Abstract
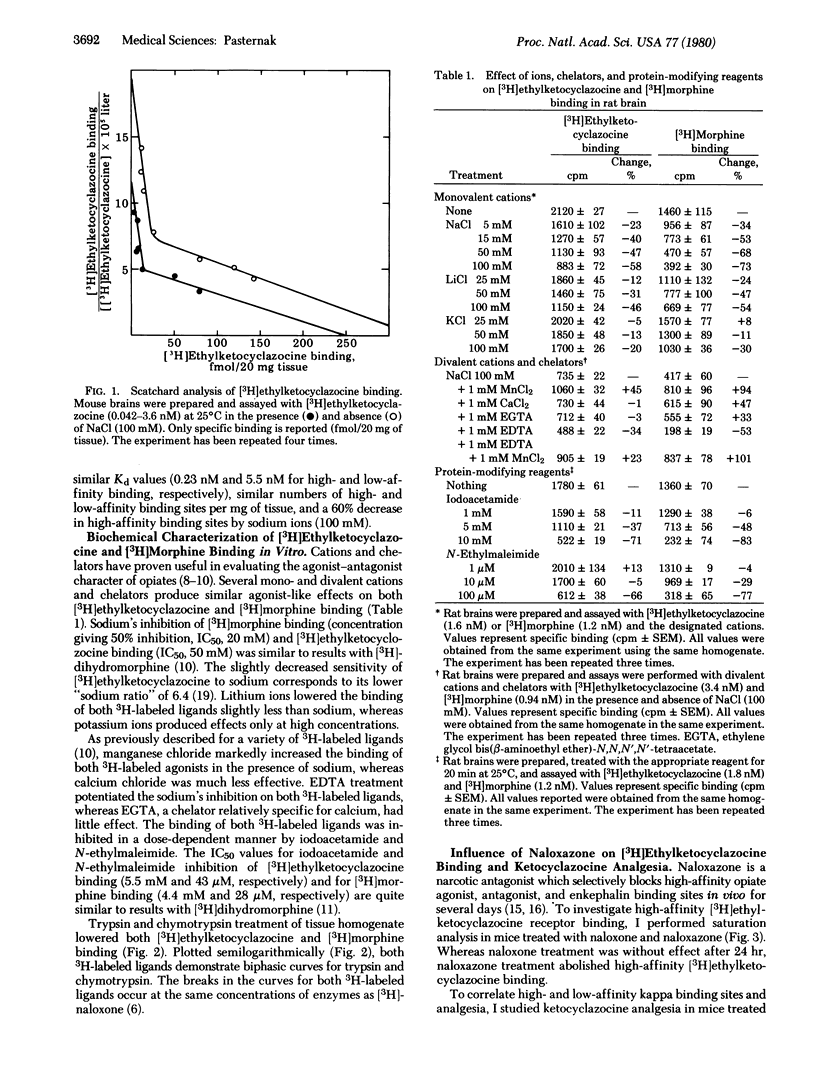
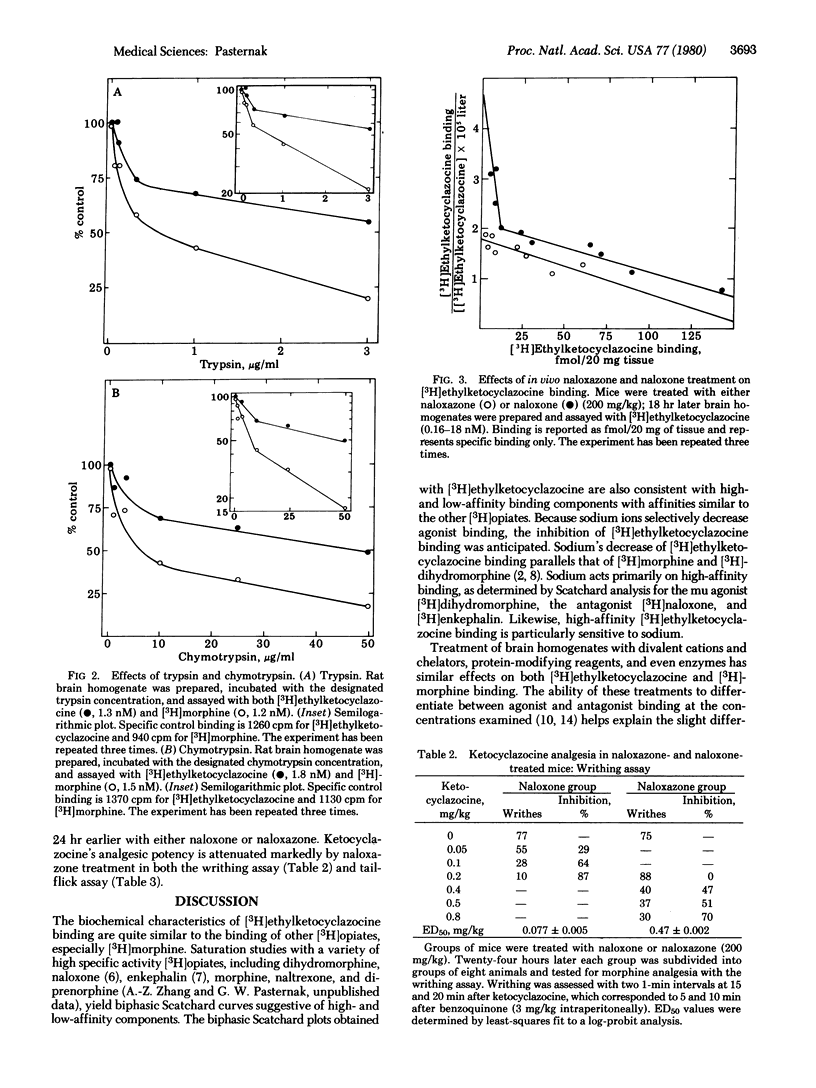
The receptor binding of the kappa agonist [3H]ethylketocyclazocine to brain homogenates in vitro and ketocyclazocine (kappa) analgesia in vivo has been investigated and compared to morphine, a mu agonist. Saturation analysis of [3H]ethylketocyclazocine binding in both mice and rats yielded biphasic Scatchard plots similar to those of opiate mu agonists, antagonists, enkephalins, and endorphins. Treatment of brain membranes with monovalent and divalent cation, chelating agents, protein-modifying reagents, and enzymes affected [3H]ethylketocyclazocine binding in a manner similar to that of [3H]morphine. Naloxazone, a long-acting antagonist that selectively abolished high-affinity [3H-DAla2,Met5]enkephalinamide binding in vivo, also selectively blocked high-affinity [3H]ethylketocyclazocine binding. Evaluation of analgesia with writhing and tail-flick assays in animals whose high-affinity binding sites were blocked by naloxazone demonstrated a 6- to 7-fold increase in median effective dose (ED50) values of ketocyclazocine. This decrease in analgesic potency was comparable to morphine's decreased potency in similarly treated mice. These biochemical and pharmacological results suggest that the analgesic properties of both kappa and mu agonists may be mediated through the same subpopulation of receptors, the high-affinity binding sites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- Pasternak G. W., Childers S. R., Snyder S. H. Opiate analgesia: evidence for mediation by a subpopulation of opiate receptors. Science. 1980 May 2;208(4443):514–516. doi: 10.1126/science.6245448. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W., Snowman A. M., Snyder S. H. Selective enhancement of [3H]opiate agonist binding by divalent cations. Mol Pharmacol. 1975 Nov;11(6):735–744. [PubMed] [Google Scholar]
- Pasternak G. W., Snyder S. H. Identification of novel high affinity opiate receptor binding in rat brain. Nature. 1975 Feb 13;253(5492):563–565. doi: 10.1038/253563a0. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W., Snyder S. H. Opiate receptor binding: effects of enzymatic treatments. Mol Pharmacol. 1974 Mar;10(2):183–193. [PubMed] [Google Scholar]
