary Material online.
2.1. Chemicals and reagents
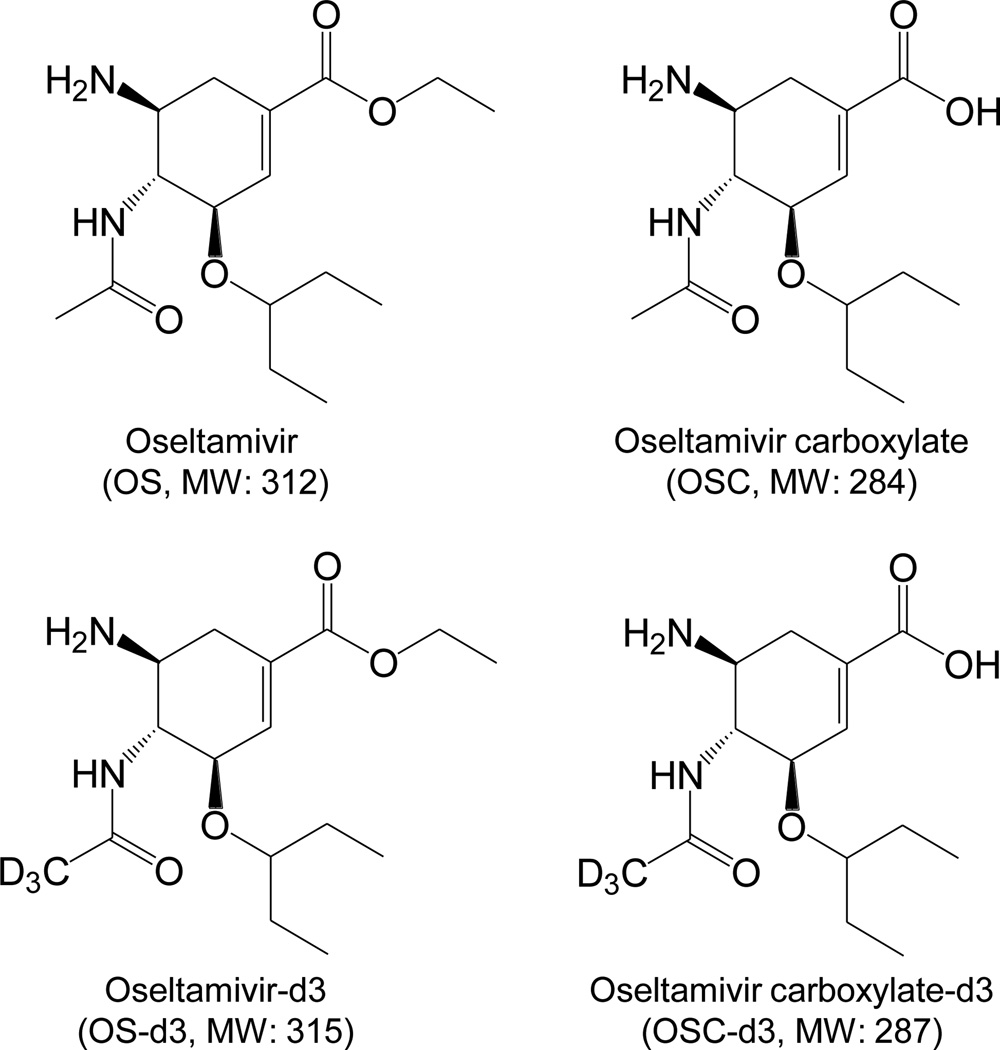
Oseltamivir (OS), oseltamivir carboxylate (OSC), deuterated oseltamivir (OS-d3), and deuterated oseltamivir carboxylate (OSC-d3) (Figure 1) were obtained from Toronto Research Chemicals (North York, ON, Canada).
Fig. 1.
Chemical structures of oseltamivir (OS) and oseltamivir carboxylate (OSC), as well as those of the two internal standards deuterated oseltamivir (OS-d3) and deuterated oseltamivir carboxylate (OSC-d3).
