Abstract
Major salivary glands of both humans and rodents consist of three pairs of macroscopic glands: parotid, submandibular, and sublingual. These glands secrete serous, mucous or mixed saliva via the proper main excretory ducts connecting the glandular bodies with the oral cavity. A series of discoveries about the salivary ducts in the 17th century by Niels Stensen (1638–1686), Thomas Wharton (1614–1673), and Caspar Bartholin (1655–1738) established the concept of exocrine secretion as well as salivary glands. Recent investigations have revealed the endocrine functions of parotin and a variety of cell growth factors produced by salivary glands.
The present review aims to describe macroscopic findings on the major salivary glands of rodents and the microscopic differences between those of humans and rodents, which review should be of interest to those researchers studying salivary glands.
Keywords: salivary glands, mouse, rat, human, immunohistochemistry
I. Introduction
The oral cavity is underlined by a mucosal membrane and is always moistened by the saliva secreted by the associated major and minor salivary glands. Major salivary glands in humans as well as mice and rats (referred to as ‘rodents’ in this review) are composed of three pairs of macroscopic glandular organs: parotid, sublingual, and submandibular [2, 36, 49]. Differing from the other alimentary tract-associated glands, the salivary glands are innervated by both sympathetic and parasympathetic nerves, resulting in the constitutive secretion of saliva under any physiological condition [32]. Both the flow rate and composition of saliva are altered by the stimulation from autonomic nerves and mastication [48]. The secretory cells making up the acini of these glandular tissues produce serous, mucous or mixed saliva. Saliva is secreted into the oral cavity via a series of ducts in the ductal system, and plays diverse roles by having digestive, antibacterial, buffering, lubricant, and water-balance functions [16]. Dysfunction of salivary secretion (hyposalivation) causes xerostomia (dry mouth) and sequentially leads to severe dental caries as well as oral mucosal disorders [14, 47]. Hyposalivation is caused by systemic diseases such as Sjögren’s syndrome and by pharmaceutical side effects, salivary stones, and tumors as well as medical treatments including radiotherapy [11, 15, 40].
Clinical and pathological researchers learn the anatomy and histology of human tissues; on the other hand, researchers are interested in normal morphology and/or function use animals for experimental treatments and procedures. Technical and bioethical limitations with respect to the handling of human tissues, as well as advancements in gene analytical studies using knockout animals, have heightened the need for a good understanding of the tissues of experimental animals, especially those of rodents. Thus, for clinical and basic researchers there is a need for broader knowledge about salivary glands of both humans and rodents.
However, the differences in salivary gland morphology between humans and rodents are not common knowledge [2, 7, 9]. In textbooks of histology for medical and dental students, of course, human salivary glands, but not rodent glands, are described. Many beginners in salivary gland research, therefore, could have the misunderstanding that rodent glands are the same as human ones. In this review article, we describe the basic morphology of rodent salivary glands and the morphological differences between the human and rodent glands.
II. Macroscopic Anatomy of Salivary Glands
Parotid gland
Human parotid gland, the largest salivary glands, locates within the triangle surrounded superiorly by the zygomatic arch, anteriorly by the masseter, and posteriorly by the sternocleidomastoid. The inferior and medial poles are mostly confined to the angle of mandible and to the temporomandibular joints, respectively. The parotid (Stensen’s or Stenon’s) duct leaves the anterior border, passes anteriorly on the masseter, penetrates the buccinator, and opens finally into the buccal cavity. The facial nerves (cranial nerve VII) penetrate through the parotid gland [2, 36, 49].
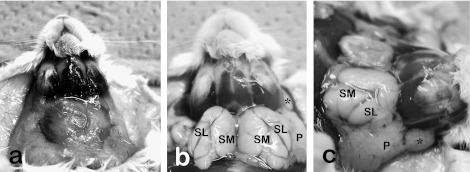
In rodents, the parotid gland is located behind and below the ear, caudally bordering the submandibular gland [19]. This parotid gland is embedded in subcutaneous adipose tissues underlining the integument of the lateral neck. Being removed from the skin, the gland seems like a “pancreas” in the mesentery. The extraorbital lacrimal gland is the most notable to be distinguished from the parotid gland, and is found under the skin on the lateral side of the face near the ear in rodents (Fig. 1).
Fig. 1.
Macroscopic anatomy of the anterior and lateral neck portions of mice before (a) and after (b, c) the perfusion of fixative and removal of fat tissues. Anterior neck is occupied by large white fat tissues including salivary glands (a). Upon removing fat tissues and submandibular lymph nodes, salivary and extraorbital lacrimal glands were exposed. The extraorbital lacrimal gland (*) is located anterior to the parotid gland (P). The submandibular (SM) and sublingual (SL) glands are encapsulated with common fascia.