- Pasternak G. W., Wilson H. A., Snyder S. H. Differential effects of protein-modifying reagents on receptor binding of opiate agonists and antagonists. Mol Pharmacol. 1975 May;11(3):340–351. [PubMed] [Google Scholar]
- Pearl J., Harris L. S. Inhibition of writhing by narcotic antagonists. J Pharmacol Exp Ther. 1966 Nov;154(2):319–323. [PubMed] [Google Scholar]
- Pert C. B., Pasternak G., Snyder S. H. Opiate agonists and antagonists discriminated by receptor binding in brain. Science. 1973 Dec 28;182(4119):1359–1361. doi: 10.1126/science.182.4119.1359. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Snyder S. H., May E. L. Opiate receptor interactions of benzomorphans in rat brain homogenates. J Pharmacol Exp Ther. 1976 Feb;196(2):316–322. [PubMed] [Google Scholar]
- Pert C. B., Snyder S. H. Opiate receptor: demonstration in nervous tissue. Science. 1973 Mar 9;179(4077):1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- Simon E. J., Groth J. Kinetics of opiate receptor inactivation by sulfhydryl reagents: evidence for conformational change in presence of sodium ions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2404–2407. doi: 10.1073/pnas.72.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J., Hiller J. M., Edelman I. Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1947–1949. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J., Hiller J. M., Groth J., Edelman I. Further properties of stereospecific opiate binding sites in rat brain: on the nature of the sodium effect. J Pharmacol Exp Ther. 1975 Mar;192(3):531–537. [PubMed] [Google Scholar]
- Terenius L. Characteristics of the "receptor" for narcotic analgesics in synaptic plasma membrane fraction from rat brain. Acta Pharmacol Toxicol (Copenh) 1973;33(5):377–384. doi: 10.1111/j.1600-0773.1973.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Wilson H. A., Pasternak G. W., Snyder S. H. Differentiation of opiate agonist and antagonist receptor binding by protein modifying reagents. Nature. 1975 Feb 6;253(5491):448–450. doi: 10.1038/253448a0. [DOI] [PubMed] [Google Scholar]