2.2. Chromatographic and mass spectrometric conditions
An AB SCIEX 3000 triple quadrupole mass spectrometer (Toronto, Canada) interfaced via a turbo ion spray (ESI) source with a Shimadzu HPLC separation module (Norcross, GA, USA) was used. The LC separation was achieved on a 5.0 µm Agilent Eclipse Plus C18 column (50 mm × 2.1 mm I.D.; Santa Clara, CA). Mobile phases were methanol/water, 1:99 (v/v), containing 2.5 mM formic acid, for A and methanol/water, 99:1 (v/v), modified with the same electrolyte, for B. A pulse gradient chromatographic method was used, which was modified from an earlier method we had developed for analysis of simmitecan [11].
The precursor-product ion pairs (singly protonated species) used for multiple reaction monitoring (MRM) of OS, OSC, OS-d3, and OSC-d3 were m/z 313.4→225.1, 285.3→138.0, 316.4→228.0 and 288.3→ 200.0, respectively. Two stable-isotope internal standards (IS), OS-d3 and OSC-d3, were used.
2.3. Plasma sample preparations
After deactivating blood esterases with sodium fluoride, the plasma samples (30 µL) were precipitated with 100 µL of methanol containing the internal standards OS-d3 and OSC-d3 at 100 ng/mL. After centrifugation for 8.0 min at 16,000×g, 10 µL of the resulting supernatant were injected into the LC-MS/MS. For the preparation of calibration curves, blank plasma was obtained from centrifugation of drug-free blood treated with sodium fluoride. The calibration standards were prepared using the blank plasma samples spiked with OS and OSC, the concentrations ranges of which were 0.34–1000 ng/mL both for OS and OSC.
2.4. Assessment of mobile phase modifier, matrix effects and assay validation
The effect of the formic acid concentration (0, 0.5, 2.5, and 25 mM) in the mobile phase on the signal intensity of the tested compounds was studied. In order to identify whether the human plasma components or blood additives (sodium fluoride) generate matrix effects on the ESI-based measurement of the analytes and internal standards, a post-extraction spike method was employed, which assessed the sample absolute and relative matrix effects.
Assay validation was carried out according to the US Food and Drug Administration guidance on bioanalytical method validation to demonstrate that the newly developed bioanalytical method was reliable for the intended applications [12].
2.5. Application to human pharmacokinetic study
To demonstrate its applicability, the newly developed analytical method was applied to quantify the plasma concentration-time profile of oseltamivir in one healthy human subject who received a single 150 mg oral dose of oseltamivir. This study was approved by the University of Tennessee Health Science Center Institutional Review Board and the subject provided written informed consent before participation. Serial blood samples (0, 0.5, 1.0, 1.5, 2, 3, 4, 6, 8, 10, and 24 h) were directly collected into BD Vacutainer tubes that contained 15 mg of sodium fluoride and 12 mg of potassium oxalate. After gentle shaking for 5–10 s, the plasma samples were separated and kept frozen at −70 °C until analysis.
Plasma pharmacokinetic parameters were calculated by standard non-compartmental methods using WinNonlin 3.1 (Pharsight, Mountain View, CA).
3. Results and discussion
3.1. Optimization of ionization efficiency and matrix effects by mobile phase modifier
Poor ionization efficiency of analytes [13] and interference of matrix components [14–16] in ESI source are two major problems with many LC-MS/MS-based bioanalytical assays. We suggest that optimization of the ionization efficiency and matrix effects can be readily realized by adjusting the mobile phase modifier, which has been largely ignored by the earlier reported assays for OS.
