Abstract
The CXCR4/CXCL12 pathway has recently been reported to be involved in stimulating the metastasis of many different neoplasms, in which CXCR4 activates various phenomena such as chemotaxis, invasion, angiogenesis and proliferation. The purpose of this study was to analyze a possible association between the expression of chemokine receptors CXCR4, CCR6 and CCR7 with the clinicopathological features of cutaneous malignant melanoma, and to assess the usefulness of these chemokine receptors for diagnosis and prognosis. In our study, a percentage of immunoexpression of both CXCR4 and its ligands CXCL12 was associated with high clinical risk. In contrast, the patients with a low immunoexpression of CXCR4 and CXCL12 had low clinical risk. CCR6 and CCR7 immunoexpressions were also correlated with some clinical parameters, but seemed no more useful than CXCR4. These data suggest that the assessment of CXCR4 immunoexpression is a novel tool for predicting tumor aggressiveness in malignant melanomas, and in particular, a high immunoexpression percentage of CXCR4 and CXCL12 might be a sign of a poor prognosis.
Keywords: chemokine, CXCR4, CXCL12, malignant melanoma, immunohistochemistry
I. Introduction
Various chemokine receptors, namely CXC chemokine receptor 4 (CXCR4), CC chemokine receptor 6 (CCR6) and CC chemokine receptor 6 (CCR7), have recently been shown to be involved in the regulation of metastasis in malignant tumors [8, 11, 16, 22]. However, little is known about the role of these receptors in promoting tumor metastasis. Chemokines are molecules that are structurally and functionally similar to growth factors. They bind to G-protein-coupled receptors on leukocytes and stem cells, and work through guanine-nucleotide-binding (G) proteins to initiate intracellular signaling cascades that promote migration towards the chemokine source [11].
CXCR4 is expressed on tumor cells such as melanomas, breast, prostate, colon, pancreatic and ovarian cancer [8, 16, 22]. Its ligand, Chemokine ligand 21 (CXCL12) also called Stromal cell-derived factor (SDF-1), is expressed in the lymph nodes, the lungs, the bone marrow, and the liver [11]. The importance of the CXCR4/CXCL12 axis in cancer is exemplified by the fact that blocking CXCR4 function leads to an inhibition of metastasis in in vivo mouse models of breast and pancreatic cancer [11, 16]. Previously, our report showed that the assessment of CXCR4 immunoreactivity in dermatofibrosarcoma protuberans (DFSP) was a useful tool for predicting tumor aggressiveness [20]. Similar enhanced expression was also observed in skin squamous cell carcinomas [4].
Melanoma is the most aggressive skin cancer once metastasis begins; therefore, it is important to characterize the molecular players involved in melanoma dissemination. The expression of CXCR4 in human melanomas has been detected in the vertical growth phase and in regional lymph nodes [9, 17]. CCR7 and its ligand CCL21 are required for the efficient migration of mature, peripheral dendritic cells to the lymphatic vessels, and subsequently to the draining lymph nodes (LN) [21]. Therefore, CCR7 is considered to play an important role in lymphocyte cell trafficking and homing to the lymph nodes. Furthermore, previous studies have shown a downregulation of CCR6 but an upregulation of CCR7 and CXCR4 in metastatic tumor tissue [15, 18].
In this study, we immunohistochemically analyzed formalin-fixed, paraffin-embedded primary malignant melanoma tissues to determine the relative incidence and pattern of the expression of four chemokine receptors: CXCR4, CCR6, CCR7 and CXCL12. We also investigated the usefulness of assaying the immunoexpression of these chemokine receptors for both diagnosis and prognosis.
II. Materials and Methods
Patients and tumor samples
Nineteen specimens of malignant melanomas were obtained from patients who underwent surgery at the Wakayama Medical University Hospital, Wakayama, Japan between 2006 and 2008. All specimens were primary tumors, and had complete documentation of the histopathology and clinical course. All of these primary tumor specimens were obtained before adjuvant therapy such as chemotherapy and radiotherapy. The tissue specimens were fixed in neutral buffered formalin, and then paraffin-embedded sections were stained with hematoxylin-eosin (HE). The histological diagnosis was confirmed by the corresponding paraffin-embedded materials, and when necessary, immunohistochemical panels such as S-100, HMB45, and Melan A were performed. The specimens consisted of 7 nodular melanomas, 10 acral lentiginous melanomas, 1 superficial spreading melanoma, and 1 desmoplastic melanoma. Detailed clinicopathological data including age, site, tumor thickness and tumor stage were also obtained (Table 1). Control non-neoplastic tissues (6 samples from 6 patients) were also obtained from the same patients at sites distant from their surgically resected tumors. The normal histological status of these tissues was confirmed by routine histological examination. This study was approved by the ethics committee of Wakayama Medical University
Table 1.