Sublingual and submandibular glands
Human sublingual and submandibular glands locate superior or inferior space of the mylohyoid, respectively. The superior border of sublingual gland represents the sublingual fold in the oral floor. The numerous orifices of minor sublingual ducts open on the sublingual fold. The submandibular (Wharton’s) duct runs forward along the lingual nerve in the sublingual space to open in the sublingual caruncle with the major sublingual (Bartholin’s) duct [2, 36, 49].
Rodent sublingual glands are located together with the submandibular glands in the anterior neck spaces between the submandibular lymph nodes and the sternum (Fig. 1). The sublingual gland occupies the latero-rostral one fourth of the submandibular-sublingual complex. The rostral margin of both glands borders the submandibular lymph nodes. Both glands are encapsulated with a common fascia; however, those in living animals and those perfused with a fixative solution are discriminable by a minute difference in shade of appearance (Fig. 1). Nomenclatures of salivary glands are based on the location of duct openings, histology, and parasympathetic innervation of human salivary glands [52]. Main excretory ducts of the sublingual and submandibular glands are separate.
III. The Duct and Acinar Systems of Salivary Glands
Duct system and excretory duct
Like the human salivary glands [2, 49], the duct system of rodents is composed of the intercalated (ID), striated (SD), excretory (ED), and main excretory ducts. The main excretory ducts of the major salivary glands were designated originally as Stensen’s or Stenon’s (parotid), Wharton’s (submandibular), and Bartholin’s (major sublingual) ducts, which are visible macroscopically. Saliva produced by acinar secretory cells in the glandular body flows sequentially through the ID, SD, and ED.
The epithelium of the ED consists of various types of columnar cells, the functions of which are unknown [37, 38]. In this epithelium brush (tuft) cells possessing microvilli at their luminal cell membrane are present, and they could be involved in secretion, resorption or sensation of salivary flow [38, 39].
Striated ducts
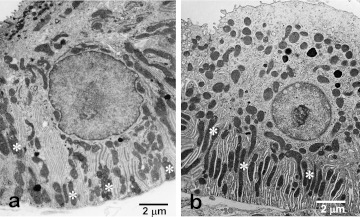
The SD is a specialized portion of the ductal system and functions for the secretion and resorption of electrolytes transported bidirectionally between the ductal lumen and the extracellular spaces. Transmembrane active pumping is mainly carried out by various types of ion channels requiring energy [16]. An increase in the basolateral surface and higher yield of energy are permitted by the basal infolding and numerous mitochondria sandwiched between enfolded basal membranes, respectively. These structural features form ‘the basal striation’ seen light-microscopically, which is the origin of the term “striated duct.” A similar structure is also present in the distal urinary tubules in the kidney (Fig. 2).
Fig. 2.
Electron microscopy of striated duct cells of the submandibular gland (a) and distal urinary tubule cells of the kidney (b) of rats. Both cells develop numerous vertically-arranged mitochondria (*) and the basal infoldings of cell membrane (the basal striation).
There is much less morphological evidence reflecting the functions of the SD besides the evidence indicating the transfer of electrolytes and water, but ultrastructural and histochemical studies have demonstrated that SD cells of mammals including humans and rodents contain numerous small clear (empty-appearing) vesicles in their apical cytoplasm [10]. These vesicles express histochemical markers for endocytosis and transcytosis. Salivary products produced by the acinar cells are incorporated by SD cells under both physiological and pathological conditions. Moreover, these clear vesicles in the apical cytoplasm are involved in the transcytosis of secretory immunoglobulin (IgA) from the basolateral to apical lumen [43]. In human salivary glands, immunostaining for the secretory component (SC) was generally faint but increased in intensity at the cell periphery and particularly at the luminal face of striated and intercalated ducts and serous acini. IgA is also distributed similar to that of the SC [24].
Intercalated ducts
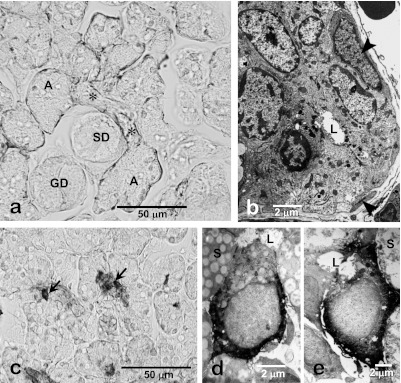
The ID is located between the acinus and the SD and receives the primary saliva directly from the acini. Cuboidal epithelial cells form the ductal epithelium, which is partially covered by myoepithelial cells (Fig. 3). The ID cells had been mentioned to be hidden stem cells of both acinar and ductal cells. The most reliable and traditional evidence suggesting this stem cell hypothesis is the higher incorporation of labeled nucleic acid bases such as tritiated thymidine or 5-bromodeoxyuridine (BrdU) in the ID than in any other portion of the ductal system [13]. Furthermore, endodermal stem cells that can differentiate into pancreatic cells are induced in the duct-ligated submandibular gland of mice [29], and stem cell-related factors have been recognized in IDs [20, 34, 35]. In the neck portion of the ID, clusters of the BrdU label-retaining cells that lack acinar or ductal markers are found and these certainly include salivary gland stem cells [21].