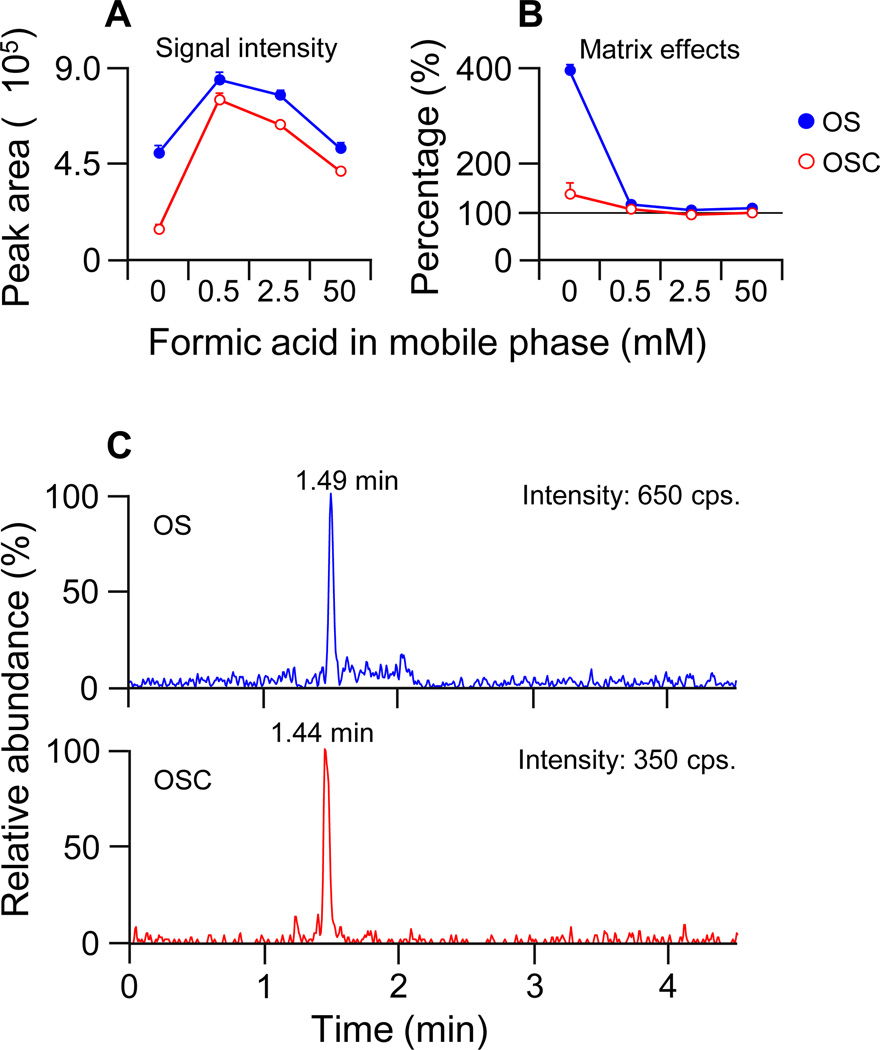
The effect of the formic acid concentration in the LC mobile phase on the signal intensity of the tested compounds is shown in Fig. 2A. The highest signal intensity was achieved when the concentration of formic acid in the mobile phase was 0.5 mM (1.7- to 5.3-fold higher than that in electrolyte-free mobile phase). Inclusion of a high concentration (50 mM) of mobile phase additive was shown to reduce the ESI response (Fig. 2A). Furthermore, formic acid in the mobile phase dramatically decreased the interference by matrix effects (Fig. 2B). The absolute matrix effects were minimized when the mobile phase contained 2.5 mM (or more) formic acid (Fig. 2B). Considering both the signal intensity and matrix effects, 2.5 mM formic acid was used as the mobile phase modifier in the subsequent validation study. This mobile phase modifier concentration is much lower than the 50 to 500 mM concentrations of electrolytes (formic or acetic acid) used in the reported assays for OS [3–10]. Therefore, it is likely that the ESI-based responses of analytes were suppressed by using high concentrations of mobile phase modifiers in the earlier assays for OS.
Fig. 2.
The effect of the formic acid concentration in LC mobile phase on the signal intensity (A) and absolute matrix effects (B) of oseltamivir (solid circle, OS) and oseltamivir carboxylate (open circle, OSC) (average values and standard deviations of triplicate measurements), and exemplified chromatogram of OS and OSC at the lower limit of quantification (LLOQ) in matrix-matched sample, monitored with scheduled MRMs for the following mass transitions OS m/z 313.4→225.1 and OSC m/z 285.3→138.0 (C).
The underlying mechanism concerning the effect of the formic acid on the signal intensity and matrix effects needed to be considered. Analytes tend to have higher signal intensities in mobile phase modified with formic acid as compared with electrolyte-free mobile phase, because formic acid is believed to facilitate the formation of protonated species in ESI droplets. However, further increasing the formic acid concentration was found to decrease the ESI response. This can be explained by the fact that there is a limited amount of excess charge and surfaces available on the ESI droplets [15]. High concentration of formic acid would compete with the target analytes for the limited charge or space on the surface droplet. It was interesting to note that serious matrix effects (signal enhancement) were observed when using electrolyte-free mobile phase. We propose that electrolyte-like component in plasma may facilitate the formation of protonated analytes in ESI droplets when no electrolyte exists in the mobile phase. This effect would be minimal after a certain amount of formic acid was added to the mobile phase.
