Abstract
Purpose
The aim of this study was to identify clinical predictive factors for tumor response after preoperative chemoradiotherapy (CRT) in rectal cancer.
Materials and Methods
The study involved 51 patients who underwent preoperative CRT followed by surgery between January 2005 and February 2012. Radiotherapy was delivered to the whole pelvis at a dose of 45 Gy in 25 fractions, followed by a boost of 5.4 Gy in 3 fractions to the primary tumor with 5 fractions per week. Three different chemotherapy regimens were used (5-fluorouracil and leucovorin, capecitabine, or tegafur/uracil). Tumor responses to preoperative CRT were assessed in terms of tumor downstaging and pathologic complete response (ypCR). Statistical analyses were performed to identify clinical factors associated with pathologic tumor response.
Results
Tumor downstaging was observed in 28 patients (54.9%), whereas ypCR was observed in 6 patients (11.8%). Multivariate analysis found that predictors of downstaging was pretreatment relative lymphocyte count (p = 0.023) and that none of clinical factors was significantly associated with ypCR.
Conclusion
Pretreatment relative lymphocyte count (%) has a significant impact on the pathologic tumor response (tumor downstaging) after preoperative CRT for locally advanced rectal cancer. Enhancement of lymphocyte-mediated immune reactions may improve the effect of preoperative CRT for rectal cancer.
Keywords: Rectal cancer, Preoperative chemoradiotherapy, Predictive factors, Tumor response
Introduction
Preoperative chemoradiotherapy (CRT) followed by surgery as the standard of care for rectal cancer can produce tumor downstaging, resulting in a reduced rate of postoperative local recurrence and a higher rate of sphincter-preserving surgery [1-4]. A prospective randomized trial confirmed the superiority of preoperative over adjuvant CRT in terms of local control and toxicity [5].
In contrast to the adjuvant setting, preoperative CRT allows a relatively short-term evaluation because it offers alternative endpoints based on pathologic tumor response. Many studies have reported that a pathologic complete response or tumor downstaging to preoperative radiotherapy with or without chemotherapy is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision [6,7]. Recently, the Korean Radiation Oncology Group (KROG) 09-01 trial concluded that rectal cancer patients achieving pathologic complete response (ypCR) after preoperative CRT had favorable long-term outcomes, whereas positive ypN status had a poor prognosis even after total regression of primary tumor [8]. Therefore, determination of factors predicting pathologic tumor response is of considerable importance in that it may provide additional information for permitting tailored treatment options as well as for assessing the individual prognosis [9].
Previous studies have suggested clinical factors such as the tumor volume, carcinoembryonic antigen (CEA) level, distance from the anal verge [10], temporal pattern of fatigue during CRT [11] and treatment interval between radiation and surgical resection [12] to correlate significantly with clinical response. And it was recently suggested that radiosensitivity depends not only on the biological characteristics of tumor cells but also on the tumor microenvironment [13]. Many factors may predict tumor response to CRT, but until now, there has been no way to propose an exact model that would predict pathologic tumor response after preoperative CRT.
In this setting, the aim of this study was to identify pretreatment clinical factors that may predict pathologic tumor response after preoperative CRT.
Materials and Methods
1. Patient characteristics
Between January 2005 and February 2012, 66 patients underwent preoperative CRT at Chungbuk National University Hospital, Cheongju, Korea. Inclusion criteria for this study are as follows: biopsy-proven rectal cancer, tumor located within 8 cm of the anal verge in digital rectal examination, cT3-T4 with or without regional lymph node metastasis, adequate bone marrow, hepatic, and renal function, the Eastern Cooperative Oncology Group (ECOG) performance status 0-2, and no evidence of distant metastasis.
Among reviewed 66 patients, 7 patients had no curative surgery because of their private affairs. Five patients who were transferred to other hospitals could not be traced by medical records. Therefore, 51 patients who met the inclusion criteria were analyzed in this study.