Patient and tumor characteristics
| Patient Characteristics | |
|---|---|
| Total number | 19 |
| Median age (years) | 68.4 |
| range | 33–89 |
| Gender | |
| Female | 5 (26) |
| Male | 14 (74) |
| Location | |
| Head/neck | 3 (16) |
| Trunk/extremities | 8 (42) |
| Feet | 8 (42) |
| Tumor thickness (mm) | |
| 0.5~2.0 | 9 (47) |
| >2.0 | 10 (53) |
| Histopathologic type | |
| Nodular melanoma | 7 (37) |
| Acral lentiginous melanoma | 10 (53) |
| Superficial spreading melanoma | 1 (5) |
| Dysplastic melanoma | 1 (5) |
| Stage | |
| I | 4 (21) |
| II | 5 (26) |
| III | 10 (53) |
| IV | 0 (0) |
The values in parentheses are percentages.
Immunohistochemistry
Immunohistochemistry was performed on deparaffinized 4 µm sections according to our previous report [4]. Prior to staining, the tissue sections were de-melanized with 0.25% potassium permanganate and 2% oxalic acid. They were then incubated for 30 min in 3% H2O2 at room temperature (RT) to block endogenous peroxidase activity, and in a blocking solution (1% normal rabbit serum and 1% bovine serum albumin) to block non-specific binding. In addition, we employed any antigen retrieval sequence using heat-assisted epitope retrieval. But the antigenicity of the antigens we evaluated was the same the sensitivity and specificity. Next, the specimens were incubated for 2 hr at RT with a polyclonal goat anti-CXCR4 antibody (1:400 dilution; GeneTex, Incorporated, San Antonio, Tx, USA), an anti-CCR6 antibody (1:300 dilution; GeneTex) or an anti-CCR7 antibody (1:300 dilution; GeneTex) [2]. The specimens were then incubated for 1 hr at RT with a biotinylated secondary antibody (1:400 dilution; DAKO, Denmark). After extensive rinsing, they were developed using the streptavidin-biotin-peroxidase complex technique (LSAB2 kit/HRP, DAKO).
The peroxidase reaction was visualized with 0.2 mg/ml 3,3'-diaminobenzidine tetrahydrochloride. The sections were then counter-stained with hematoxylin. The control sections were not exposed to the primary antibody.
Evaluation of immunolabeling
Score of the percentage of positively immunostained tumor cells
The immunohistochemical scoring in the relative number of immunopositive cells was performed blindly by 3 dermatologists or a pathologist who had no clinical knowledge of the patients [4]. The immunostained sections were scanned by light-microscopy. In the tumor tissues, 5 fields of ×400 high power view were assessed randomly in all specimens. The necrotic areas and edges of the tissue sections were not included in the counting. The staining was categorized into four semi-quantitative classes based on the percentage of immunostained (positive) tumor cells: absence of staining, <10% positive cells (low), 10% to 50% positive cells (moderate), and >50% positive cells (high), based on the report of Scale et al. [17]. Samples with immunohistochemical scores of negative or weak staining with low to moderate distributions were considered to have ‘low’ expression whereas high distributions were considered to have ‘high’ expression.
Furthermore, the correlation in the relationship between CXCL12 and CXCR4 was studied by scoring the number of CXCL12 and CXCR4 immunopositive cells.
Score of the intensity of positively immunostained tumor cells
Another qualitative assessment for CXCL12 and CXCR4 was adopted to assess the proper immunohistochemical intensity of the tumors based on the strength of their immunoexpression: negative immunostaining, weak immunostaining, moderate immunostaining, or strong immunostaining [8].