The post-extraction spike-based assessment indicated that absolute matrix effects of sodium fluoride-treated plasma samples on ESI-based measurement of OS and OSA were between 88% and 105% (n = 3) and the associated relative matrix effects were between 3.0% and 14.5% (n = 3) (Table 1). With regard to the previously published assays for OS in human plasma or blood [3–7, 9, 10]; one study assessed the matrix effects appropriately and found no significant matrix effect [4], three studies did not evaluate the matrix effects [3, 6, 10], and the other three studies either did not assess the matrix effect properly or found significant matrix effects without attempting to adjust the assay conditions to reduce them [5, 7, 9].
Table 1.
Matrix effects of oseltamivir (OS), oseltamivir carboxylate (OSC), deuterated oseltamivir (OS-d3), and deuterated oseltamivir carboxylate (OSC-d3) in human plasma
| Compounds | Concentration (ng/mL) |
Blank A (%), absolute ME |
Blank B (%), absolute ME |
Blank C (%), absolute ME |
Average (%), absolute ME |
CV (%), relative ME |
|---|---|---|---|---|---|---|
| 4.1 | 107 | 97 | 99 | 101 | 4.9 | |
| OS | 37.1 | 85 | 107 | 114 | 102 | 14.5 |
| 333 | 103 | 109 | 103 | 105 | 3.1 | |
| 4.1 | 83 | 90 | 91 | 88 | 5.3 | |
| OSC | 37.1 | 92 | 97 | 97 | 96 | 3.0 |
| 333 | 91 | 98 | 94 | 95 | 3.8 | |
| OS-d3 | 100 | 99 | 105 | 102 | 102 | 3.0 |
| OSC-d3 | 100 | 90 | 99 | 94 | 94 | 5.1 |
ME, matrix effect; Blank A (or B, C), blank plasma from three different human subjects.
3.2. The fast LC gradient
LC-MS sensitivity can be improved through increases in chromatographic performance (narrowing of the peak widths) [17]. In addition, the LC-MS-based assay sensitivity is normally better for analytes that are eluted at higher organic modifier content [18]. The increased signal intensity may result from more efficient desolvation of the analyte in organic modifier than in water, and from improved ESI spray stability due to the decreased droplet surface tension [19]. Therefore, a very fast LC gradient was used here to increase the sensitivity of analytes by narrowing the peak width and eluting the analytes at higher organic modifier content. The fast LC gradient first retains the analytes with a mobile phase containing 95% water (start proportion) allowing the unretained sample solvent and polar matrix components to be washed from the column. The analytes are then eluted from the column with a strong mobile phase containing 99% methanol (elution proportion), which also results in favorable band compression on a conventional C18 column (5 µm particle size). The peak widths for OS and OSC were around 6 and 9 seconds, respectively (Fig. 2C). In contrast, the peak widths of most reported assays are about 18–60 seconds (obtained by visual assessment of the figures). Only one published assay showed comparable peak widths to those of our assay. It should be pointed out that this assay was the most sensitive of those previously published, with a LLOQ of 1 ng/mL for both analytes [5].
In summary, taking advantage of the fast LC gradient allowed us to further increase the sensitivity of the analytes.
3.3. Selectivity, linearity, lower limit of quantification, and accuracy and precision
In the initial test, we found the precursor-product ion pair used for multiple reaction monitoring of OS-d3 (288.3→200.0) could interfere with the detection of OS (313.4→166.1). Therefore, we used an alternative precursor-product ion pair (313.4→225.1) for OS to avoid the interference. The signal intensity of the two ion pairs for OS was comparable.
Blank plasma samples from five donors were analyzed to determine whether plasma components interfered with the detection of the compounds of interest (selectivity). No significant interferences (≤ 20% of LLOQ) were observed at the retention times of OS and OSC.