Patients underwent preoperative staging workups, including digital rectal examination, full blood counts, biochemical tumor markers (serum CEA and carbohydrate antigen [CA] 19-9), colonoscopy with biopsy, chest radiography, abdominopelvic computed tomography (CT), pelvic magnetic resonance imaging (MRI), and/or positron emission tomography (PET)/CT. Endorectal ultrasonography (ERUS) was not routinely executed. Pretreatment blood data were obtained using samples collected within 0-7 days before the start of RT. Clinical T classification was determined using pelvic MRI or ERUS. Positive lymph node involvement was defined as a lymph node ≥5 mm in the smallest diameter observed on CT or MRI [14]. The lesion volumes were displayed automatically in a three-dimensional format and were calculated by summing each of the cross-sectional volumes of the entire lesion. The sixth edition of the American Joint Committee on Cancer TNM system was used for staging [15].
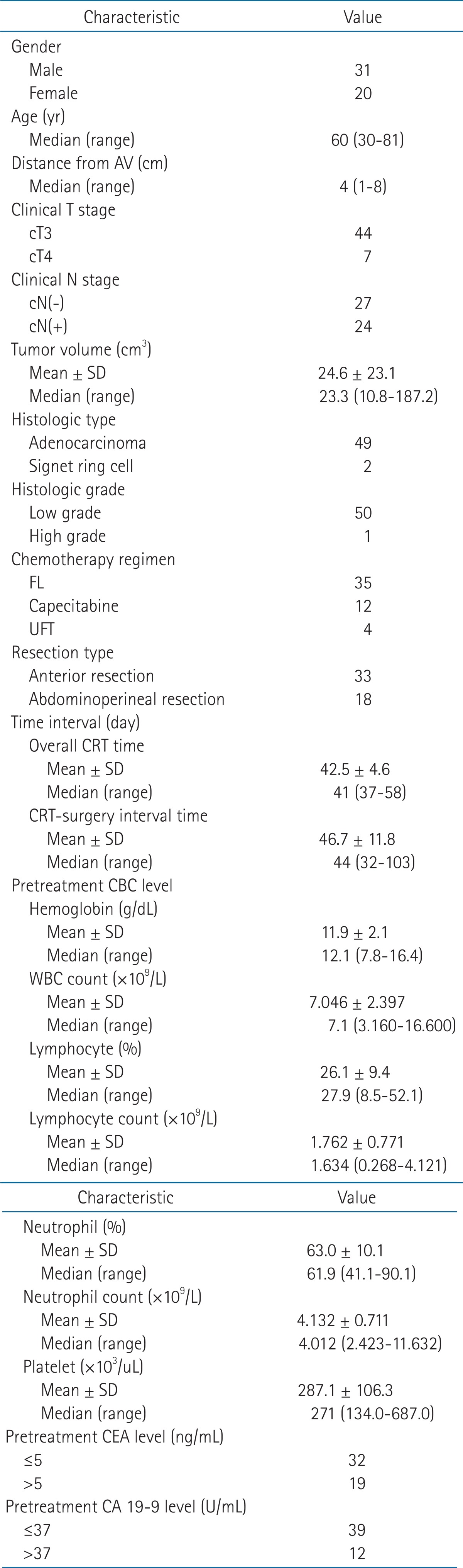
Clinical and pathological characteristics of population are described in Table 1. The study population was mostly male (60.8%) and had a median age of 60 years (range, 31 to 81 years). Almost all patients had a cT3 classification of their primary tumor (86.3%), and the major type was adenocarcinoma (96.1%). Elevated serum CEA levels were observed in 37.3% of patients at diagnosis (the upper limit of normal was defined as 5 ng/mL). Elevated serum CA 19-9 levels were observed in 23.5% of patients at diagnosis (the upper limit of normal was defined as 37 U/mL).
Table 1.
Patient characteristics (n = 51)
AV, anal verge; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CRT, chemoradiotherapy; FL, 5-fluorouracil and leucovorin; UFT, tegafur/uracil; WBC, white blood cell.