Statistical analysis
The correlations among the immunohistochemical expression, baseline patient features, and tumors were studied by contingency tables and Mann-Whitney U analysis.
The Spearman rank correlation was used to evaluate the correlation between the expression level of CXCL12 and CXCR4. A P<0.05 was accepted as statistically significant.
The differences of the immunohistochemical expression with malignant melanoma tissues and control tissues was estimated using Student’s t-test.
III. Results
CXCR4 and CCR7 expression was significantly higher in malignant melanomas when compared with control tissue (CXCR4: p=0.011, CCR7: p=0.0001). CCR6 expression was significantly lower in malignant melanomas when compared with control tissue (p=0.017). Downregulation of the CCR6 expression and upregulation of the CCR7 and CXCR4 expressions were observed in malignant melanoma but not in control tissues.
CXCR4 expression in malignant melanomas
Representative results from the immunohistochemical staining for CXCR4 are shown in Figure 1. CXCR4 immunoexpression was detected in the cytoplasm in most cases. The immunolabeling intensity in the tumor cell varied widely, and there were no significant relationships among the intensity and several parameters.
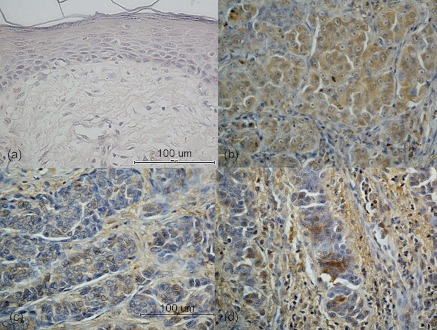
Fig. 1.
Immunohistochemical staining for CXCR4 in malignant melanoma. The immunoexpression was detected diffusely in the cytoplasm. All patients showing nodular melanomas showed a high CXCR4 immunoexpression percentage (b). No expression of CXCR4 was observed in the normal skin (control tissue) (a). On the other hand, acral lentiginous melanoma presented with low to moderate CXCR4 immunoexpression. The immunoexpression was observed partially in the cytoplasm, and infiltrating lymphocytes were also positively immunostained (c: low expression, d: moderate expression). Original magnification ×400. Bar=100 µm.
The correlation between the clinicopathological parameters and CXCR4 immunoexpression is summarized in Table 2. A high immunoexpression percentage was correlated with the tumor thickness (p=0.049), a nodular type (p=0.014) and developing distant metastasis within two years (p=0.014). The site of metastasis included the lung in all 7 cases. There was no significant correlation between the involvement of the sentinel lymph nodes and CXCR4 immunoexpression (p=0.15).
Table 2.
Clinicopathologic features and CXCR4 expression
| n (%) | Number of CXCR4 expression | P | ||||
|---|---|---|---|---|---|---|
| Low | Moderate | High | ||||
| Age (yrs) | <70 | 9 (47) | 0 (0) | 6 (67) | 3 (33) | 0.43 |
| ≥70 | 10 (53) | 4 (40) | 2 (20) | 4 (40) | ||
| Gender | Male | 14 (74) | 3 (21) | 5 (36) | 6 (43) | 0.55 |
| Female | 5 (26) | 1 (20) | 3 (60) | 1 (20) | ||
| Location | Head/neck | 3 (16) | 0 (0) | 2 (67) | 1 (33) | 0.41 |
| Trunk/extremities | 8 (42) | 1 (13) | 3 (50) | 4 (38) | ||
| Feet | 8 (42) | 3 (38) | 3 (50) | 2 (13) | ||
| Tumor thickness (mm) | 0.5~2.0 | 9 (47) | 4 (44) | 3 (33) | 2 (22) | 0.049* |
| >2.0 | 10 (53) | 0 (0) | 5 (50) | 5 (50) | ||
| Clinical type | nodular | 7 (37) | 0 (0) | 2 (29) | 5 (71) | 0.014* |
| non-nodular | 12 (63) | 4 (33) | 6 (50) | 2 (17) | ||
| Sentinel lymph node | Not involved | 11 (58) | 4 (36) | 4 (36) | 3 (27) | 0.15 |
| Involved | 6 (32) | 0 (0) | 3 (50) | 3 (50) | ||
| Not investigated | 2 (11) | 0 (0) | 1 (50) | 1 (50) | ||
| Distant metastasis within 2 years | yes | 7 (37) | 0 (0) | 2 (28) | 5 (72) | 0.014* |
| no | 12 (63) | 4 (33) | 6 (50) | 2 (17) | ||
*P<0.05 between groups.