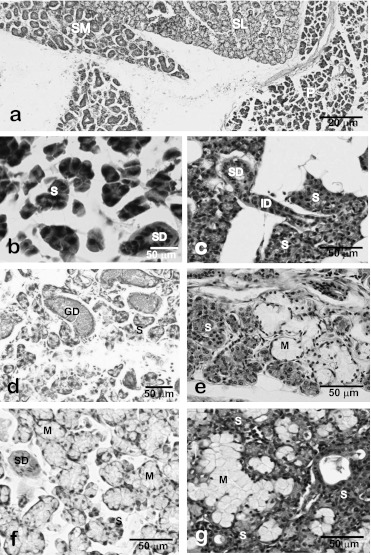
Fig. 3.
Immunohistochemistry and electron microscopy of the intercalated duct of the rat submandibular gland. Smooth muscle-action-immunohistochemistry shows distribution of myoepithelial cells along the intercalated duct (*) as well as acini (A). No myoepithelial cells are associated with other duct portions such as the striated (SD) and granular ducts (GD). The intercalated duct cells are cuboidal in shape and partially surrounded by myoepithelial cells (arrowhead, b). Hsp27-immunohistochemistry of 3-week-old rat submandibular gland shows the centrally-located immunopositive terminal tubule cells (arrows, d). Electron micrograms of Hsp27-immunohistochemistry of 4-week-old rat submandibular gland show that Hsp27-immunoreactive terminal tubule cells differentiated into immature acinar cells (d) and granular intercalated duct cells (e). L, lumen; S, secretory granules in acinar cells.
Each cell of an ID appears to be uniform but slightly heterogeneous upon histochemical and electron microscopic observations. Both granular and agranular types of ID cells are distinguishable [8], and the former represents a remnant of the perinatal secretory cell phenotype of the terminal tubules (TT) forming the terminal portions of embryonic and immature submandibular glands [8, 31, 52]. One of our earlier studies demonstrated that a 25–27 kDa heat shock protein (Hsp27) is localized in both postnatal TT cells located at the center of developing acini (Fig. 3) and in granular ID cells, in addition to being detected in apoptotic immature acinar cells [8]. These TT cells exhibit substantial proliferative activity until 2–3 weeks after birth and then differentiate into mature acinar cells [12]. Moreover, the profiles of the secretory granules in the TT and ID cells are very similar, thus suggesting that granular ID cells are of TT origin and may hide potent proliferative and differentiation capability in mature salivary glands under physiological conditions. In duct-ligated submandibular glands of rats, Hsp27-positive epithelial cells are substantially but temporarily induced after the ligation is stopped [41]. These data strongly suggest that the ID include the precursor or stem cells of the salivary glands.
Granular ducts
Principal GCT cells and pillar cells
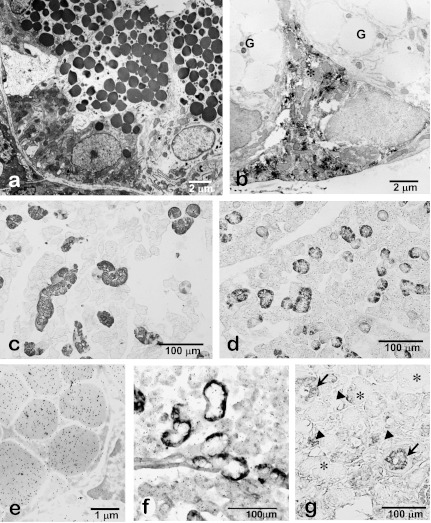
The granular duct (granular convoluted tubule, GCT) is located between the ID and SD in the rodent submandibular gland. The duct wall is composed of a simple columnar epithelium (Fig. 4). The principal cell type of the GCT is a high-columnar secretory cell containing many secretory granules in its supranuclear cytoplasm. These granules contain a variety of biologically active polypeptides such as cell growth factors and hormones and are exocytosed in response to neural and hormonal stimuli. In addition, “pillar cells” with a narrow lumen and wide base exist sandwiched between principal GCT cells (Fig. 4b). The functional roles of these pillar cells are unclear, but a paracrine function based on FGF2 immunolocalized in their cytoplasm has been suggested [5].