2. Treatment
Preoperative radiotherapy was delivered to the whole pelvis at a dose of 45 Gy in 25 fractions, followed by a boost of 5.4 Gy in 3 fractions to the primary tumor with 5 fractions per week. All patients underwent CT simulation for three-dimensional radiotherapy planning. Delineation of the clinical target volume (CTV) included the gross tumor volume, mesorectum, presacral space, whole of the sacral hollow, and regional lymphatics. The boost CTV included the gross tumor volume and mesorectum with ≥2 cm margins in all directions. CTV to planning target volume expansions of 1 cm were applied. The relevant organs at risk volumes for this study were the bladder, femoral bones, pelvic bones, and small bowel. The 6-MV or 10-MV photon beams were used for treatment plan. Dosimetric parameters were calculated using cumulative dose volume histogram data. Preoperative chemotherapy was initiated on the first day of pelvic radiotherapy and was delivered concurrently with radiotherapy. Three different chemotherapy regimens were used. The 5-fluorouracil and leucovorin group received two cycles of an intravenous bolus injection of 5-fluorouracil (400 mg/m2/day) and leucovorin (20 mg/m2/day) for 5 days in the first and fifth weeks of radiotherapy. The capecitabine group received 825 mg/m2 capecitabine orally twice daily during radiotherapy with or without weekend breaks. The oral tegafur/uracil (UFT) group received 300-600 mg/m2/day (as tegafur components) in two divided doses during radiotherapy without weekend breaks (Table 1). At the range of 32 to 103 days after the completion of preoperative CRT, all patients underwent a proctectomy, including high or low ligation of the inferior mesenteric vessels and total mesorectal excision. Anterior resection was performed in 33 patients (64.7%) and abdominoperineal resection in 18 patients (35.3%). Lateral node dissection was not performed in the surgical procedure.
3. Response evaluation
Downstaging was defined as the lowering of the T classification (as ypT2 or lower) by comparing pretreatment (before CRT) clinical and postoperative pathological stage. ypCR was defined as the absence of any tumor cells in the operative pathology specimen defined by ypT0pN0.
The following parameters were evaluated as potential clinical predictive factors of tumor response: age, sex, clinical T stage, clinical lymph node status, tumor volume, distance from the anal verge, histologic type, histologic grade, chemotherapy regimen, overall CRT time, interval time between CRT and surgery, pretreatment biochemical tumor marker level, and pretreatment complete blood count (CBC) level (Table 1).
4. Statistical analysis
Data were summarized by frequencies and percentages for categorical variables. To determine the association between response and covariates, univariate analysis was performed using the nonparametric Pearson's chi-square or Fisher's exact test. A receiver operating characteristic curve was used to define the cutoff point for the various continuous variables that were relative to the predicting tumor response. Multivariate analysis was performed to determine the independence of all variables identified as possibly significant by using a stepwise logistic regression model. All analyses were performed with IBM SPSS statistics trial ver. 20.0 (IBM, Armonk, NY, USA). A p-values of <0.05 were considered to indicate a significant difference.
Results
1. Pathologic tumor response after preoperative CRT
Pathologic examination of resected specimens revealed ypCR (ypT0pN0) in 6 patients (11.8%). Downstaging to ypT2 or less was observed in 28 patients (54.9%).