The calibration curves for quantification of OS and OSC in plasma showed good linear relationships, ranging up to 1000 ng/mL with correlation coefficients >0.99. The within-run (n = 3) and between-run (n = 3) precision of the assay were quite good for both of the analytes, i.e., 2.2–11.0% and 1.8–9.9%, respectively, while the assay accuracy was also satisfactory, i.e., 95–104% and 96–110%, respectively (Table 2). The LLOQ of a 30 µL initial plasma sample size was 0.34 ng/mL for both analytes (Fig. 2C). The plasma sample size utilized in this study is less than those of reported assays for human plasma (50–500 µL) [3–6, 9, 10].
Table 2.
Accuracy and precision (RSD) for assay of oseltamivir (OS) and oseltamivir oseltamivir carboxylate (OSC) in human plasma
| Nominal concentration (ng/mL) |
Within-run (n = 3) | Between-run (n = 3) | ||
|---|---|---|---|---|
| Measured concentration (ng/mL) mean ± SD (RSD) |
Accuracy | Measured concentration (ng/mL) mean ± SD (RSD) |
Accuracy | |
| OS | ||||
| 0.34 (LLOQ) | 0.33 ! 0.04 (11.0%) | 97% | 0.36 ! 0.04 (9.9%) | 106% |
| 4.1 | 3.90 ! 0.09 (2.2%) | 95% | 3.93 ± 0.07 (1.8%) | 96% |
| 37.1 | 37.1 ! 1.0 (2.7%) | 100% | 37.1 ± 0.8 (2.2%) | 100% |
| 333 | 339 ! 9 (2.5%) | 102% | 336 ± 8 (2.5%) | 101% |
| OSC | ||||
| 0.34 (LLOQ) | 0.36 ! 0.03 (9.7%) | 104% | 0.37 ! 0.03 (7.7%) | 110% |
| 4.1 | 4.04 ! 0.15 (3.7%) | 98% | 4.11 ± 0.14 (3.4%) | 100% |
| 37.1 | 37.5 ! 1.0 (2.6%) | 101% | 37.3 ± 0.8 (2.2%) | 101% |
| 333 | 339 ± 10 (3.0%) | 102% | 336 ± 8 (2.4%) | 101% |
The human plasma was treated with sodium fluoride before use.
3.4. Stability of OS and OSC
In this study, the esterase inhibitor sodium fluoride was utilized to deactivate blood esterases to prevent the hydrolysis of OS during and after sampling. After sodium fluoride-based treatment of blood samples, both OS and OSC, as well as their respective internal standards, were stable under analytical conditions mimicking those experienced by real samples. For example, both OS and OSC were stable for at least 4.5 h in plasma samples at 24 °C, and after three freeze-thaw cycles. The processed samples were also stable in the autosampler for at least 18 h. The results of the long-term storage stability study showed the analytes were stable for at least three weeks at −70°C. The stock solution stability (in water) was not assessed in this study because previous studies demonstrated that both analytes were stable in the stock solution for two years (−20 °C) [9].
3.5. Application to the human pharmacokinetic study
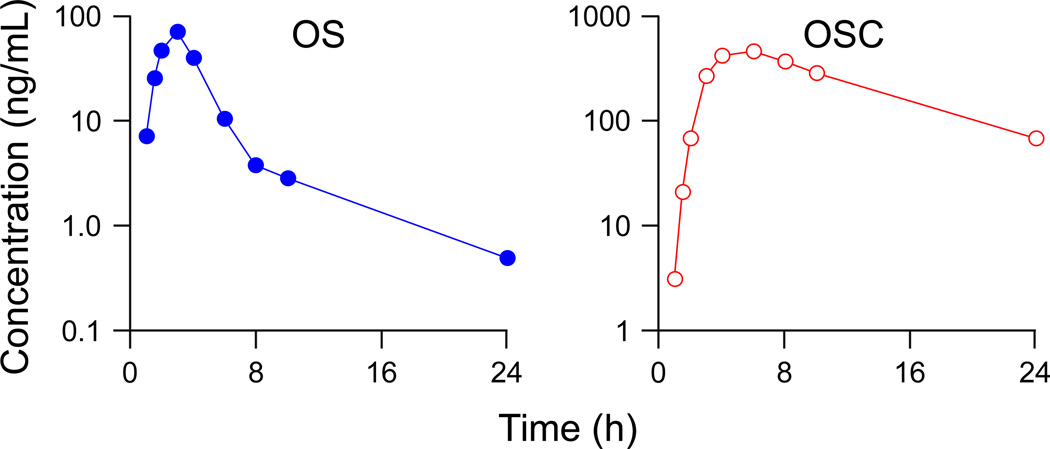
After assay validation, the newly developed bioanalytical method was applied to the pharmacokinetic study of OS in one human subject. As shown in Fig. 3, both OS and the metabolite OSC were measurable in all plasma samples up to 24 h. In general, the pharmacokinetic parameters were similar to the reported values except for t1/2 of OS [20]. The t1/2 of OS presented here (6.5 h) was more than 2-fold higher than the reported values (1.7 ± 0.5 h). We suggest this may be caused by the insufficient sensitivity of the earlier assays which failed to measure the 24-h plasma samples with the concentration of OS lower than 1 ng/mL. As shown in Fig. 3, the estimated t1/2 will be underestimated if we omit the 24-h point and recalculate the t1/2 based on the last three points (6-, 8-, and 10-h plasma samples). This explanation is borne out by the results of our study of 10 healthy volunteers that document an OS t1/2 of 4.2 ± 1.1 h. In addition, AUC0→∞ will also be underestimated by 10% if we omit the 24-h point. Therefore, a more sensitive assay will provide a more accurate estimation of the OS pharmacokinetic parameters.