Fig. 4.
Photomicrographs of rat (a, b, e, f), mouse (c, d) and human (g) submandibular glands. Electron microscopy shows supranuclear localization of numerous exocrine secretory granules in the granular duct cells (a). FGF2-immunoelectron microscopy shows an immunopositive pillar cell (*) without large secretory granules present between the principal granular duct cells without FGF2-immunoreactivity (G) (b). EGF-immunohistochemistry of the submandibular glands shows specific localization of EGF in the granular duct in male (c) and female (d) mouse. Note that EGF-immunopositive granular ducts are less developed in the female submandibular glands than the male glands. Colloidal-gold particles representing the immunoreactivity for EGF are exclusively localized in the secretory granules of the granular duct cells (e). Expression of EGF-mRNA is specifically localized in the basal cytoplasm of the granular duct cells (f). In human submandibular glands, EGF-immunoreactivity is dominantly localized in the striated duct (arrows) and partially in the serous acinar cells (arrowheads). No immunoreaction is detected in mucous acini (*) (g).
Cell growth factors
The GCT produces and secretes a variety of bioactive polypeptides, hormones, and cell growth factors including EGF, NGF [10, 17, 18, 26], BDNF [22, 45], HGF [2, 6, 7], IGF-I [2, 4, 7], TGF-α [50], and TGF-β [2, 3, 7]. Also, FGF2 is contained in the cytoplasm of the pillar cells [2, 5, 7]. There is no doubt that these factors are produced by GCT cells and secreted into the saliva in an exocrine fashion (Fig. 4c–f) and thus pass into the oral cavity and then permeate the upper digestive tract. Salivary gland-derived growth factors could act directly on the mucosal membranes for their protection and repair. For example, EGF inhibits acid secretion, protects gastric mucosa against injury, and accelerates mucosal ulcer healing by stimulating cell migration and proliferation [23, 44]. Further suggestions have been made that such factors are reabsorbed by the mucosa and transported to remote organs via the bloodstream [7, 9, 10, 17, 18]. Removal of salivary glands in rodents causes a reduction in the EGF content in plasma [44]. Labeled-EGF supplemented via the oral cavity can be detected in the lungs and skin, as well as in the digestive tract and craniofacial organs [33]. Salivary gland-derived growth factors can function as hormones outside the oral cavity and digestive tract.
Before the discovery of salivary gland-derived growth factors, e.g., NGF and EGF, salivary endocrine factors had been explored; and the salivary gland hormone “parotin” was purified from the bovine parotid gland [28] and evaluated for its immunolocalization in duct cells [42]. Although parotin is effective for epithelial growth and repair and for calcification, advancement of our understanding as to how it functions as a salivary gland hormone is limited. However, the endocrine function of salivary glands has been established by the detection of many growth factors in the salivary glands of rodents as well as humans.
Growth factors in human salivary glands, which lack GCTs
Human salivary glands also secrete growth factors from portions within the glands other than the GCTs, which are not present in humans. However, morphological analyses have been much fewer than in the case of the rodent glands. EGF is immunolocalized in human serous acinar cells (Fig. 4g) and may be released into the primary saliva, and then partially delivered by transcytosis across SD cells to the interstitium and ultimately into the blood circulation [10, 25], in addition to EGF that is delivered in exocrine fashion and reabsorbed in the digestive tract. Endocrinological functions of the salivary glands by BDNF is mentioned by Dr. Tsukinoki in the present issue.
Sexual dimorphism
The GCT cells are differentiated from the striated duct cells and maintained by male sex hormones such as testosterone [31]. GCT cells are dedifferentiated by castration or hypophysectomy and are recovered by the administration of testosterone [7, 9]. GCT is poorly developed in female submandibular glands (Fig. 4c, d). Hyper-differentiation of GCT cells is induced by testosterone administration [31]. Male-dominant growth factors in mice acts on spermatogenesis [46], as well as on the adrenal medulla to promote aggressive behavior [1]. Sexual dimorphism of the mouse submandibular gland is more prominent than that of the rat gland.
Acinus
The acinus, the secretory endpiece of salivary glands, produces and secretes the primitive saliva into the central lumen. An acinus is composed of a number of excretory secretory cells and surrounding myoepithelial cells. Major types of acinar secretory cells are serous and mucous in both human and rodent salivary glands. However, histochemical and biochemical studies have revealed that the chemical composition and morphological profiles of secretory granules in these cells are much different between these two types of secretory cells [30]. Mucous secretory granules contain appreciable amounts of mucin and glycoconjugates, whereas serous secretory granules contain little glycoconjugates and a large amount of water and ions. Serous granules containing acidic glycoconjugates are termed ‘seromucous’ [27]. In both human and rodent salivary glands, serous cells in salivary glands are correctly termed ‘seromucous’.