2. Clinical factors predicting pathologic tumor response
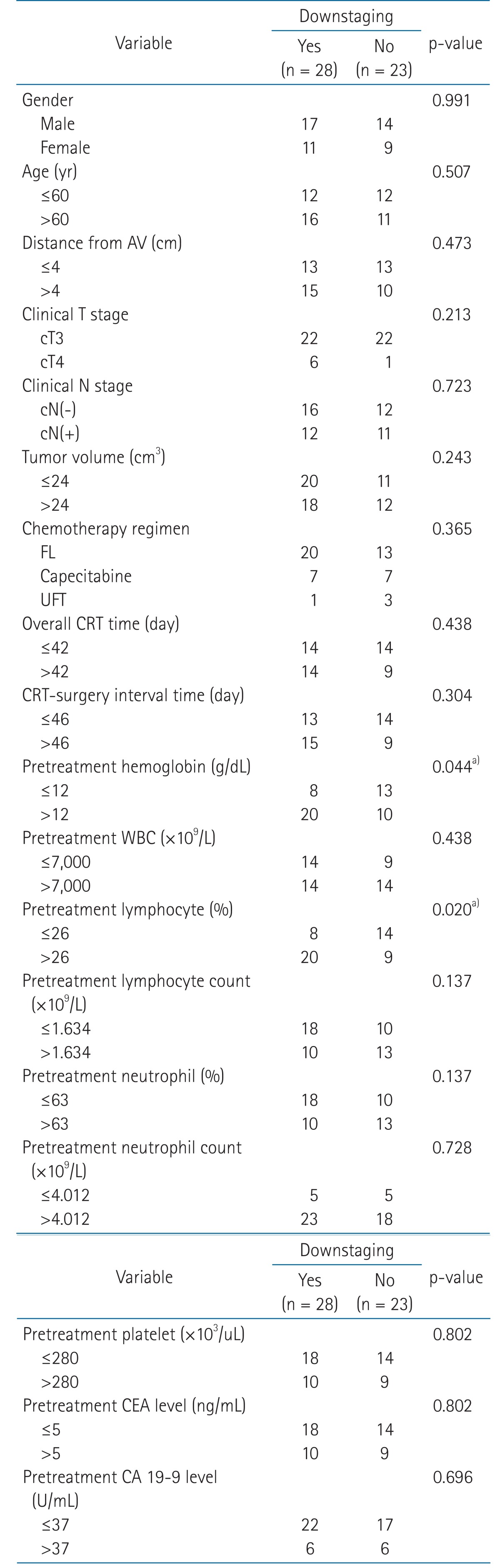
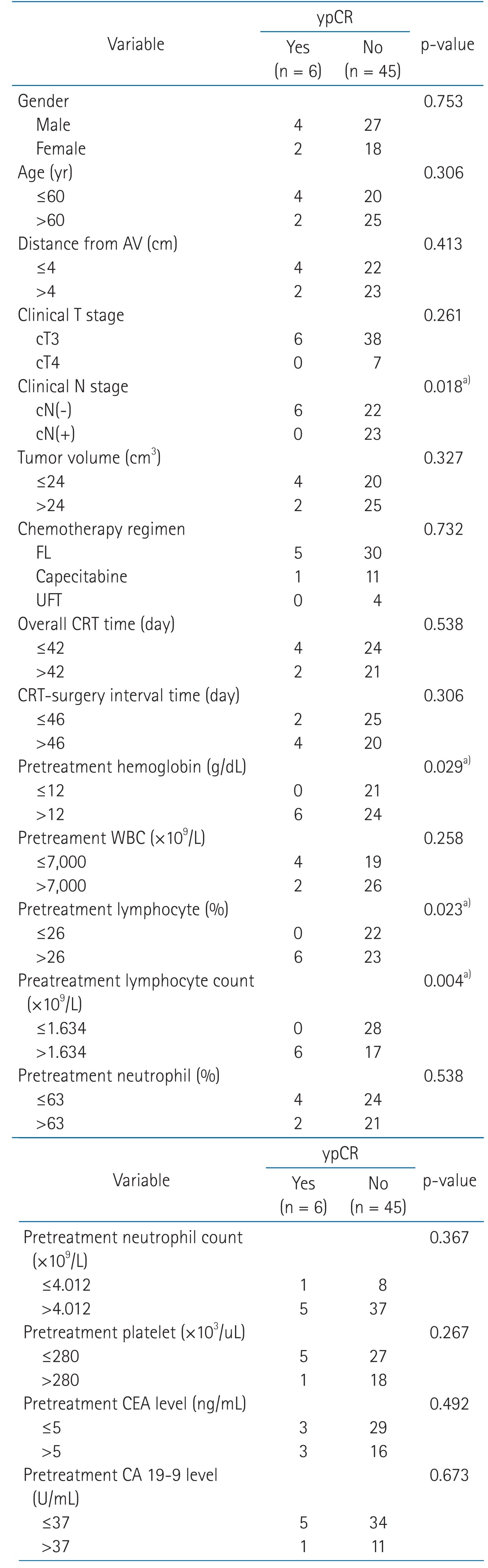
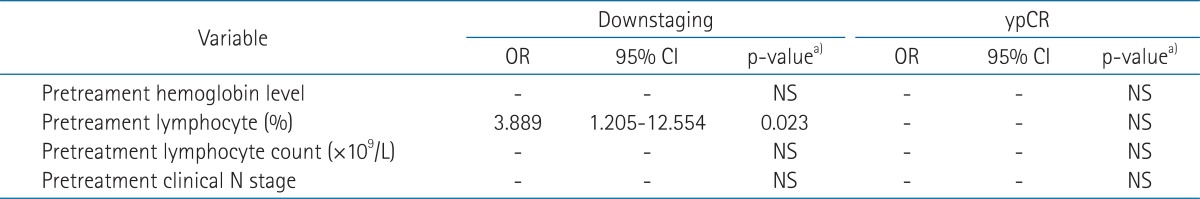
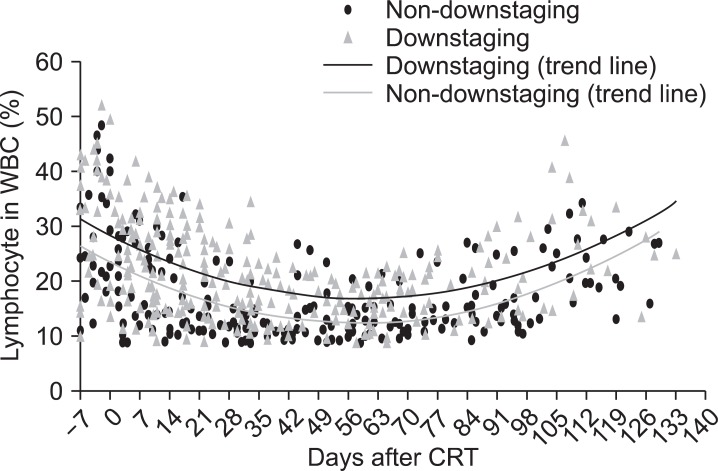
Univariate predictors of downstaging (ypT0-2) were found to be a pretreatment hemoglobin level >12.0 g/dL (p = 0.044), and pretreatment relative lymphocyte count (pretreatment lymphocyte percentage in WBC) >26% (p = 0.020). Univariate predictors of ypCR were found to be a cN(-) classification (p = 0.018), relative lymphocyte count >26% (p = 0.023), pretreatment absolute lymphocyte count (×109/L) >1.634 (p = 0.004) and pretreatment hemoglobin level >12.0 g/dL (p = 0.029) (Tables 2 and 3). Multivariate analysis revealed that pretreatment relative lymphocyte count >26% (p = 0.023) were predictors of downstaging, and that none was a statistically significant predictor of ypCR (Table 4). The change of relative lymphocyte count (%) during CRT and after CRT from the day 0, the initiation of CRT until surgery was plotted in all of the 51 patients. Relative lymphocyte counts were markedly reduced during CRT and increased gradually up to the time of surgery (Fig. 1).
Table 2.
Univariate analysis to identify predictors of downstaging
Determined by Pearson's chi-square or Fisher's extract test.
AV, anal verge; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CRT, chemoradiotherapy; FL, 5-fluorouracil and leucovorin; UFT, tegafur/uracil; WBC, white blood cell.
a)p < 0.05.
Table 3.