Furthermore, a high immunoexpression percentage was observed in 5 out of 7 cases (71%) of nodular melanoma. The CXCR4 immunoexpression was diffuse in the cytoplasm of spindle-shaped cells, epithelioid cells and isolated populations with balloon cell changes in nodular melanoma (Fig. 1b). However, this immunoexpression was not observed in peripheral vascular endothelial cells or lymphocytes. In non-nodular melanomas, high CXCR4 immunoexpression was observed in 2 out of 12 (17%) cases, and was partially observed in the cytoplasm and at the peripheral areas of the tumors (Fig. 1c, d).
Correlation with CXCR4 and CXCL12 expression
CXCL12 immunostaining was mainly distributed in the cytoplasm and peripheral vessels.
All 4 patients with a high immunoexpression percentage of both CXCR4 and CXCL12 had nodular melanomas, and their overall survival was 0%. In contrast, all 4 patients with the low immunoexpression percentage of both CXCR4 and CXCL12 were the non-nodular type, with a thin tumor thickness, early stage, and a 100% 5-year overall survival (Fig. 2, Table 3).
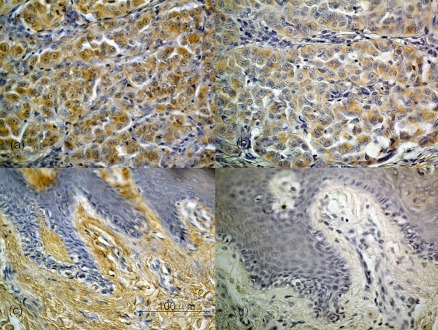
Fig. 2.
Immunohistochemical staining for CXCR4 and CXCL12 in malignant melanoma. Nodular melanomas showing a high CXCR4/CXCL12 immunoexpression percentage (a: CXCR4, b: CXCL12). The immunoexpression was detected diffusely in the cytoplasm. In most cases, the CXCL12 distribution was similar to that of CXCR4. However, in acral lentiginous melanomas, CXCR4/CXCL12 immunoexpression (c: CXCR4, d: CXCL12) was at very low percentage, and was partially observed in the cytoplasm of tumor cells (c, d). Original magnification ×400. Bar=100 µm.
Table 3.
Relationship between CXCL12 and CXCR4 expression in malignant melanoma (n=19)
| CXCL12 expression level | Total | ||||
|---|---|---|---|---|---|
| Low | Moderate | High | |||
| CXCR4 expression level | Low | 4 | 0 | 0 | 4 |
| Moderate | 5 | 2 | 1 | 8 | |
| High | 2 | 1 | 4 | 7 | |
| Total | 11 | 3 | 5 | 19 | |
There was a significant and positive correlation between the expression level (score: 1–3) of CXCR4 and its ligand CXCL12 (rs=0.57 by Spearman rank correlation coefficient).
Spearman’s rank correlation coefficient was calculated to analyze the association between the immunoexpression of CXCR4 and CXCR12. There was a significant and positive correlation between the immunoexpression levels of CXCR4 and CXCL12 (rs=0.57 by Spearman rank correlation coefficient).
Correlation between CCR6 & CCR7 expression and clinicopathological parameters in malignant melanoma
The correlations among the clinicopathological parameters and the CCR6 & CCR7 immunoexpression are summarized in Tables 4 and 5. A high CCR6 immunoexpression percentage was correlated with a nodular type of melanoma (p=0.012), but no significant associations were detected among the other parameters. Similarly, a higher CCR7 immunoexpression percentage was correlated with the tumor thickness (p=0.037), but no significant associations were detected among the other parameters.
Table 4.