The distal ends of mucous acini are surrounded by serous demilunes. These demilunes are observed in the submandibular glands of humans and in the sublingual glands of both humans and rodents. Saliva secreted by the demilune cells is delivered by intercellular canaliculi between the mucous cells. Yamashina and colleagues have demonstrated that the serous demilune is formed artificially by chemical fixation, and they revealed by rapid-freezing fixation that the ‘vital’ end portion of the mixed acini are composed of irregularly-arranged cells of both types [51].
IV. Microscopic Anatomy of Human and Rodent Salivary Glands
Parotid gland
Both human and rodent parotid glands are composed of pure serous acini (Fig. 5a–c). The acinus is composed of serous secretory cells that contain many secretory granules having an electron-lucent profile and are situated in the supranuclear cytoplasm. These cells have a well-developed rough endoplasmic reticulum in the infranuclear region and are surrounded by myoepithelial cells. The ID and SD are prominent. The human parotid gland is well characterized intralobular adipose tissues, whereas the adipocytes are not prominent in the rodent parotid gland.
Fig. 5.
Light micrographs of major salivary glands of the rat (a, b, d, f) and human (c, e, g). Major salivary glands of rats are easily distinguishable light-microscopically by the histological features of acinar and ductal structures (a). In rat (b) and human (c) parotid glands, acini are composed of serous (or seromucous) cells. In rat submandibular glands (d), the granular ducts (GCT) are markedly developed but mucous acini and serous demilunes are not recognized. In human submandibular glands (e), mixed acini accompanying serous demilunes are observed whereas no GCT portions are found. In rat (f) and human (g) sublingual glands, mixed acini accompanying the serous demilunes are observed. P, parotid gland; SL, sublingual gland; SM, submandibular gland; S, serous acini; M, mucous acini; SD, striated duct; ID, intercalated duct; GD, granular duct; D, demilune.
Submandibular gland
The human submandibular gland is a mixed gland composed of both serous and mucous acinar cells, whereas the rodent one has only the serous type (Fig. 5a, d, e). Whereas serous demilunes are marked in the mixed acini of the human gland, no demilunes or mucous cells are present in the rodent gland. The GCT portions of rodent submandibular glands have often been mistaken for mucous acini or tubules. The ID and SD are also well developed in both species. A myoepithelium covers the serous acini as well as the ID.
Sublingual gland
Sublingual glands of both humans and rodents are mixed glands (Fig. 5a, f, g). Their acini are composed of centrally-located mucous cells and peripheral serous demilunes. Therefore, sublingual and submandibular glands are easy to distinguish from one another light-microscopically. The borderline connective tissue between the sublingual and submandibular glands is clear and includes parasympathetic neurons of the submandibular ganglion.
V. Immunohistochemical Issues Regarding Rodent Salivary Glands
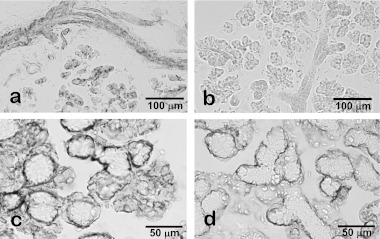
In addition to general immunohistochemical issues, non-specific reactions of rodent salivary glands to secondary antibodies are notable. Such dubious staining is remarkable in the ductal portions (Fig. 6). Non-specific bindings of the secondary antibodies to the salivary duct cells could be the result of the secretory immunoglobulin during transcytosis. We strongly recommend carefully selecting secondary antibodies suitable for the tissues used in experiments. Conventional control staining procedures may result in misjudgment as to the immunohistochemical specificity. If a weak immunoreaction is observed in the ducts, careful consideration is necessary to judge the specificity of the immunoreaction. Other histochemical methods such as in situ hybridization and/or biochemical experiments are strongly recommended to confirm the immunohistochemical results.
Fig. 6.
Non-specific immunoreactions in the rodent salivary glands. Without specific primary antibodies anti-mouse IgG secondary antibody reacted with duct cells and luminal membrane of acinar cells in rat parotid glands (a) whereas no non-specific reaction was detected by using pre-absorbed secondary antibody for rat tissues (b). The immunohistochemical procedure for the rat sublingual glands by the combination of an anti-smooth muscle actin mouse monoclonal antibody, an established marker for myoepithelial cells, and conventional anti-mouse IgG antibody triggers a broader immunoreaction than expected, including serous demilunes (c). After replacing the secondary antibody with the pre-absorbed one for rat tissue, the immunoreaction is confirmed to localize in myoepithelial cells (d).