Univariate analysis to identify predictors of pathologic complete response (ypCR)
Determined by Pearson's chi-square or Fisher's extract test.
AV, anal verge; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CRT, chemoradiotherapy; FL, 5-fluorouracil and leucovorin; UFT, tegafur/uracil; WBC, white blood cell.
a)p < 0.05.
Table 4.
Multivariate analysis to identify predictors of downstaging and ypCR
pCR, pathologic complete response; OR, odds ratio; CI, confidence interval; NS, not significant.
a)Determined by stepwise logistic regression analysis.
Fig. 1.
The change of relative lymphocyte count (%) during the period of treatment. The start of radiotherapy (RT) was set at day 0 and the values obtained at the day from the start of RT were plotted. The trend line represents a trend, the long-term movement in time series data. It tells whether a particular data set have increased or decreased over the period of time. CRT, chemoradiotherapy; WBC, white blood cell.
Discussion and Conclusion
In patients with locally advanced rectal cancer (LARC), preoperative CRT is actually the standard of care. However, clinical predictive factors of pathologic tumor response after preoperative CRT, which would be essential for the optimal management of patients with rectal cancer, have never been fully explained. Knowledge of such factors might be useful to clinicians for predicting treatment outcomes and take part in therapeutic decisions allowing development of risk-adapted treatment strategies. For example, more aggressive preoperative regimens may be considered in patients who are less likely to respond to standard preoperative therapy.
It was recently suggested that radiosensitivity depends not only on the biological characteristics of tumor cells but also on the tumor microenvironment [12]. Although peripheral lymphocyte may not necessarily reflect adequate immune function, a number of reports documented a correlation between baseline peripheral lymphocyte counts and survival in patients with various types of malignancies, including carcinoma of the uterine cervix and breast [16]. In addition, the relevant predictive role of peripheral lymphocyte was independent of the main clinical factors such as tumor extent, performance status, or weight loss [17,18]. The degree of recovery of lymphocyte counts after RT reportedly correlates with tumor recurrence in bladder cancer [19] as well as head and neck squamous cell carcinoma [20]. A recent retrospective study suggested that baseline lymphocyte count is factor predicting tumor response and progression-free survival in patients with locally advanced cervical carcinoma treated with concurrent CRT [21]. Kitayama et al. [22] suggested that circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in LARC. Many previous reports suggested that a high number of tumor infiltrating lymphocytes (TIL) in colorectal cancer is strongly associated with a favorable outcome in the patients with colorectal cancer [23-26]. These observations allow us to speculate that lymphocyte-mediated immune reactions are supposed to have positive roles on clinical efficacy of preoperative CRT in the treatment of the patients with LARC.
Although the results obtained from our retrospective study has a limitation, the significant association between the peripheral lymphocyte level and downstaging rate suggests that the death of tumor cells after CRT is partially dependent on host immune reaction. CD8+ TIL from cancer patients have been shown to undergo apoptosis in response to tumor-derived microvesicles expressing tumor antigens, Fas ligand and MHC class I [27]. The decline in lymphocytes observed in cancer patients may reflect both reduced production and increased apoptosis of lymphoyctes. This, in another way, suggests that peripheral lymphocytes reflect the total condition of the host to fight with cancer and can be a good marker to tumor response. At least, the lymphocyte level before preoperative CRT is not largely affected by the timing of blood sampling and thus can be a good clinical predictive factor [22].
When lymphocyte levels were divided into relative and absolute lymphocyte count variables, multivariate analysis revealed that pretreatment relative lymphocyte count was only a predictor of downstaging. The number of peripheral lymphocytes is very prone to be affected by various factors such as age, nutrition, and some stresses. For example, the elevation of total white blood cell (WBC) counts may be caused by the increase in cortisol levels during an acute stress reaction. The altered WBC differential by elevating the relative neutrophil count and decreasing the relative lymphocyte count can cause relative lymphocytopenia with or without the change of total absolute lymphocyte count. Changes in lymphocyte subpopulations underlie much of the age-related decline in the immune response [28]. Considering that cancer patients could be influenced by age or other stresses, relative lymphocyte count might offer more sensitive value than total absolute lymphocyte count for evaluation of the host immune function.