Clinicopathologic features and CCR6 expression
| n (%) | Number of CCR6 expression | P | ||||
|---|---|---|---|---|---|---|
| Low | Moderate | High | ||||
| Age (yrs) | <70 | 9 (47) | 4 (44) | 3 (33) | 2 (22) | 0.59 |
| ≥70 | 10 (53) | 6 (60) | 2 (20) | 2 (20) | ||
| Gender | Male | 14 (74) | 8 (57) | 4 (29) | 2 (14) | 0.36 |
| Female | 5 (26) | 2 (40) | 1 (20) | 2 (40) | ||
| Location | Head/neck | 3 (16) | 2 (0) | 0 (67) | 1 (33) | 0.23 |
| Trunk/extremities | 8 (42) | 2 (25) | 4 (50) | 2 (25) | ||
| Feet | 8 (42) | 6 (75) | 1 (13) | 1 (13) | ||
| Tumor thickness (mm) | 0.5~2.0 | 9 (47) | 7 (77) | 0 (0) | 2 (22) | 0.14 |
| >2.0 | 10 (53) | 3 (30) | 5 (50) | 2 (20) | ||
| Clinical type | nodular | 7 (37) | 1 (0) | 3 (14) | 3 (86) | 0.012* |
| non-nodular | 12 (63) | 9 (75) | 2 (17) | 1 (8) | ||
| Sentinel lymph node | Not involved | 11 (58) | 8 (72) | 1 (9) | 2 (18) | 0.097 |
| Involved | 6 (32) | 1 (17) | 4 (67) | 1 (17) | ||
| Not investigated | 2 (11) | 1 (50) | 0 (0) | 1 (50) | ||
| Distant metastasis within 2 years | yes | 7 (37) | 2 (29) | 3 (43) | 2 (29) | 0.16 |
| no | 12 (63) | 8 (67) | 2 (17) | 2 (17) | ||
*P<0.05 between groups.
Table 5.
Clinicopathologic features and CCR7 expression
| n (%) | Number of CCR7 expression | P | ||||
|---|---|---|---|---|---|---|
| Low | Moderate | High | ||||
| Age (yrs) | <70 | 9 (47) | 1 (11) | 4 (44) | 4 (44) | 0.38 |
| ≥70 | 10 (53) | 1 (10) | 7 (70) | 2 (20) | ||
| Gender | Male | 14 (74) | 2 (14) | 8 (57) | 4 (29) | 0.46 |
| Female | 5 (26) | 0 (0) | 3 (60) | 2 (40) | ||
| Location | Head/neck | 3 (16) | 0 (0) | 1 (33) | 2 (67) | 0.37 |
| Trunk/extremities | 8 (42) | 1 (13) | 4 (50) | 3 (38) | ||
| Feet | 8 (42) | 1 (13) | 6 (75) | 1 (13) | ||
| Tumor thickness (mm) | 0.5~2.0 | 9 (47) | 2 (22) | 6 (67) | 1 (11) | 0.037* |
| >2.0 | 10 (53) | 0 (0) | 5 (50) | 5 (50) | ||
| Clinical type | nodular | 7 (37) | 0 (0) | 4 (57) | 3 (43) | 0.27 |
| non-nodular | 12 (63) | 2 (17) | 7 (58) | 3 (25) | ||
| Sentinel lymph node | Not involved | 11 (58) | 2 (18) | 6 (55) | 3 (27) | 0.24 |
| Involved | 6 (32) | 0 (0) | 3 (50) | 3 (50) | ||
| Not investigated | 2 (11) | 0 (0) | 2 (100) | 0 (0) | ||
| Distant metastasis within 2 years | yes | 7 (37) | 0 (0) | 3 (43) | 4 (57) | 0.055 |
| no | 12 (63) | 2 (17) | 8 (67) | 2 (17) | ||
*P<0.05 between groups.
IV. Discussion
The expression of chemokine receptors by malignant cells could represent a mechanism for increased proliferation and cell motility, thus leading to tumor growth and metastasis. It has been postulated that organ-specific metastasis might be governed, in part, by interactions between chemokine receptors on cancer cells with metastatic potential and chemokine gradients in the target organs.
Many retrospective studies have now documented that the expression of various chemokine receptors (particularly CXCR4) is associated with a poor prognosis [6, 17, 18]. Our previous study showed that CXCR4 immunoreactivity was significantly higher in relapsed DFSP as compared to non-relapsed DFSP [20].