VI. Conclusions
Rodent salivary glands used in animal experiments show a similar but different histology compared with the human glands. Especially, rodent submandibular glands develop GCTs producing a variety of cell growth factors. Immunohistochemical staining of rodent salivary glands requires a careful choice of secondary antibodies and negative control staining.
VII. Acknowledgments
The authors thank Prof. Shoichi Iseki, Kanazawa University School of Medicine, for his constant encouragement of our research in our salivary gland studies.
VIII. References
- 1.Aloe L., Alleva E., Böhm A., Levi-Montalcini R. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc. Natl. Acad. Sci. U S A. 1986;83:6184–6187. doi: 10.1073/pnas.83.16.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano O. The salivary gland: anatomy for surgeons and researchers. Jpn. J. Oral Maxillofac. Surg. 2011;57:384–393. [Google Scholar]
- 3.Amano O., Tsuji T., Nakamura T., Iseki S. Expression of transforming growth factor beta-1 in the submandibular gland of the rat. J. Histochem. Cytochem. 1991;39:1707–1711. doi: 10.1177/39.12.1940322. [DOI] [PubMed] [Google Scholar]
- 4.Amano O., Iseki S. Expression, localization and developmental regulation of insulin-like growth factor I mRNA in rat submandibular gland. Archs. Oral Biol. 1993;38:671–677. doi: 10.1016/0003-9969(93)90006-8. [DOI] [PubMed] [Google Scholar]
- 5.Amano O., Yoshitake Y., Nishikawa K., Iseki S. Basic fibroblast growth factor in rat salivary glands. Cell Tissue Res. 1993;273:467–474. doi: 10.1007/BF00333701. [DOI] [PubMed] [Google Scholar]
- 6.Amano O., Matsumoto K., Nakamura T., Iseki S. Expression and localization of hepatocyte growth factor in rat submandibular gland. Growth Factors. 1994;10:145–151. doi: 10.3109/08977199409010988. [DOI] [PubMed] [Google Scholar]
- 7.Amano O., Iseki S. Expression and localization of cell growth factors in the salivary gland: a review. Acta Anat. Nippon. 2001;71:201–211. [PubMed] [Google Scholar]
- 8.Amano O., Kudo Y., Shimada M., Wakayama T., Yamamoto M., Iseki S. Transient occurrence of 27 kDa heat-shock protein in the terminal tubule cells during postnatal development of the rat submandibular gland. Anat. Rec. 2001;264:358–366. doi: 10.1002/ar.10023. [DOI] [PubMed] [Google Scholar]
- 9.Amano O., Iseki S. Cell growth factors in salivary glands. Microscope. 2005;40:1–6. [Google Scholar]
- 10.Barka T. Biologically active polypeptides in mouse submandibular glands. Acta Histochem. Cytochem. 1980;13:9–22. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- 11.Burlage F. R., Coppes R. P., Meertens H., Stokman M. A., Vissink A. Parotid and submandibular/ sublingual flow during high dose radiotherapy. Radiother. Oncol. 2001;61:271–274. doi: 10.1016/s0167-8140(01)00427-3. [DOI] [PubMed] [Google Scholar]
- 12.Chang W. W. Cell population changes during acinus formation in the postnatal rat submandibular gland. Anat. Rec. 1974;178:187–201. doi: 10.1002/ar.1091780204. [DOI] [PubMed] [Google Scholar]
- 13.Denny P. C., Ball W. D., Redman R. S. Salivary glands: a paradigm for diversity of gland development. Crit. Rev. Oral Biol. Med. 1997;8:51–75. doi: 10.1177/10454411970080010301. [DOI] [PubMed] [Google Scholar]
- 14.Featherstone J. D. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 15.Fox P. C. Acquired salivary dysfunction. Ann. New York Acad. Sci. 1998;842:132–137. doi: 10.1111/j.1749-6632.1998.tb09641.x. [DOI] [PubMed] [Google Scholar]
- 16.Genkins G. N. In “The Physiology and Biochemistry of the Mouth” 4th ed. Blackwell Scientific; Oxford: 1978. Saliva; pp. 284–359. [Google Scholar]
- 17.Gresik E. W. The granular convoluted tubule (GCT) cell of rodent submandibular glands. Microsc. Res. Tech. 1994;27:1–24. doi: 10.1002/jemt.1070270102. [DOI] [PubMed] [Google Scholar]
- 18.Gresik E. W., Hosoi K., Kurihara K., Maruyama S., Ueha T. The rodent granular convoluted tubule cell—an update. Eur. J. Morphol. 1996;34:221–224. doi: 10.1076/ejom.34.3.221.13033. [DOI] [PubMed] [Google Scholar]