When relative lymphocyte count was compared between downstaging and non-downstaging cases, samples derived from patients of downstaging group tended to contain more lymphocytes than those from non-downstaging group (data not shown). This raises a possibility that the peripheral lymphocytes may have significant immunologic effects on antitumor response of CRT. Transient lymphopenia after CRT may activate homeostatic mechanisms that finally stimulate TIL. Thus, CRT-induced transient lymphopenia, combined with additional immunopharmacological interventions, may achieve enhanced antitumor response. We used total lymphocyte level in WBC as a baseline immunologic parameter because no details concerning peripheral lymphoid subpopulations (B, T, or natural killer cells) were available in medical records. Total or relative lymphocyte count values were not directly associated with the presence of TIL. Therefore, we need to analyze phenotype and function of peripheral lymphocyte subpopulations.
In this study, the analysis endpoints were limited to the pathologic response and not extended to long-term clinical outcomes, such as disease-free and overall survival. But, recent the KROG 09-01 trial concluded that rectal cancer patients achieving ypCR after preoperative CRT had favorable long-term outcomes, whereas positive ypN status had a poor prognosis even after total regression of primary tumor [7]. In addition, Kitayama et al. [22] showed that patients with high lymphocyte group showed significantly better outcome in overall and disease-free survival.
Overstaging a tumor remains a possibility in staging analysis. Thus, to enhance the accuracy, only the T classification was used in downstaging analysis in the present study. However, in our study, ERUS was not routinely executed. Although MRI are more reliable methods for determining T classification than evaluation of lymph node status, staging failures still occur, owing to difficulties in accurate discrimination between T2 and T3 lesions, which are caused by perirectal fat desmoplastic reactions on MRI [29]. Garcia-Aquilar et al. [30] showed also that overall accuracy of ERUS in assessing the level of rectal wall invasion was 69%, with 18% of the tumors overstaged and 13% of tumors understaged. These results show the difficulty in evaluating the tumor response after preoperative CRT and therefore in finding predictive factors of response.
Although the small population of this study could lower statistical power to exclude clinically significant differences, downstaging and ypCR rates did not differ significantly between 5-FU and oral fluoropyrimidines in univariate analysis. A potential advantage of oral fluoropyrimidines in patients with rectal cancer is the use of a more convenient oral treatment that might be at least as effective as intravenous 5-FU. Oral fluoropyrimidines mimic continuously infused FU and simplify chemoradiation. Nevertheless, randomized phase III studies revealed that the administration of oral fluoropyrimidines with preoperative radiotherapy achieved similar rates of ypCR and tumor downstaging compared to 5-FU [31,32].