Previous research has demonstrated a downregulation of CCR6 and an upregulation of CCR7 and CXCR4 in metastatic tumor tissues [18, 21]. Melanoma cell lines express the functional chemokine receptors CCR7, CXCR3, CCR10 and CXCR4, which are able to activate cell motility during invasion, cell proliferation, and survival [1, 7, 12–14].
We hypothesized that the expression of CXCR4, CCR6 and CCR7 in malignant melanomas would correlate with the clinicopathologic indicators of tumor progression. We found a significant and positive correlation between CXCR4 expression and tumor thickness, a nodular type and developing distant metastasis in patients with primary melanoma. In particular, those patients with a high immunoexpression percentage of both CXCR4 and CXCL12 presented with high clinical risk and a poor prognosis, whereas patients with a low immunoexpression percentage of CXCR4 and CXCL12 presented with low clinical risk and a better prognosis.
Much evidence has reported that CXCR4/CXCL12 interactions play key roles in cancer cell survival, proliferation, chemotaxis, homing, adhesion, tumor angiogenesis, and resistance to conventional and targeted therapies. However, the effect of CXCL12 on the proliferation of CXCR4-expressing tumor cells has not been fully defined, and moreover, a small number of studies have demonstrated a proliferative effect in vitro and in vivo. Our results support the idea that an autocrine mechanism may be responsible for both CXCR4 and CXCL12. These findings support the hypothesis that CXCL12/CXCR4 may play important roles in prognostic expectation.
There was no significant correlation between CCR6 or CCR7 expression and developing distant metastasis. Previous studies reported that the overexpression of CXCR4 dramatically enhanced the metastatic accumulation of B16 melanoma cells in mouse lungs, but metastasis to the lymph nodes, liver and kidney were not affected [1], whereas others have shown the involvement of other chemokine receptors such as CCR7 and CXCR3 in lymph node metastasis [5, 19].
Like CXCR4, CCR7 is well known to stimulate cell proliferation and directional migration in benign and malignant cells, and are associated with metastasis and decreased survival in gastric carcinomas [10], as well as squamous cell carcinomas of the pharynx, esophagus, and cervix [3]. However, there was no association between CCR7 expression and the development of lymph node or distant metastasis in our present study. We also obtained similar results regarding CCR6 expression.
Although our study showed similar results as previous reports in Caucasian patients, it can be concluded that the assessment of CXCR4 immunoreactivity in cutaneous malignant melanomas may be a useful tool for predicting tumor aggressiveness in Japanese patients. Additionally, our results suggest that the assessment of CXCR4/CXCL12 expression in malignant melanomas might help in prognostic expectation.
V. Acknowledgments
We are grateful to Prof. Toshikazu Kondo from the Forensic Medicine Department, Wakayama Medical University. This work was supported in part by grants from the Japan Society for the Promotion of Science.
VI. References
- 1.Bartolomé R. A., Gálvez B. G., Longo N., Baleux F., Van Muijen G. N., Sánchez-Mateos P., Arroyo A. G., Teixidó J. Stromal dell-derived factor-1α promotes melanoma cell invasion across basement membranes involving stimulation of membrane-type 1 matrix metalloproteinase and Rho GTPase activities. Cancer Res. 2004;64:2534–2543. doi: 10.1158/0008-5472.can-03-3398. [DOI] [PubMed] [Google Scholar]
- 2.Bleul C. C., Farzan M., Choe H., Parolin C., Clark-Lewis I., Sodroski J., Springer T. A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 3.Ding Y., Shimada Y., Maeda M., Kawabe A., Kaganoi J., Komoto I., Hashimoto Y., Miyake M., Hashida H., Imamura M. Association of CC chemokine 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin. Cancer Res. 2003;9:3406–3412. [PubMed] [Google Scholar]
- 4.Kaminaka C., Toyozawa S., Yonei N., Kunimoto K., Furukawa F., Yamamoto Y. Phenol peels for the treatment of actinic keratosis and Bowen’s disease: correlation with chemokine receptors expression. Skin Cancer. 2011;26:327–332. (Abstract in English) [Google Scholar]