- 19.Jonjic S. Surgical removal of mouse salivary glands. Curr. Protoc. Immunol. 2001 doi: 10.1002/0471142735.im0111s43. Chapter 1; Unit 1.11. [DOI] [PubMed] [Google Scholar]
- 20.Kawanami T., Matsuzaki Y., Sawaki T., Sakai T., Jin Z. X., Masaki Y., Fukushima T., Tanaka M., Umehara H. Identification of human salivary stem cells from cultured labial minor salivary cells. Jpn. J. Clin. Immunol. 2007;30:455–460. doi: 10.2177/jsci.30.455. [DOI] [PubMed] [Google Scholar]
- 21.Kimoto M., Yura Y., Kishino M., Toyosawa S., Ogawa Y. Label-retaining cells in the rat submandibular gland. J. Histochem. Cytochem. 2008;56:15–24. doi: 10.1369/jhc.7A7269.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo Y., Saruta J., To M., Shiiki N., Sato C., Tsukinoki K. Expression and role of the BDNF receptor-TrkB in rat adrenal gland under acute immobilization stress. Acta Histochem. Cytochem. 2010;43:139–147. doi: 10.1267/ahc.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konturek S. J. Role of epidermal growth factor in gastroprotection and ulcer healing. Scand. J. Gastroenterol. 1988;23:129–133. doi: 10.3109/00365528809103956. [DOI] [PubMed] [Google Scholar]
- 24.Korsrud F. R., Brandtzaeg P. Characterization of epithelial elements in human major salivary glands by functional markers: localization of amylase, lactoferrin, lysozyme, secretory component, and secretory immunoglobulins by paired immunofluorescence staining. J. Histochem. Cytochem. 1982;30:657–666. doi: 10.1177/30.7.6179983. [DOI] [PubMed] [Google Scholar]
- 25.Lantini M. S., Cossu M. Immunocytochemical investigation of the subcellular distribution of some secretory products in human salivary glands. Eur. J. Morphol. 1998;36:230–234. [PubMed] [Google Scholar]
- 26.Mori M., Takai Y., Kunikata M. Review: biologically active polypeptides in the submandibular gland—roles of the granular convoluted tubule—. Acta Histochem. Cytochem. 1992;25:325–341. [Google Scholar]
- 27.Munger B. L. Histochemical studies on seromucous- and mucous-secreting cells of human salivary glands. Am. J. Anat. 1964;115:411–429. doi: 10.1002/aja.1001150303. [DOI] [PubMed] [Google Scholar]
- 28.Ogata E., Suzuki H., Shimazawa E., Nakanowatari K., Asano H. On the hypocalcemic effect in rabbits of a bovine parotid extract (parotin) Endocrinol. Jpn. 1971;18:235–342. doi: 10.1507/endocrj1954.18.235. [DOI] [PubMed] [Google Scholar]
- 29.Okumura K., Nakamura K., Hisatomi Y., Nagano K., Tanaka Y., Terada K., Sugiyama T., Umeyama K., Matsumoto K., Yamamoto T., Endo F. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38:104–113. doi: 10.1053/jhep.2003.50259. [DOI] [PubMed] [Google Scholar]
- 30.Philips C. J., Tandler B., Nagato T. In “Biology of the Salivary Glands”, ed. by K. Dobrosielski-Vergona. CRC Press; Boca Raton: 1993. Evolution and divergence of salivary gland acinar cells: a format for understanding molecular evolution; pp. 39–80. [Google Scholar]
- 31.Pinkstaff C. A. The cytology of salivary glands. Int. Rev. Cytol. 1980;63:141–261. doi: 10.1016/s0074-7696(08)61759-3. [DOI] [PubMed] [Google Scholar]
- 32.Proctor G. B., Carpenter G. H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Purushotham K. R., Offenmüller K., Bui A. T., Zelles T., Blazsek J., Schultz G. S., Humphreys-Beher M. G. Absorption of epidermal growth factor occurs through the gastrointestinal tract and oral cavity in adult rats. Am. J. Physiol. 1995;269:867–873. doi: 10.1152/ajpgi.1995.269.6.G867. [DOI] [PubMed] [Google Scholar]
- 34.Purwanti N., Azlina A., Karabasil M. R., Hasegawa T., Yao C., Akamatsu T., Hosoi K. Involvement of the IL-6/STAT3/Sca-1 system in proliferation of duct cells following duct ligation in the submandibular gland of mice. J. Med. Invest. 2009;56:253–254. doi: 10.2152/jmi.56.253. [DOI] [PubMed] [Google Scholar]