In conclusion, despite the small number of patients and possible wide biologic variations in lymphocyte subset number and activity, the pretreatment relative lymphocyte count (%) was a strong predictive factor of pathologic tumor response (tumor downstaging) after preoperative CRT for LARC. Lymphocyte-mediated immune reactions may be involved in the clinical response in CRT. Enhancement of antitumor immune mediated mechanisms may improve the effect of preoperative CRT for rectal cancer.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 2.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results: EORTC 22921. J Clin Oncol. 2005;23:5620–5627. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 3.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15–24. doi: 10.1016/j.radonc.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Yoon MS, Nam TK, Kim HR, et al. Results of preoperative concurrent chemoradiotherapy for the treatment of rectal cancer. J Korean Soc Ther Radiol Oncol. 2008;26:247–256. [Google Scholar]
- 5.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298–304. doi: 10.1007/s10350-004-6545-x. [DOI] [PubMed] [Google Scholar]
- 7.Kaminsky-Forrett MC, Conroy T, Luporsi E, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys. 1998;42:935–941. doi: 10.1016/s0360-3016(98)00345-9. [DOI] [PubMed] [Google Scholar]
- 8.Yeo SG, Kim DY, Kim TH, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01) Ann Surg. 2010;252:998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1. [DOI] [PubMed] [Google Scholar]
- 9.Yoon SM, Kim DY, Kim TH, et al. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1167–1172. doi: 10.1016/j.ijrobp.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Das P, Skibber JM, Rodriguez-Bigas MA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. 2007;109:1750–1755. doi: 10.1002/cncr.22625. [DOI] [PubMed] [Google Scholar]
- 11.Park HC, Janjan NA, Mendoza TR, et al. Temporal patterns of fatigue predict pathologic response in patients treated with preoperative chemoradiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75:775–781. doi: 10.1016/j.ijrobp.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250:582–589. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 13.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment: tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52:78–83. doi: 10.1016/j.ejrad.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Green FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 16.Riesco A. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer. 1970;25:135–140. doi: 10.1002/1097-0142(197001)25:1<135::aid-cncr2820250120>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Kim US, Papatestas AE, Aufses AH., Jr Prognostic significance of peripheral lymphocyte counts and carcinoembryonic antigens in colorectal carcinoma. J Surg Oncol. 1976;8:257–262. doi: 10.1002/jso.2930080312. [DOI] [PubMed] [Google Scholar]
- 18.Lavin PT, Bruckner HW, Plaxe SC. Studies in prognostic factors relating to chemotherapy for advanced gastric cancer. Cancer. 1982;50:2016–2023. doi: 10.1002/1097-0142(19821115)50:10<2016::aid-cncr2820501007>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.O'Toole C, Unsgaard B. Clinical status and rate of recovery of blood lymphocyte levels after radiotherapy for bladder cancer. Cancer Res. 1979;39:840–843. [PubMed] [Google Scholar]
- 20.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:3755–3762. doi: 10.1158/1078-0432.CCR-04-0054. [DOI] [PubMed] [Google Scholar]
- 21.Choi CH, Kang H, Kim WY, et al. Prognostic value of baseline lymphocyte count in cervical carcinoma treated with concurrent chemoradiation. Int J Radiat Oncol Biol Phys. 2008;71:199–204. doi: 10.1016/j.ijrobp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer. 2011;11:64. doi: 10.1186/1471-2407-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canadas-Garre M, Becerra-Massare P, Lopez de la Torre-Casares M, et al. Reduction of false-negative papillary thyroid carcinomas by the routine analysis of BRAF(T1799A) mutation on fine-needle aspiration biopsy specimens: a prospective study of 814 thyroid FNAB patients. Ann Surg. 2012;255:986–992. doi: 10.1097/SLA.0b013e31824e8d70. [DOI] [PubMed] [Google Scholar]
- 24.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 25.Morris M, Platell C, Iacopetta B. Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res. 2008;14:1413–1417. doi: 10.1158/1078-0432.CCR-07-1994. [DOI] [PubMed] [Google Scholar]
- 26.Laghi L, Bianchi P, Miranda E, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 27.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 29.Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology. 2004;232:335–346. doi: 10.1148/radiol.2322021326. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Aguilar J, Pollack J, Lee SH, et al. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45:10–15. doi: 10.1007/s10350-004-6106-3. [DOI] [PubMed] [Google Scholar]
- 31.de la Torre A, Garcia-Berrocal MI, Arias F, et al. Preoperative chemoradiotherapy for rectal cancer: randomized trial comparing oral uracil and tegafur and oral leucovorin vs. intravenous 5-fluorouracil and leucovorin. Int J Radiat Oncol Biol Phys. 2008;70:102–110. doi: 10.1016/j.ijrobp.2007.05.068. [DOI] [PubMed] [Google Scholar]
- 32.Roh MS, Yothers GA, O'Connel MJ, et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04 [abstract] J Clin Oncol. 2011;29(Suppl):3503. [Google Scholar]