- 5.Kawada K., Sonoshita M., Sakashita H., Takabayashi A., Yamaoka Y., Manabe T., Inaba K., Minato N., Oshima M., Taketo M. M. Pivotal rale of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 6.Kim J., Takeuchi H., Lam S. T., Turner R. R., Wang H. J., Kuo C., Foshag L., Bilchik A. J., Hoon D. S. Chemokine receptor CXCR4 expression in colorectal cancer patients increase the risk for reccurence and for poor survival. J. Clin. Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 7.Laverdiere C., Hoang B. H., Yang R., Sowers R., Qin J., Meyers P. A., Huvos A. G., Healey J. H., Gorlick R. Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin. Cancer Res. 2005;11:2561–2567. doi: 10.1158/1078-0432.CCR-04-1089. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y., Ji R., Li J., Gu Q., Zhao X., Sun T., Wang J., Li J., Du Q., Sun B. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J. Exp. Clin. Cancer Res. 2010;29:16–25. doi: 10.1186/1756-9966-29-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longo-Imedio M. I., Longo N., Treviño I., Lázaro P., Sánchez-Mateos P. Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int. J. Cancer. 2005;117:861–865. doi: 10.1002/ijc.21269. [DOI] [PubMed] [Google Scholar]
- 10.Mashino K., Sadanaga N., Yamaguchi H., Tanaka F., Ohta M., Shibuta K., Inoue H., Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- 11.Müller A., Homey B., Soto H., Ge N., Catron D., Buchanan M. E., McClanahan T., Murphy E., Yuan W., Wagner S. N., Barrera J. L., Mohar A., Verástegui E., Zlotnik A. Involvement of chemokine receptor in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 12.Murakami T., Maki W., Cardones A. R., Fang H., Tun Kyi A., Nestle F. O., Hwang S. T. Expression of CXC chemokine receptor-4 enhance the pulmonary metastasis potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 13.Murakami T., Cardones A. R., Hwang S. T. Chemokine receptors and melanoma metastasis. J. Dermatol. Sci. 2004;36:71–78. doi: 10.1016/j.jdermsci.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Robledo M. M., Bartolome R. A., Longo N., Rodríguez-Frade J. M., Mellado M., Longo I., van Muijen G. N., Sánchez-Mateos P., Teixidó J. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J. Biol. Chem. 2001;276:45098–45105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- 15.Saeki H., Moor A. M., Brown M. J., Hwang S. T. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- 16.Saur D., Seidler B., Schneider G., Algül H., Beck R., Senekowitsch-Schmidtke R., Schwaiger M., Schmid R. M. CXCR4 expression increases liver lung metastasis in a mouse model of pancreatic cancer. Gastroenterology. 2005;129:1237–1250. doi: 10.1053/j.gastro.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 17.Scala S., Ottaiano A., Ascierto P. A., Cavalli M., Simeone E., Giuliano P., Napolitano M., Franco R., Botti G., Castello G. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin. Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 18.Scala S., Giuliano P., Ascierto P. A., Ieranò C., Franco R., Napolitano M., Ottaiano A., Lombardi M. L., Luongo M., Simeone E., Castiglia D., Mauro F., De Michele I., Calemma R., Botti G., Caracò C., Nicoletti G., Satriano R. A., Castello G. Human melanoma metastases express functional CXCR4. Clin. Cancer Res. 2006;12:2427–2433. doi: 10.1158/1078-0432.CCR-05-1940. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi H., Fujimoto A., Tanaka M., Yamano T., Hsueh E., Hoon D. S. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin. Cancer Res. 2004;10:2351–2358. doi: 10.1158/1078-0432.ccr-03-0195. [DOI] [PubMed] [Google Scholar]
- 20.Toyozawa S., Yamamoto Y., Ishida Y., Kondo T., Nakamura Y., Furukawa F. Immunohistochemical analysis of CXCR4 expression in fibrohistiocytic tumors. Acta Histochem. Cytochem. 2010;43:45–50. doi: 10.1267/ahc.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Xi L., Hunt J. L., Gooding W., Whiteside T. L., Chen Z., Godfrey T. E., Ferris R. L. Expression pattern of chemokine receptor (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies novel metastatic potential. Cancer Res. 2004;64:1861–1866. doi: 10.1158/0008-5472.can-03-2968. [DOI] [PubMed] [Google Scholar]
- 22.Zlotnik A. Chemokines and cancer. Int. J. Cancer. 2006;119:2026–2029. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]