- 35.Purwanti N., Tsuji D., Azlina A., Karabasil M. R., Javkhlan P., Hasegawa T., Yao C., Akamatsu T., Itoh K., Hosoi K. Induction of Sca-1 in the duct cells of the mouse submandibular gland by obstruction of the main excretory duct. J. Oral Pathol. Med. 2011;40:651–658. doi: 10.1111/j.1600-0714.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 36.Saracco C. G., Crabill E. V. In “Biology of the Salivary Glands”, ed. by K. Dobrosielski-Vergona. CRC Press; Boca Raton: 1993. Anatomy of the human salivary glands; pp. 1–14. [Google Scholar]
- 37.Sato A., Goto F., Miyoshi S. Ultrastructure of the main excretory duct epithelium of the female mouse submandibular gland with special reference to sexual dimorphism. Cell Tissue Res. 1994;277:407–415. doi: 10.1007/BF00300213. [DOI] [PubMed] [Google Scholar]
- 38.Sato A., Miyoshi S. Cells in the duct system of the rat submandibular gland. Eur. J. Morphol. 1998;36:61–66. [PubMed] [Google Scholar]
- 39.Sato A., Suganuma T., Ide S., Kawano J., Nagato T. Tuft cells in the main excretory duct of the rat submandibular gland. Eur. J. Morphol. 2000;38:227–231. doi: 10.1076/0924-3860(200010)38:4;1-o;ft227. [DOI] [PubMed] [Google Scholar]
- 40.Ship J. A. In “Saliva and Oral Health” 3rd ed., ed. by M. Edgar, C. Dawes and D. O’Mullane. British Dental Association; London: 2004. Xerostomia: aetiology, diagnosis, management and clinical implications; pp. 41–55. [Google Scholar]
- 41.Takahashi-Horiuchi Y., Sugiyama K., Sakashita H., Amano O. Expression of heat shock protein 27 with the transition from proliferation to differentiation of acinar precursor cell in regenerating submandibular gland of rats. Tohoku J. Exp. Med. 2008;214:221–230. doi: 10.1620/tjem.214.221. [DOI] [PubMed] [Google Scholar]
- 42.Takano K., Suzuki T. Localization of parotin in bovine parotid gland, demonstrated by the immunohistochemical method. Acta Histochem. Cytochem. 1977;4:1–10. [Google Scholar]
- 43.Tandler B., Gresik E. W., Nagato T., Phillips C. J. Secretion by striated ducts of mammalian major salivary glands: review from an ultrastructural, functional, and evolutionary perspective. Anat. Rec. 2001;264:121–145. doi: 10.1002/ar.1108. [DOI] [PubMed] [Google Scholar]
- 44.Tarnawski A. S., Jones M. K. The role of epidermal growth factor (EGF) and its receptor in mucosal protection, adaptation to injury, and ulcer healing: involvement of EGF-R signal transduction pathways. J. Clin. Gastroenterol. 1998;27 Suppl 1:S12–20. doi: 10.1097/00004836-199800001-00004. [DOI] [PubMed] [Google Scholar]
- 45.Tsukinoki K., Saruta J., Sasaguri K., Miyoshi Y., Jinbu Y., Kusama M., Sato S., Watanabe Y. Immobilization stress induces BDNF in rat submandibular glands. J. Dent. Res. 2006;85:844–848. doi: 10.1177/154405910608500913. [DOI] [PubMed] [Google Scholar]
- 46.Tsutsumi O., Kurachi H., Oka T. A physiological role of epidermal growth factor in male reproductive function. Science. 1986;233:975–977. doi: 10.1126/science.3090686. [DOI] [PubMed] [Google Scholar]
- 47.Vissink A., Mitchell J. B., Baum B. J., Limesand K. H., Jensen S. B., Fox P. C., Elting L. S., Langendijk J. A., Coppes R. P., Reyland M. E. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:983–991. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe S., Dawes C. Salivary flow rates and salivary film thickness in five-year-old children. J. Dent. Res. 1990;69:1150–1153. doi: 10.1177/00220345900690050601. [DOI] [PubMed] [Google Scholar]
- 49.Williams P. L., Warwick R., Dyson M., Bannister L. H. 37th ed. Churchill Livingstone; Edinburgh: 1989. Gray’s Anatomy; pp. 1290–1298. [Google Scholar]
- 50.Wu H. H., Kawamata H., Wang D. D., Oyasu R. Immunohistochemical localization of transforming growth factor alpha in the major salivary glands of male and female rats. Histochem. J. 1993;25:613–618. doi: 10.1007/BF00157875. [DOI] [PubMed] [Google Scholar]
- 51.Yamashina S., Tamaki H., Katsumata O. The serous demilune of rat sublingual gland is an artificial structure produced by conventional fixation. Arch. Histol. Cytol. 1999;62:347–354. doi: 10.1679/aohc.62.347. [DOI] [PubMed] [Google Scholar]
- 52.Young J. A., van Lennep E. W. Academic Press; London: 1978. The Morphology of Salivary Glands. [Google Scholar]