Fig. 3.
Plasma concentration-time profile of oseltamivir (OS, solid circle) and the metabolite oseltamivir carboxylate (OSC, open circle) after a single oral 150 mg dose of OS to a human subject.
4. Conclusions
To our knowledge, this is the first assay for the measurement of OS and OSC in human plasma that integrates both the high sensitivity and simplicity. The newly developed assay was successfully applied to the human pharmacokinetic study of OS, and has the potential to accurately estimate the pharmacokinetic parameters of OS with more confidence. The approach utilized in the optimization of sensitivity and matrix effect is extremely straightforward, and can be extended to the development of assays for other drugs in biological samples.
Supplementary Material
A simple and sensitive assay for plasma oseltamivir and its metabolite was described.
This is the first assay that integrates both the high sensitivity and simplicity.
The approach presented here can be used in the optimization of assays for other drugs.
Acknowledgement
This study was financially supported by grant R15GM096074 from the National Institute of General Medical Sciences.
Abbreviations
- OS
oseltamivir
- OSC
oseltamivir carboxylate
- OS-d3
deuterated oseltamivir
- OSC-d3
deuterated oseltamivir carboxylate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dutkowski R. Oseltamivir in seasonal influenza: cumulative experience in low- and high risk patients. J. Antimicrob. Chemother. 2010;65(Suppl 2):ii11–ii24. doi: 10.1093/jac/dkq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy D. Responding to pandemic (H1N1) 2009 influenza: the role of oseltamivir. J. Antimicrob. Chemother. 2010;65(Suppl 2):ii35–ii40. doi: 10.1093/jac/dkq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiltshire H, Wiltshire B, Citron A, Clarke T, Serpe C, Gray D, Herron W. Development of a high-performance liquid chromatographic-mass spectrometric assay for the specific and sensitive quantification of Ro 64-0802, an anti-influenza drug, and its pro-drug, oseltamivir, in human and animal plasma and urine. J. Chromatogr. B. 2000;745:373–388. doi: 10.1016/s0378-4347(00)00300-5. [DOI] [PubMed] [Google Scholar]
- 4.Lindegardh N, Hanpithakpong W, Wattanagoon Y, Singhasivanon P, White NJ, Day NP. Development and validation of a liquid chromatographic-tandem mass spectrometric method for determination of oseltamivir and its metabolite oseltamivir carboxylate in plasma, saliva and urine. J. Chromatogr. B. 2007;859:74–83. doi: 10.1016/j.jchromb.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Heinig K, Bucheli F. Sensitive determination of oseltamivir and oseltamivir carboxylate in plasma, urine, cerebrospinal fluid and brain by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 2008;876:129–136. doi: 10.1016/j.jchromb.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Kanneti R, Bhavesh D, Paramar D, R S, Bhatt PA. Development and validation of a high throughput and robust LC-MS/MS with electrospray ionization method for simultaneous quantitation of oseltamivir phosphate and its oseltamivir carboxylate metabolite in human plasma for pharmacokinetic studies. Biomed. Chromatogr. 2011;25:727–733. doi: 10.1002/bmc.1509. [DOI] [PubMed] [Google Scholar]
- 7.Hooff GP, Meesters RJ, van Kampen JJ, van Huizen NA, Koch B, Al Hadithy AF, van Gelder T, Osterhaus AD, Gruters RA, Luider TM. Dried blood spot UHPLC-MS/MS analysis of oseltamivir and oseltamivircarboxylate-a validated assay for the clinic. Anal. Bioanal. Chem. 2011;400:3473–3479. doi: 10.1007/s00216-011-5050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinig K, Wirz T, Bucheli F, Gajate-Perez A. Determination of oseltamivir (Tamiflu®) and oseltamivir carboxylate in dried blood spots using offline or online extraction. Bioanalysis. 2011;3:421–437. doi: 10.4155/bio.11.4. [DOI] [PubMed] [Google Scholar]
- 9.Kromdijk W, Rosing H, van den Broek MP, Beijnen JH, Huitema AD. Quantitative determination of oseltamivir and oseltamivir carboxylate in human fluoride EDTA plasma including the ex vivo stability using high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Chromatogr. B. 2012;891–892:57–63. doi: 10.1016/j.jchromb.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Chang Q, Chow MS, Zuo Z. Studies on the influence of esterase inhibitor to the pharmacokinetic profiles of oseltamivir and oseltamivir carboxylate in rats using an improved LC/MS/MS method. Biomed. Chromatogr. 2009;23:852–857. doi: 10.1002/bmc.1195. [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Sun Y, Du F, Niu W, Xu F, Huang Y, Li C. Accurate determination of the anticancer prodrug simmitecan and its active metabolite chimmitecan in various plasma samples based on immediate deactivation of blood carboxylesterases. J. Chromatogr. A. 2011;1218:6646–6653. doi: 10.1016/j.chroma.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. [last accessed on May, 2012];Bioanalytical Method Validation. 2001 http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdf.
- 13.Gao S, Zhang ZP, Karnes HT. Sensitivity enhancement in liquid chromatography/atmospheric pressure ionization mass spectrometry using derivatization and mobile phase additives. J. Chromatogr. B. 2005;825:98–110. doi: 10.1016/j.jchromb.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Gosetti F, Mazzucco E, Zampieri D, Gennaro MC. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A. 2010;1217:3929–3937. doi: 10.1016/j.chroma.2009.11.060. [DOI] [PubMed] [Google Scholar]
- 15.Trufelli H, Palma P, Famiglini G, Cappiello A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2011;30:491–509. doi: 10.1002/mas.20298. [DOI] [PubMed] [Google Scholar]
- 16.Chambers E, Wagrowski-Diehl DM, Lu Z, Mazzeo JR. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J. Chromatogr. B. 2007;852:22–34. doi: 10.1016/j.jchromb.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Churchwell MI, Twaddle NC, Meeker LR, Doerge DR. Improving LC-MS sensitivity through increases in chromatographic performance: comparisons of UPLC-ES/MS/MS to HPLC-ES/MS/MS. J. Chromatogr. B. 2005;825:134–143. doi: 10.1016/j.jchromb.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Dams R, Benijts T, Gunther W, Lambert W, De Leenheer A. Influence of the eluent composition on the ionization efficiency for morphine of pneumatically assisted electrospray, atmospheric-pressure chemical ionization and sonic spray. Rapid Commun. Mass Spectrom. 2002;16:1072–1077. doi: 10.1002/rcm.683. [DOI] [PubMed] [Google Scholar]
- 19.Niu W, Zhu X, Yu K, Li L, Sun Y, Li C. Nebulizing conditions of pneumatic electrospray ionization significantly influence electrolyte effects on compound measurement. J. Mass Spectrom. 2012;47:370–380. doi: 10.1002/jms.2050. [DOI] [PubMed] [Google Scholar]
- 20.He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin. Pharmacokinet. 1999;37:471–484. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.